Abstract
The interaction between receptors for the Fc portion of IgG (FcγRs) from monocytes/macrophages and immune complexes (IC) triggers regulatory and effector functions. Recently, we have demonstrated that IC exert a drastic inhibition of basal and IFN-γ-induced expression of MHC class II on human monocytes. Taking into account that the regulation of MHC class II molecules is a crucial event in the immune response, in this report we extend our previous studies analysing the effect of STAT-1 phosphorylation in the down-regulatory process, the fate of the intracellular pool of MHC class II molecules and the effect of complement on MHC class II down-regulation induced by IC. We also studied the effect of IC on the expression of MHC class II (I-Ad) in macrophages using a mouse model of chronic inflammation. We demonstrate that IC induce a depletion not only on surface expressed but also on intracellular MHC class II content and that IC-induced down-regulation of MHC class II is not mediated by the inhibition of STAT-1 phosphorylation. On the other hand, the effect of IC is not specific for the down-regulation of MHC class II, for it could be restricted to other molecules involved in inflammatory processes. Our experiments also show that the activation of the complement system could be a crucial step on the regulation of the effect of IC on MHC class II expression. In agreement with our in vitro experiments using human monocytes, IC treatment reduces the expression of MHC class II in a mouse model of chronic inflammation.
Keywords: inflammation, IC, MHC class II, monocyte
INTRODUCTION
The formation of circulating immune complexes (IC) is the physiological consequence of antibody response to different antigens, including microorganisms [1], or the result of autoimmune disorders such as vasculitis, lupus erythematosus, or rheumatoid arthritis [2]. These IC can interact with receptors for the Fc portion of IgG (FcγRs) present on cells of the immune system, triggering a variety of regulatory and effector functions [3–8].
Three distinct types of FcγRs (FcγRI, FcγRII and FcγRIII) are distributed widely and heterogeneously on different cells including B and T lymphocytes, neutrophils and monocytes/macrophages [9–11]. Monocytes, which express FcγRI and FcγRII constitutively, are known as one of the most important cells involved in inflammatory processes. Thus, the interaction between FcγRs from monocytes/macrophages and IC, their natural ligands, enables these cells to induce the secretion of different substances such as enzymes, oxygen species, prostaglandins, leukotrienes [3–5], a variety of cytokines such as interleukin-1β (IL-1α), IL-6, IL-10, tumour necrosis factor alpha (TNF-α) [6,7] and also to increase intracellular cAMP levels [8].
Interferon-γ (IFN-γ) activates monocytes/macrophages by enhancing the expression of FcγRI and class II molecules from the major histocompatibility complex (MHC class II) [12–17]. Recently, we have demonstrated that precipitating ovalbumin (OA)-rabbit IgG anti-OA IC exerted a drastic and reversible inhibition of basal and IFN-γ-induced expression of MHC class II on human monocytes. In parallel with MHC class II down-regulation, the interaction of IC with both FcγRI and FcγRII induced the inhibition of antigen presentation. These effects were abrogated by the protease inhibitors pepstatin and phosphoramidon, supporting the role of aspartic and metalloprotease(s) in this process. Moreover, similar results were obtained using supernatants from IC-treated peripheral blood mononuclear cells (PBMC) [18].
Taking into account that the regulation of MHC class II molecules is a crucial event in the immune response, in this report we extend our previous studies analysing the effect of STAT-1 phosphorylation in the down-regulatory process as well as the fate of the intracellular pool of MHC class II molecules. In addition, as the activation of complement is a crucial step in the regulation of IC–FcγRs interactions [19–21], we evaluated the effect of complement on MHC class II down-regulation induced by IC. We also studied the effect of IC on the expression of MHC class II (I-Ad) in macrophages using a mouse model of chronic inflammation.
