Abstract
Antineutrophil cytoplasmic antibodies (ANCA) of IgG class have been described at high prevalence in autoimmune hepatitis (AIH) and primary sclerosing cholangitis (PSC). Data on IgA class ANCA in these diseases are limited. The aim of this study was to determine the prevalence and fluorescence patterns of IgA class ANCA in AIH and PSC and to examine a relationship between the presence of IgA ANCA and clinical characteristics in these patients. Sera from 35 patients with PSC (21 with concomitant inflammatory bowel disease), 40 patients with AIH and 10 healthy controls were studied. ANCA were detected on ethanol-fixed neutrophils using an indirect immunofluorescence technique. ANCA of the IgA class were found in 20% of sera from patients with PSC and in 50% of AIH sera. The majority of AIH patients with IgA class ANCA showed a ‘classical’ perinuclear staining pattern, whereas the ‘classical’ and ‘atypical’ perinuclear fluorescence patterns were distributed equally in PSC. In sera containing IgG and IgA class ANCA simultaneously, IgG class ANCA showed an ‘atypical’ pANCA fluorescence pattern whereas IgA class ANCA produced a ‘classical’ perinuclear staining. The presence of IgA class ANCA was not associated with disease-specific clinical characteristics. IgA class ANCA are more frequently detected in sera of patients with AIH than PSC. The diversity of fluorescence patterns points to different target antigens of IgA class ANCA with distinct subcellular localizations.
Keywords: ANCA, IIF, immunoglobulin A, liver disease
INTRODUCTION
Antineutrophil cytoplasmic antibodies (ANCA) are a heterogeneous group of autoantibodies directed against cellular components of the polymorphonuclear leucocyte. The role of IgG class ANCA as diagnostic markers of autoimmune liver disorders is accepted widely [1]. Detected by indirect immunofluorescence microscopy on ethanol-fixed neutrophils, ANCA were described first in vasculitic disorders, particularly in Wegener's granulomatosis and microscopic polyangiitis [2–4]. Later on, ANCA were also found in autoimmune-mediated liver diseases, such as primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH). ANCA prevalences of 65–87% in patients with PSC and up to 96% in sera from AIH patients have been reported [5–9]. Among the three fluorescence patterns of ANCA, a diffuse cytoplasmic staining pattern (cANCA) is characteristic of Wegener's granulomatosis, whereas ANCA in patients with PSC or AIH either produce a ‘classical’ perinuclear (pANCA) fluorescence pattern with highlighting of the perinuclear cytoplasm or an ‘atypical’ perinuclear (‘atypical’ pANCA) fluorescence pattern with staining of the nuclear periphery and multiple intranuclear fluorescent foci [10–12].
ANCA of IgG class have been studied extensively in patients with PSC or AIH, whereas little information is available about the prevalence and characteristics of IgA class ANCA in these disorders. In PSC, ANCA of IgA class have been reported in up to 55% of patients [13–15]. However, the prevalence of IgA class ANCA in patients with AIH has not yet been evaluated. Recent reports have demonstrated IgA class ANCA in 28–52% of patients with ulcerative colitis (UC) [13,16–16]. Up to 80% of patients with PSC are diagnosed simultaneously with inflammatory bowel disease (IBD), particularly UC, thus the prevalence of IgA class ANCA might differ with respect to concomitant IBD [16,20]. Furthermore, IgA has long been accepted as an important factor in mucosal immunity, particularly of the gut-associated lymphoid tissue (GALT). GALT involvement in ANCA production would suggest the presence of IgA class ANCA.
To determine whether ANCA of IgA class are useful in the diagnosis of PSC and AIH, we investigated their prevalence and characteristic fluorescence patterns and analysed our study cohort for a putative relationship between the presence of IgA class ANCA and clinical characteristics of PSC or AIH.