MATERIALS AND METHODS
Reagents
Ficoll 400, tissue culture medium (RPMI-1640), saponin, human recombinant IFN-γ, mouse recombinant IFN-γ and chromatographically purified ovalbumin (OA) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Percoll was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). Fluorescein isothiocyanate (FITC)-labelled anti-HLA-DR (clone Immu-357), phycoerythrin (PE)-labelled anti-CD14 (clone RMO52) monoclonal antibodies (MoAb) and mouse IgG1 (clone 679·1Mc7) and IgG2a (clone U7·27) control isotype antibodies were obtained from Immunotech (Marseille, France). FITC-labelled antimouse I-Ad (clone 39-10-8) MoAb and mouse IgG3, κ control isotype were ordered from PharMingen, USA. Rabbit antihuman Phospho-STAT-1 (Tyr701) polyclonal antibody was from Cell Signaling Technology (Beverly, MA, USA). FITC-labelled goat F(ab′)2 antirabbit IgG (H + L) was obtained from Caltag Laboratories (Burlingame, CA, USA). FITC-labelled anti-HLA-ABC (clone B9·12·1), FITC-labelled anti-CD54 (ICAM-1) (clone 84H10) and FITC-labelled anti-CD11a (clone 25·3) were from Immunotech. FITC-labelled anti-CD11b (clone Bear1) was from Caltag Laboratories. Mouse antihuman Phospho-STAT-1 (Tyr701) MoAb was from Santa Cruz Biotechnology (CA, USA). Mouse anti-STAT-1 (C-terminus) (clone 42) was from Transduction Laboratories (Lexington, USA).
Preparation of immune complexes, heat-aggregated IgG and IgG-coated plates
Immune complexes (IC) were prepared using rabbit IgG antiovalbumin (OA) isolated from heat-inactivated serum by affinity chromatography. Precipitating ovalbumin (OA)-rabbit IgG anti-OA IC were prepared at threefold antigen excess, based on equivalence points determined by quantitative precipitation curves. Antigens and antibodies were incubated first for 30 min at 37°C and then for 1 h at 4°C. After this period, the IC formed were centrifuged at 10 000 g for 5 min, the supernatant was discarded and precipitating IC were resuspended at a concentration of 1 mg antibody/ml. Soluble human heat-aggregated IgG (agg-IgG) was prepared by heating purified human IgG at a concentration of 5 mg/ml for 12 min at 63°C. Then, heat-aggregated human IgG was centrifuged at 10 000 g for 5 min and the precipitate was discarded. This soluble agg-IgG was diluted in medium at a concentration of 1 mg/ml. IgG-coated plates were prepared by adding 40 µl aliquots of purified human IgG at a concentration of 0·5 mg/ml in saline to 96-well tissue culture-treated plates. Plates were then incubated at 37°C for 18 h and unbound antibodies were removed by washing with saline, just before the addition of the cells.
Preparation of human monocytes
Fresh human blood was obtained by venipuncture from healthy adult volunteers and collected on citrate/dextrose/adenine. Blood was diluted 1 : 2 with saline, layered on a Ficoll-Hypaque cushion and centrifuged at 400 g for 25 min as described previously [22]. PBMC were collected, centrifuged on a Percoll gradient and resuspended in RPMI 10% heat-inactivated (56°C, 30 min) fetal calf serum (FCS) and 50 µg/ml gentamycin. Viability of monocytes was >95% as determined by the trypan blue exclusion test.
Preparation of fixed, permeabilized monocytes for cytofluorometric analysis
Permeabilization of monocytes was performed in order to evaluate intracellular MHC class II, as described previously [23]. Briefly, monocytes were incubated with medium (control) or IC (100 µg/ml). After 1 h of incubation at 37°C, the cells were incubated with medium or IFN-γ (240 U/ml) for 24 h. After this period, aliquots of these cells were fixed with cold paraformaldehyde 0·5% for 30 min, washed, permeabilized in 0·04% saponin, 0·1% ovalbumin, phosphate buffered saline (PBS) and glycine buffer for 10 min and then stained with an anti-HLA-DR antibody for 30 min. The cells were washed with the same buffer and resuspended in Isoflow before cytometric analysis. Non-permeabilized control cells were treated identically but in the presence of a buffer without saponin. Non-specific binding was determined using mouse IgG1 isotype matched control antibody.
Detection of intracellular phosphorylated STAT-1 by flow cytometry
Human monocytes (2·5 × 106 cells/ml) were allowed to adhere onto γ-globulin-coated or uncoated plastic dishes for 1 h at 37°C. Then, the cells were treated with medium or IFN-γ (240 U/ml) for 15 min. After this period, the cells were removed, washed and resuspended in 100 µl of Reagent A (Fix & Perm) for 2–3 min at room temperature. The cells were then washed with PBS 2% FCS and resuspended in 3 ml of cold metanol 70% for 10 min at 0°C. Afterwards, the cells were washed with PBS 2% FCS and incubated with 100 µl of Reagent B (Fix & Perm) and the rabbit antihuman Phospho-STAT-1 (Tyr701) polyclonal antibody or normal rabbit IgG as control for 30 min at room temperature. After this period, the cells were washed with PBS 2% FCS and incubated with FITC-labelled goat F(ab′)2 antirabbit IgG (H + l) for 30 min at room temperature. Finally, the cells were washed with PBS 2% FCS, fixed with paraformaldehyde 0·5% and resuspended in Isoflow [24]. The phosphorylation of the transcription factor STAT-1 induced by IFN-γ was evaluated by flow cytometry.
Immunoprecipitation
For each condition, human monocytes at 2 × 106 cells/ml were used. At the end of the experimental treatment, the cells were washed with PBS. They were lysed by incubation on ice for 20 min in 0·5 ml 100 mm Tris HCl (pH 8·0), 100 mm NaCl, 2 mm EDTA, 1% Nonidet P-40 (RIPA buffer), 1 mm Na3VO4, 50 mm NaF, 0·3 U/ml aprotinin, 2 mm PMSF and 1 µg/ml each of leupeptin and pepstatin A. Lysates were centrifuged for 15 min at 14 000 g. Protein concentrations were determined using a micro Bradford assay (Pierce, Rockford, IL, USA). Lysates were incubated overnight at 4°C with anti-STAT-1 with rotation and then with protein G-Sepharose for 2 h with rotation. The beads were washed thoroughly with lysis buffer, and adsorbed proteins were solubilized in sample buffer and separated on 8% SDS-PAGE minigels using standard Tris-glycine buffers. Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA) for 1 h at 300 mA and blocked with PBS 3% non-fat dry milk for 1 h. The membrane was probed with primary antibody in PBS 0·4% BSA (antiphospho-STAT-1 MoAb or anti-STAT-1 MoAb for control) overnight. After washing three times with PBS 0·2% Tween 20, blots were incubated for 1 h with a HRP-conjugated goat antimouse IgG (Amersham, Aylesbury, UK). Immunoreactivity was detected using the enhanced chemiluminescence Western blotting detection reagent (ECL, Amersham).
In vivo studies
Open-ended glass cylinders of 2 cm long and 8 mm wide were introduced subcutaneously into adult BALB/c mice [25]. The cylinders caused a chronic inflammatory process and after 20 days appeared with fibrotic tissue ‘closing’ the open ends of the cylinders and also with an important infiltration of macrophages. At that moment, 50 µl of IC and/or 50 µl of IFN-γ (3000 U/ml) were injected into the cylinder with a tuberculin syringe. Twenty-four h later the cylinders were extracted from mice and the cells that had been recruited were collected and stained with an antibody against murine MHC class II (anti-I-Ad). Flow cytometry was performed and the macrophage population was analysed using macrophage-specific forward light scatter (FSC) and side light scatter (SSC) gates.
Flow cytometry
After different treatments, 0·5 × 106 human monocytes/ml or 0·35 × 106 cells obtained from the glass cylinders introduced into BALB/c mice were washed and incubated with the monoclonal antibodies indicated above. The cells were washed and resuspended in Isoflow and flow cytometry was performed on a FACS Analyser (Becton-Dickinson Immunocytometry System, San Jose, CA, USA). The results were expressed as the percentage of median of fluorescence intensity (% MFI) of untreated cells (controls). Purity of human monocytes, determined as CD14 positive cells, was > 90% in all donors. Non-specific binding was determined using the appropriate mouse IgG isotype matched control antibody.
Statistical analysis
Statistical significance of results was calculated using the non-parametric Mann–Whitney test (two-tailed). For the in vivo experiment the unpaired t-test was used.