MATERIALS AND METHODS
Patients
Primary sclerosing cholangitis
Sera of 35 patients with PSC were examined. The diagnosis of PSC was based on the presence of elevated serum markers of cholestasis such as alkaline phosphatase, gamma-glutamyltransferase and bilirubin along with characteristic findings in endoscopic retrograde cholangiography (diffuse narrowing, irregularities, budding of extra- and intrahepatic bile ducts). Histological characteristics obtained from liver biopsy included the presence of portal tract inflammation, periductal fibrosis and loss of small bile ducts [21–22]. The clinical and biochemical characteristics of the patients with PSC are summarized in Table 1.
Table 1.
Clinical and biochemical characteristics of patients with primary sclerosing cholangitis
| Characteristics | |
|---|---|
| No. of patients | 35 |
| Sex (m/f) | 29/6 |
| Median age in years (range) | 42 (16–70) |
| Presence of cirrhosis | 3 |
| Biochemical characteristics | |
| Alanine aminotransferase (5–19 U/l) | 42 ± 20 |
| Alkaline phosphatase (40–190 U/l) | 532 ± 261 |
| Total bilirubin (0·1–1·1 mg/dl) | 1·3 ± 1·1 |
| Mayo risk score1 | −1·26 ± 0·84 |
| Associated IBD | 21 |
| Ulcerative colitis | 16 |
| Crohn's disease | 4 |
| Indeterminate colitis | 1 |
| Disease activity of associated IBD | |
| Active | 15 |
| Remission | 6 |
| Localization of associated IBD | |
| Pancolitis | 15 |
| Partial involvement of the gut | 5 |
| No detectable inflammation | 1 |
| Treatment at time of testing | |
| No treatment | 20 |
| Azulfidine/5-ASA | 13 |
| 5-ASA plus prednisolone | 2 |
IBD, inflammatory bowel disease.
According to [23].
Autoimmune hepatitis
We investigated sera of 40 patients with AIH. The diagnosis of AIH was based on the criteria proposed by the International Autoimmune Hepatitis Study Group [24]. According to these diagnostic criteria, all patients had ‘definite AIH’. Table 2 shows the clinical and serological characteristics of the cohort of AIH patients. The study was approved by the Institutional Review Board of the University of Bonn.
Table 2.
Clinical and biochemical characteristics of patients with autoimmune hepatitis
| Characteristics | |
|---|---|
| No. of patients | 40 |
| Sex (m/f) | 8/32 |
| Median age in years (range) | 45 (1–69) |
| Presence of cirrhosis | 7 |
| Biochemical characteristics | |
| Alanine aminotransferase (5–19 U/l) | 253 ± 271 |
| Alkaline phosphatase (40–190 U/l) | 168 ± 63 |
| Total bilirubin (0·1–1·1 mg/dl) | 1·3 ± 0·6 |
| Treatment at time of testing | |
| Active disease | 28 |
| No treatment1 | 12 |
| Prednisolone | 3 |
| Prednisolone plus azathioprine | 13 |
| Disease in remission | 12 |
| Prednisolone, low-dose | 4 |
| No immunosuppressive therapy | 8 |
Treatment had not been initiated as sera were obtained when diagnosis was first established.
Healthy controls
Sera from 10 healthy volunteers (seven females, three males, median age 32 years, range 20–42 years) were included. All serum samples were obtained by standard venous puncture, centrifuged and stored at −20°C.
ANCA detection
ANCA were detected using indirect immunofluorescence (IIF) microscopy according to the guidelines of the First International Workshop on ANCA [2]. Commercially available slides with ethanol-fixed human neutrophils (Inova Diagnostics, San Diego, CA, USA) were used as substrate. Briefly, the neutrophil slides were incubated with serum samples, diluted 1 : 20 in phosphate-buffered saline (PBS), for 20 min at room temperature in a humidified chamber. After extensive washing with PBS, bound ANCA were detected using affinity-purified polyclonal fluorescein isothiocyanate (FITC)-conjugated goat antihuman IgG(1−4) (Inova Diagnostics) or affinity-purified polyclonal FITC-conjugated rabbit antihuman IgA(1+2) (Inova Diagnostics) for 20 min at room temperature in a humidified chamber, followed by extensive washing in PBS. After mounting with an antifading medium, slides were viewed with a Leitz SM-Lux microscope (Wetzlar, Germany) equipped with epifluorescent optics and photographed using Kodak Ektachrome 320 JT colour slide films (Rochester, NY, USA). Slides were viewed by two independent observers, who were unaware of the clinical diagnosis of the patients.
Propidium iodide staining
Ethanol-fixed neutrophil slides (Inova Diagnostics) were incubated with sera containing ANCA as described above. Counterstaining with the DNA-specific dye propidium iodide (Sigma, St Louis, MO, USA; diluted 1 : 2·500 for 20 min, 20°C) was used to mark the nuclear borders clearly and to detect possible overlapping of the fluorescence signals given by FITC-conjugated secondary anti-IgG or -IgA antibodies with propidium iodide.
Statistical analysis
Statistical analysis was performed using the Fisher's exact test. P-values of <0·05 were considered as significant.
RESULTS
Prevalence of ANCA with respect to their immunoglobulin class
All sera were investigated for the presence of ANCA with respect to their immunoglobulin classes IgG and IgA. ANCA of the IgG class were found in 77% (27/35) of sera from patients with PSC and in 85% (34/40) from patients with AIH. ANCA of the IgA class were detected significantly more frequently in patients with AIH than in patients with PSC [50% (20/40) compared to 20% (7/35); P < 0·05] (Table 3). Except for a single serum from a PSC patient with concomitant IBD and two sera from AIH patients, all remaining sera, which were positive for IgA class ANCA, also contained ANCA of the IgG class. In none of the sera from healthy controls, ANCA of either class were detected (data not shown).
Table 3.
Prevalence of ANCA detected by anti-IgG and anti-IgA secondary antibodies in patients with primary sclerosing cholangitis (with IBD/without IBD) and autoimmune hepatitis
| Primary sclerosing cholangitis | ||||
|---|---|---|---|---|
| total (n = 35) | with IBD (n = 21) | without IBD (n = 14) | Autoimmune hepatitis (n = 40) | |
| IgA ANCA only | 1/35 (3%) | 1/21 (5%) | 0/14 (0%) | 2/40 (5%) |
| IgG ANCA only | 21/35 (60%) | 11/21 (52%) | 10/14 (71%) | 16/40 (40%) |
| IgA and IgG ANCA | 6/35 (17%) | 4/21 (19%) | 2/14 (14%) | 18/40 (45%) |
| Total ANCA positive | 28/35 (80%) | 16/21 (76%) | 12/14 (86%) | 36/40 (90%) |
| Total ANCA negative | 7/35 (20%) | 5/21 (24%) | 2/14 (14%) | 4/40 (10%) |
ANCA, antineutrophil cytoplasmic antibody; IBD, inflammatory bowel disease.
Fluorescence staining patterns of IgA and IgG class ANCA
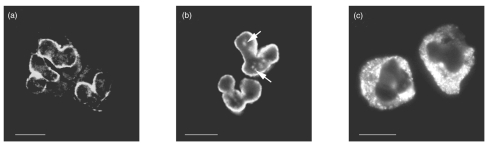
In our study cohort, three well-established staining patterns of ANCA were detected with respect to the IgA and IgG class of the autoantibody: (1) a perinuclear fluorescence pattern with a staining of the perinuclear cytoplasm (‘classical’ p-ANCA, Fig. 1a); (2) a so-called ‘atypical’ perinuclear staining pattern with highlighting of the nuclear periphery and scattered intranuclear fluorescent spots (‘atypical’ p-ANCA, Fig. 1b); and (3) a diffuse cytoplasmic fluorescence (c-ANCA, Fig. 1c). The atypical p-ANCA staining emerged as the predominant fluorescence pattern of IgG class ANCA in PSC (81%) and AIH (91%) (Table 4). In patients with PSC, IgA class ANCA yielded either a perinuclear (43%) or an atypical perinuclear (43%) staining pattern, whereas the majority of IgA class ANCA in sera from AIH patients showed a ‘classical’ p-ANCA fluorescence pattern (83%). A cytoplasmic staining pattern of IgG or IgA class ANCA was not found in any of the sera from AIH patients. In PSC patients, only a single serum containing IgG and IgA class ANCA yielded a cytoplasmic fluorescence.