RESULTS
Kinetics of the inhibition of MHC class II expression by IC
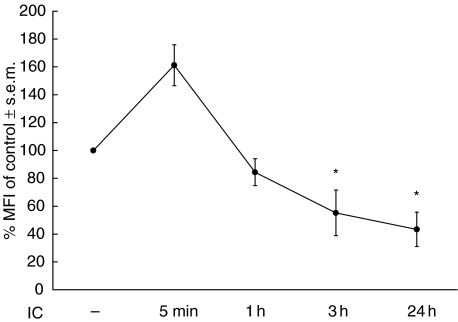
In view of the observation that after 24 h incubation of human monocytes with IC there is a down-regulation of the basal surface expression of MHC class II [18], it was of interest to study the kinetics of this effect. For this purpose, monocytes were incubated with IC for 5 min, 1 h, 3 h and 24 h. At these points, the expression of MHC class II was analysed by flow cytometry. As shown in Fig. 1, after 5 min of incubation with IC the basal expression of MHC class II was increased. However, after 1 h of incubation this expression reaches similar values to untreated cells, while at 3h- incubation of monocytes in the presence of IC the basal expression of MHC class II was reduced significantly. This IC-mediated down-regulation after 3 h of incubation was similar to that reached after 24 h of incubation, indicating that 3 h of incubation with IC is sufficient for the down-regulation of the basal expression of MHC class II. Untreated monocytes did not display any variation in class II expression during the 24 h of in vitro culture.
Fig. 1.
Kinetics of the effect of IC on basal MHC class II expression. Human monocytes (0·5 × 106 cells/ml) were incubated with medium (control) or IC for 5 min, 1 h, 3 h or 24 h. After these periods, the cells were stained with an anti-HLA-DR antibody and the expression of this molecule was evaluated by flow cytometry. Data are expressed as % MFI ± s.e.m. of control cells. Statistical significance was calculated using the Mann–Whitney test, two-tailed; n= 5. *P < 0·05 significantly different from control.
Effect of IC on intracellular MHC class II
The next experiment was designed to study whether the IC-mediated down-regulation also occurs on the intracellular pool of MHC class II. For this purpose, specific anti-HLA-DR staining of saponin permeabilized cells was used to evaluate the total amount of MHC class II, while staining of non-permeabilized cells was used to determine membrane-associated MHC class II. The differences between these two fluorescence intensity values represent the amount of intracellular of MHC class II [23]. As shown in Table 1, the preincubation of monocytes with precipitating ovalbumin (OA)-rabbit IgG anti-OA IC exerts a drastic reduction of the basal and IFN-γ-induced expression of both intracellular and surface MHC class II.
Table 1.
Effect of IC on intracellular MHC class II
| % MFI of control ± s.e.m. | |||
|---|---|---|---|
| Treatment | Surface | Intracellular | |
| – | IFN-γ | 316·3 ± 40·46* | 181·0 ± 46·51* |
| IC | – | 57·0 ± 8·6Ω | 56·5 ± 7·18Ω |
| IC | IFN-γ | 67·3 ± 16·38Ω | 65·25 ± 6·05Ω |
Human monocytes (0·5 × 106 cells/ml) were incubated with medium (control) or IC (100 µg/ml). After 1 h of incubation at 37°C, the cells were incubated with medium or IFN-γ(240 U/ml) for 24 h. After this period, human monocytes were permeabilized and stained with an anti-HLA-DR antibody to evaluate total MHC class II, or non-permeabilized and stained with the same antibody to evaluate surface MHC class II. The level of expression of MHC class II molecules was evaluated by flow cytometry. The difference between these two values of fluorescence intensity represents the intracellular MHC class II. Data are expressed as the percentage of median of fluorescence intensity (% MFI) ± s.e.m. of control cells. Statistical significance was calculated using the Mann–Whitney test, two-tailed; n= 5.
P < 0·05 significantly different from control. Ω,P < 0·05 significantly different from control and IFNγ-treated cells.
Because the down-regulation of MHC class II can be mediated by enzymes released by monocytes after IC–FcγR interaction [18], we studied the effect of supernatants (spn) obtained from IC-stimulated monocytes on the intracellular pool of MHC class II. For this purpose, monocytes were incubated with medium or IC. After 24 h the cell suspension was centrifuged and the spn were collected and added to naive monocytes for 3 h. The results indicate that spn from IC-treated cells are capable of reducing the basal surface expression of MHC class II but not the expression of MHC class II present in the intracellular stores [% MFI ± s.e.m. of control cells (a) surface: 68 ± 7*; (b) intracellular: 103 ± 9; *P < 0·05 versus monocytes incubated with spn from untreated monocytes]. Supernatants obtained from precipitating IC incubated with medium alone for 24 h were unable to down-regulate surface MHC class II expression, indicating the absence of IC in these spn. On the other hand, essentially the same down-regulatory effects of spn was obtained using IFN-γ-treated monocytes which have an over-expressed amount of MHC class II molecules [% MFI ± s.e.m. of control cells (a) surface: 116 ± 8*; (b) intracellular: 225 ± 47; *P < 0·05 versus IFN-γ-treated monocytes incubated with spn from untreated monocytes].