Fig. 1.
Fluorescence patterns of IgA class ANCA in patients with primary sclerosing cholangitis or autoimmune hepatitis. IgA class ANCA were detected using FITC-conjugated rabbit antihuman IgA-antibodies producing a green signal at 525 nm wavelength and were visualized by indirect immunofluorescence microscopy on ethanol-fixed neutrophils. (a) A perinuclear staining pattern (‘classical’ p-ANCA) with an intense highlighting of the perinuclear cytoplasm, detected in a serum of a patient with autoimmune hepatitis. (b) A rim-like staining of the nuclear periphery along with scattered intranuclear fluorescent foci (→) (‘atypical’ p-ANCA) as shown in a serum from a patient with primary sclerosing cholangitis. (c) A cytoplasmic staining pattern (c-ANCA) with accentuation of the fluorescence between the nuclear lobes, demonstrated in a serum from a patient with primary sclerosing cholangitis. Size bars indicate 10 µm.
Table 4.
Distribution of ANCA staining patterns as detected by anti-IgG and anti-IgA secondary antibodies in primary sclerosing cholangitis and autoimmune hepatitis
| PSC (n = 35) | AIH (n = 40) | |||
|---|---|---|---|---|
| ANCA staining pattern | IgG ANCA | IgA ANCA | IgG ANCA | IgA ANCA |
| ANCA positive | 27/35 (77%) | 7/35 (20%) | 34/40 (85%) | 18/40 (45%) |
| Perinuclear | 4/27 (15%) | 3/7 (43%) | 3/34 (9%) | 15/18 (83%) |
| Atypical perinuclear | 22/27 (81%) | 3/7 (43%) | 31/34 (91%) | 3/18 (17%) |
| Cytoplasmic | 1/27 (4%) | 1/7 (14%) | 0/34 (0%) | 0/18 (0%) |
| Negative | 8/35 (23%) | 28/35 (80%) | 6/40 (15%) | 22/40 (55%) |
IIF, indirect immunofluorescence; PSC, primary sclerosing cholangitis; AIH, autoimmune hepatitis; ANCA, antineutrophil cytoplasmic antibody.
Change of staining pattern in sera simultaneously positive for IgA and IgG class ANCA
Twenty-one per cent (6/28) of ANCA-positive sera from PSC patients and 50% (18/36) of sera from AIH patients contained simultaneously both IgA and IgG class ANCA. It is important to note that in these patients the fluorescence pattern varied according to the Ig class of ANCA. In 33% (2/6) of sera from PSC patients and 78% (14/18) of sera from AIH patients, ANCA gave an ‘atypical’ perinuclear fluorescence staining when using FITC-conjugated anti-IgG secondary antibodies, while a ‘classical’ perinuclear staining was produced when FITC-conjugated anti-IgA secondary antibodies were applied (Fig. 2a,b). This change of ANCA fluorescence pattern was confirmed by two independent investigators.
Fig. 2.
Variation of the ANCA staining pattern with respect to the immunoglobulin class specificity of the secondary antibody in sera containing simultaneously IgG and IgA class ANCA. The different staining patterns were visualized on ethanol-fixed human neutrophils by indirect immunofluorescence microscopy. A serum from a patient with autoimmune hepatitis was used. IgG ANCA were detected with FITC-conjugated goat antihuman IgG antibodies and IgA class ANCA with FITC-conjugated rabbit antihuman IgA antibodies, each of them producing a green fluorescence signal at 525 nm wavelength. The neutrophils were counterstained with propidium iodide giving a red fluorescence signal at 570 nm wavelength. When both fluorescence signals were optically superimposed, a co-localization appeared yellow. (a) The ‘atypical’ perinuclear staining pattern (‘atypical’ pANCA) detected by FITC-conjugated IgG secondary antibodies changed to (b) a ‘classical’ perinuclear staining pattern when FITC-conjugated IgA secondary antibodies were applied. (c) The fluorescence signal produced by ‘atypical’ perinuclear IgG class ANCA overlapped completely with the staining given by propidium iodide in the nuclear periphery and in the area of the intranuclear fluorescent spots. (d) In contrast, the ‘classical’ perinuclear staining detected with IgA class ANCA did not co-localize with the propidium iodide staining. A homogeneous rim-like staining of the perinuclear cytoplasm was detected. Size bars indicate 10 µm.
To support our previous hypothesis that the ‘atypical’ and ‘classical’ perinuclear fluorescence patterns of ANCA are clearly distinct and are due to different subcellular localizations of the target antigens, counterstaining with the nuclear DNA dye propidium iodide was performed [11,25]. The ‘atypical’ perinuclear staining of IgG class ANCA characterized by a perinuclear fluorescence and scattered intranuclear fluorescent spots completely overlapped with the staining given by propidium iodide in the nuclear periphery (Fig. 2c). In contrast, IgA class ANCA yielding a ‘classical’ perinuclear staining pattern gave a homogeneous rim-like fluorescence signal in the perinuclear cytoplasm not overlapping with the nuclear fluorescence signal produced by the propidium iodide staining (Fig. 2d).
Relationship between the presence of IgA class ANCA and clinical characteristics
No association was found between the presence or absence of IgA ANCA and sex, disease activity, immunosuppressive therapy, presence of other autoantibodies, concomitant IBD and extent of bowel disease in the PSC and AIH patients of our study population.
DISCUSSION
Previous reports on ANCA in autoimmune liver diseases have focused on ANCA of the IgG class, whereas the presence and role of IgA class ANCA as diagnostic and clinical seromarkers have rarely been studied in PSC [13–15] and, to our knowledge, have not so far been evaluated in AIH.
In the present study, IgA class ANCA were detected in 50% of AIH patients. A lower prevalence was found in PSC patients where only 20% of the sera showed IgA class ANCA, which is in agreement with previous reports [13,14]. Only one of 35 sera from PSC patients and two of 40 sera from AIH patients contained IgA class ANCA exclusively, whereas the remaining sera which were positive for IgA class ANCA also contained ANCA of IgG class. In ulcerative colitis, it has been proposed that the combination of IgA and IgG class ANCA testing notably increases the sensitivity of ANCA detection [17]. However, in our study cohort sensitivity of ANCA testing increased only from 77% for IgG ANCA alone up to 80% for the combination of IgA and IgG testing in PSC and from 85% for IgG alone up to 90% for combined testing of IgA and IgG ANCA in AIH.
No significant relationship between the presence of IgA ANCA and clinical parameters was detectable in our patients. This finding is in line with several other studies demonstrating no significant association between the serum titre of IgG class ANCA and the disease activity, extent, duration or the intake of immunosuppressive drugs, respectively [7,14,15,26,27]. Only a few studies were able to show an association between single clinical parameters and the presence of IgG class ANCA. In PSC, the presence of liver cirrhosis and higher liver transplantation rates have been proposed to be associated with the presence of IgG ANCA [7,28]. In AIH patients, Roozendaal et al. [20] observed recently that the presence of IgG ANCA was related to relapsing disease and longer duration of the disorder.
The staining pattern of IgA class ANCA using IIF varied with respect to the type of autoimmune liver disease. In sera from PSC patients, IgA class ANCA produced a ‘classical’ perinuclear and an ‘atypical’ perinuclear fluorescence pattern at equal prevalences. This observation points to different target antigens of IgA class ANCA with distinct subcellular localizations. In contrast, the majority of IgA class ANCA from patients with AIH showed a ‘classical’ perinuclear staining pattern. Thus, IgA class ANCA in AIH and to some extent in PSC are directed most probably against a cytoplasmic antigen.