Effect of IC on STAT-1 phosphorylation
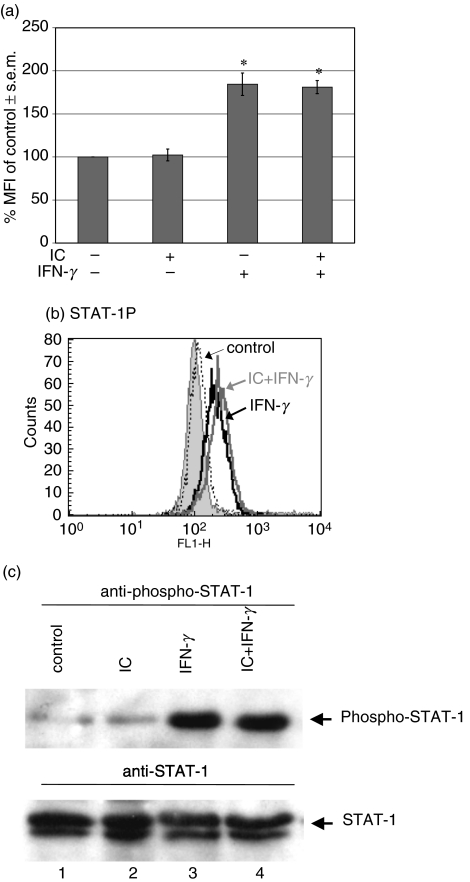
Many genes, including MHC class II and FcγRI, which are activated transcriptionally by IFN-γ, require tyrosine phosphorylation of the transcription factor STAT-1 for their expression. We investigated whether the down-regulation of MHC class II by IC was mediated by the inhibition of STAT-1 phosphorylation. Because the antibody used to reveal anti-STAT-1 was obtained from rabbit, to avoid cross-reaction we used human γ-globulin-coated plastic dishes or soluble human agg-IgG instead of OA-rabbit IgG anti-OA IC. These preparations show a similar effect to OA-rabbit IgG anti-OA IC on MHC class II expression. For this purpose, human monocytes were allowed to adhere onto human γ-globulin-coated plastic dishes for 1 h. The adhered cells were then treated with medium or IFN-γ for 15 min, and STAT-1 phosphorylation was evaluated by flow cytometry as indicated in Materials and methods. As shown in Fig. 2a,b, IFN-γ is able to induce STAT-1 phosphorylation even in the presence of IC. However, the γ-globulin-coated dishes were not capable of inducing STAT-1 phosphorylation in the absence of IFN-γ (Fig. 2a). Similar results were achieved using soluble human agg-IgG instead of γ-globulin-coated dishes.
Fig. 2.
Effect of IC on phosphorylation of STAT-1. Human monocytes (2·5 × 106 cells/ml) were allowed to adhere onto γ-globulin-coated or uncoated plastic dishes for 1 h at 37°C. Then, the cells were treated with medium or IFN-γ for 15 min After that period, the cells were removed, permeabilized and the phosphorylation of STAT-1 was evaluated by flow cytometry as indicated in Materials and methods. (a) Data are expressed as % MFI ± s.e.m. of control cells. Statistical significance was calculated using the Mann–Whitney test, two-tailed; n= 5. *P < 0·05 significantly different from control. (b) The histogram corresponds to a representative experiment of five. Non-specific binding (filled peak) was determined using normal rabbit IgG as control; x axis: fluorescence intensity (arbitrary units), y axis: cell number. (c) Human monocytes (2 × 106 cells/ml) were allowed to adhere onto γ-globulin-coated or uncoated plastic dishes for 1 h at 37°C. Then, the cells were treated with medium or IFN-γ for 5 min. After that period, the cells were lysed and the extracts immunoprecipitated with anti-STAT-1. They were then analysed by SDS-PAGE, transferred to PVDF membranes and probed with antiphospho-STAT-1 MoAb (upper panel). The lower panel represents the same membrane reprobed with anti-STAT-1 MoAb to show the amount of STAT-1 protein loaded in each lane. Untreated cells, lane 1; IC, lane 2; IFN-γ, lane 3; IC + IFN-γ, lane 4.