Interestingly, a change of staining pattern from an ‘atypical’ perinuclear fluorescence to a ‘classical’ perinuclear staining was seen in 78% of AIH patients and 33% of PSC patients, which were positive simultaneously for IgG and IgA ANCA. Additional counterstaining with propidium iodide further supported our observation that IgG and IgA class ANCA in these patients recognized target antigens of different subcellular localizations. The putative target antigen(s) of IgA class ANCA in autoimmune liver disorders has not yet been identified. For IgG class ANCA, several proteins of the cytosol (actin, catalase, enolase) and the azurophilic (bactericidal/permeability increasing protein, cathepsin G) or specific granules (lactoferrin) have been suggested as target antigens [29,32]. Recent studies described nuclear proteins, particularly of the nuclear periphery, as candidate proteins of ‘atypical’ p-ANCA [33–36]. Using enzyme-linked immunosorbent assays, we could not demonstrate any reactivity of IgA class ANCA with the aforementioned cytosolic and granular proteins (data not shown). Western blot analysis performed with whole cell lysates from neutrophils or human promyelocytic HL-60 cells and sera positive for IgG and IgA class ANCA revealed a heterogeneous pattern of reactive proteins of IgA class ANCA. Using particular subcellular fractionation methods, very recent data pointed to a family of putative candidate proteins. Further studies will focus on the molecular identification of the target antigen(s) of IgA class ANCA in autoimmune liver diseases and should analyse whether IgA and IgG class ANCA share common antigens.
Immunoglobulin A is an important factor of mucosal immunity, particularly of the gut-associated lymphoid tissue (GALT). In the mucosal response, antibodies of the IgA2 subclass are mainly involved and produced by lamina propria lymphocytes and subepithelial plasma cells [37]. In UC patients, Targan and coworkers [38] demonstrated ANCA in supernatants of intestinal lamina propria cells but not in supernatants of peripheral blood lymphocytes or extracts of mesenterial lymph nodes indicating the existence of ANCA producing B cell clones in the GALT. Detection of IgA antibodies in 24% of PSC patients with concomitant IBD may also point to an involvement of the GALT in this particular subgroup of our study cohort. This hypothesis needs further confirmation as our method did not allow differentiation of both IgA subclasses.
However, in AIH patients and PSC patients without IBD other mechanisms may be involved in the production of IgA class ANCA. Recent studies have demonstrated that the biliary epithelium is a target for immune-mediated injury and acts as an active participant in the immune response [39,40]. Furthermore, antigenic mimicry by constituents of microorganisms which activate hepatic macrophages in an immunogenetically susceptible host may trigger the autoantibody formation [41]. The resulting increase in peribiliary cytokine and chemokine secretion may attract neutrophils, monocytes, T and B lymphocytes and finally set the stage for autoantibody production [42]. Thus, the high prevalence of IgA class ANCA, particularly in AIH patients, might result from ANCA production by subepithelial plasma cells of the biliary system or plasma cells in the inflammatory infiltrates of the liver. Our observation that the prevalence of IgA class ANCA in PSC patients did not differ with respect to associated IBD would support further this hypothetical model of biliary derived ANCA production in PSC.
In conclusion, IgA class ANCA are present at significantly higher prevalence in AIH patients than in PSC patients. Nevertheless, their overall prevalence is significantly lower than that of IgG class ANCA in these disorders. In our study group, we found no significant relationship between the presence of IgA class ANCA and clinical parameters of AIH and PSC. The characteristic change of staining pattern from predominantly ‘atypical’ perinuclear fluorescence of IgG class ANCA to ‘classical’ perinuclear fluorescence of IgA class ANCA points to different target antigens with distinct subcellular localizations. Molecular identification of the responsible target antigens may provide important insights in the putative role of ANCA – and particularly their different immunoglobulin classes − in the immunopathogenesis of autoimmune liver disorders.
Acknowledgments
The study was supported by a grant of the Lise-Meitner-Foundation (to B.T.). The authors are most grateful to Ian McFarlane MD, King's College London, London UK, for providing serum samples.
REFERENCES
- 1.Roozendaal C, Kallenberg CGM. Anti-neutrophil autoantibodies (ANCA) in autoimmune liver diseases. Hepato-Gastroenterology. 1999;46:3034–40. [PubMed] [Google Scholar]
- 2.Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS. 1989;97(Suppl. 6):12–3. [PubMed] [Google Scholar]
- 3.Davies DJ, Moran JE, Niall NF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J. 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woude van der FJ, Lobatto S, Permin H, et al. Autoantibodies against neutrophils and monocytes: tools for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 5.Targan S, Landers C, Vidrich A, Czaja AL. High-titer antineutrophil cytoplasmic antibodies in type 1 autoimmune hepatitis. Gastroenterology. 1995;108:1159–66. doi: 10.1016/0016-5085(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 6.Zauli D, Ghetti S, Grassi A, et al. Anti-neutrophil cytoplasmic antibodies in type 1 and 2 autoimmune hepatitis. Hepatology. 1997;25:1105–7. doi: 10.1002/hep.510250510. [DOI] [PubMed] [Google Scholar]
- 7.Mulder AHL, Horst G, Haagsma EB, Kleibeuker JH, Kallenberg CGM. Antineutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411–7. [PubMed] [Google Scholar]
- 8.Hardarson S, Labrecque DR, Mitros FA, Neil GA, Goeken JA. Antineutrophil cytoplasmic antibody in inflammatory bowel and hepatobiliary diseases. Am J Clin Pathol. 1993;99:277–81. doi: 10.1093/ajcp/99.3.277. [DOI] [PubMed] [Google Scholar]
- 9.Bansi D, Chapman R, Fleming K. Antineutrophil cytoplasmic antibodies in chronic liver diseases: prevalence, titre, specificity and Ig G subclass. J Hepatol. 1996;24:581–6. doi: 10.1016/s0168-8278(96)80144-9. [DOI] [PubMed] [Google Scholar]
- 10.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 11.Terjung B, Herzog V, Worman HJ, et al. Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders co-localize with nuclear lamina proteins. Hepatology. 1998;28:332–40. doi: 10.1002/hep.510280207. [DOI] [PubMed] [Google Scholar]
- 12.Savige J, Gillis D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA) Am J Clin Pathol. 1999;111:507–13. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 13.Peen E, Almer S, Bodemar G, et al. Antilactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo SK, Fleming KA, Chapman RW. Prevalence of anti-neutrophil antibody in primary sclerosing cholangitis and ulcerative colitis using an alkaline phosphatase technique. Gut. 1992;33:1370–5. doi: 10.1136/gut.33.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–62. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellerbroek PM, Oudkerk Pool M, Ridwan BU, et al. Neutrophil cytoplasmic antibodies (p-ANCA) in ulcerative colitis. J Clin Pathol. 1994;47:257–62. doi: 10.1136/jcp.47.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gigase P, De Clerck LS, Van Cotthem KA, et al. Anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease with special attention for IgA-class antibodies. Dig Dis Sci. 1997;42:2171–4. doi: 10.1023/a:1018803509150. [DOI] [PubMed] [Google Scholar]
- 18.Esteve M, Mallolas J, Klaassen J, et al. Factors related to the presence of IgA class antineutrophil cytoplasmic antibodies in ulcerative colitis. Am J Gastroenterol. 1998;93:615–8. doi: 10.1111/j.1572-0241.1998.175_b.x. [DOI] [PubMed] [Google Scholar]
- 19.Müller-Ladner U, Gross V, Andus T, et al. Distinct patterns of immunoglobulin classes and IgG subclasses of autoantibodies in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1996;8:579–84. doi: 10.1097/00042737-199606000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Roozendaal C, de Jong MA, van den Berg AP, van Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J Hepatol. 2000;32:734–41. doi: 10.1016/s0168-8278(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 21.Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology. 1999;30:325–32. doi: 10.1002/hep.510300101. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner RH. Diagnostic criteria, clinical manifestations and natural history of primary sclerosing cholangitis. In: Krawitt EL, Wiesner RH, Nisioka M, editors. Autoimmune liver diseases. 2. Amsterdam: Elsevier Science Publisher BV; 1998. pp. 381–412. [Google Scholar]
- 23.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–94. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 25.Terjung B, Worman HJ, Herzog V, Sauerbruch T, Spengler U. Differentiation of antineutrophil nuclear antibodies in inflammatory bowel and autoimmune liver diseases from antineutrophil cytoplasmic antibodies using immunofluorescence microscopy. Clin Exp Immunol. 2001;126:37–46. doi: 10.1046/j.1365-2249.2001.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Targan SR, Landers CJ, LaRusso NF, Lindsay KL, Wiesner RH, Shanahan F. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385–91. [PubMed] [Google Scholar]
- 27.Roozendaal C, van Milligen de Wit AWM, Haagsma EB, et al. Antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis: defined specificities may be associated with distinct clinical features. Am J Med. 1998;105:393–9. doi: 10.1016/s0002-9343(98)00294-0. [DOI] [PubMed] [Google Scholar]
- 28.Pokorny CS, Norton ID, McCaughan GW, Selby WS. Anti-neutrophil cytoplasmic antibody: a prognostic indicator in primary sclerosing cholangitis. J Gastroenterol Hepatol. 1994;9:40–4. doi: 10.1111/j.1440-1746.1994.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 29.Orth T, Gerken G, Kellner R, Meyer zum Buesschenfelde KH, Mayet WJ. Actin is a target antigen of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune hepatitis type-1. J Hepatol. 1997;26:37–47. doi: 10.1016/s0168-8278(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 30.Orth T, Kellner R, Diekmann O, Faust J, Meyer zum Buesschenfelde KH, Mayet WJ. Identification and characterization of autoantibodies against catalase and α-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol. 1998;112:507–15. doi: 10.1046/j.1365-2249.1998.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roozendaal C, Zhao MH, Horst G, et al. Catalase and alpha-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:10–6. doi: 10.1046/j.1365-2249.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walmsley RS, Zhao MH, Hamilton MI, et al. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997;40:105–9. doi: 10.1136/gut.40.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobajima J, Ozaki S, Uesugi H, et al. High mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 are significant target antigens of perinuclear anti-neutrophil cytoplasmic antibodies in autoimmune hepatitis. Gut. 1999;44:867–73. doi: 10.1136/gut.44.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggena M, Cohavy O, Parseghian MH, et al. Identification of histone H1 as a cognate antigen of the ulcerative colitis-associated marker antibody pANCA. J Autoimmun. 2000;14:83–97. doi: 10.1006/jaut.1999.0340. [DOI] [PubMed] [Google Scholar]
- 35.Terjung B, Spengler U, Sauerbruch T, Worman HJ. Atypical p-ANCA in IBD and hepatobiliary disorders react with a 50 kD nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310–22. doi: 10.1053/gast.2000.9366. [DOI] [PubMed] [Google Scholar]
- 36.Mallolas J, Esteve M, Rius E, Cabré E, Gassull MA. Antineutrophil antibodies associated with ulcerative colitis interact with the antigen(s) during the process of apoptosis. Gut. 2000;47:74–8. doi: 10.1136/gut.47.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mestecky J, McGhee JR, Elson CO. Intestinal IgA system. Immunol Allergy Clin North Am. 1988;8:349–68. [Google Scholar]
- 38.Targan SR, Landers CJ, Cobb L, MacDermott RP, Vidrich A. Perinuclear anti-neutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells of ulcerative colitis patients. J Immunol. 1995;155:3262–7. [PubMed] [Google Scholar]
- 39.Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120:1485–501. doi: 10.1053/gast.2001.22441. [DOI] [PubMed] [Google Scholar]
- 40.Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351–7. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 41.Czaja A, Homburger HA. Antibodies in liver disease. Gastroenterology. 2001;120:239–49. doi: 10.1053/gast.2001.20223. [DOI] [PubMed] [Google Scholar]
- 42.Vierling J. Aetiopathogenesis of primary sclerosing cholangitis. In: Manns M, Chapman RW, Stiehl A, Wiesner R, editors. Primary sclerosing cholangitis. London: Kluwer Academic Publishers; 1998. pp. 37–45. [Google Scholar]