The lack of inhibition of IFN-γ-induced STAT-1 phosphorylation by IC evaluated by flow cytometry was confirmed using immunoprecipitation, as indicated in Materials and methods (Fig. 2c). These results indicate that the IC-induced down-regulation of MHC class II is not mediated by the inhibition of STAT-1 phosphorylation.
Effect of IC on the surface expression of other molecules
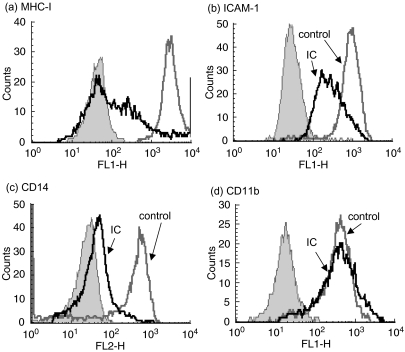
We have evaluated the effect of IC on only the expression of MHC class II antigens in human monocytes. Thus, in order to determine the specificity of this effect, we evaluated whether IC could alter the expression of other immunologically relevant molecules. The molecules studied were: class I molecules from the major histocompatibility complex (MHC class I), intercellular adhesion molecule ICAM-1 (CD54), CD14 and the integrins CD11a (LFA-1) and CD11b. As shown in Fig. 3a,b,c, IC are capable of down-regulating the basal expression of MHC class I, ICAM-1 and CD14. However, the basal expression of CD11b (Fig. 3d) and CD11a (n = 5, not shown) are not modified by IC treatment. These results indicate that although the effect of IC is not strictly specific for the down-regulation of MHC class II, it could be restricted to some other molecules involved in inflammatory processes.
Fig. 3.
Effect of IC on the surface expression of other molecules. Human monocytes (0·5 × 106 cells/ml) were incubated with medium (control) or IC (100 µg/ml) for 24 h. After that period, the cells were stained with: (a) anti-HLA-ABC (MHC class I); (b) anti-CD54 (ICAM-1); (c) anti-CD14; (d) anti-CD11b or anti-CD11a (n = 5, data not shown), and the expression of these molecules was evaluated by flow cytometry. The histograms correspond to a representative experiment of five [for (a, b and d)] or 17 (for (c). Non-specific binding (filled peak) was determined using control isotype antibodies; x axis: fluorescence intensity (arbitrary units), y axis: cell number.
Effect of complement on IC-induced down-regulation of MHC class II
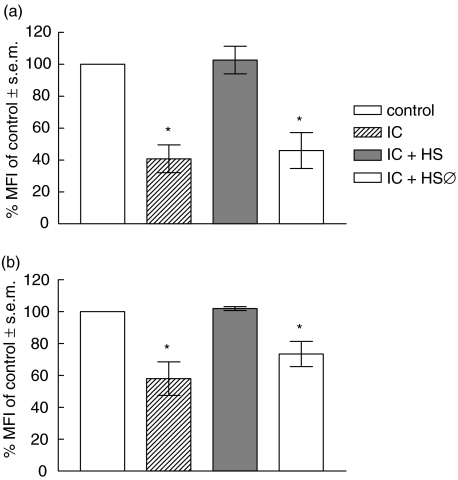
Considering that complement activation can solubilize IC or inhibit the precipitation of IC [26–29], we evaluated the effect of complement on IC-induced down-regulation of MHC class II on human monocytes. For this purpose, IC were incubated with normal or heat-inactivated (56°C, 30 min) autologous human serum. After 1 h of incubation at 37°C, these preparations were added to human monocytes for 18 h. After this period, the expression of MHC class II was analysed by flow cytometry. As shown in Fig. 4a, normal human serum is capable of abolishing the IC effect on the basal expression of MHC class II. In this experiment IC were formed in the absence of complement; however, the same effect was obtained when IC were formed in the presence of complement (Fig. 4b). These results indicate that, as shown previously, the activation of the complement system could be a crucial step on IC–FcγRs interaction-dependent effects [19–21].
Fig. 4.
Effect of complement on IC-induced down-regulation of MHC class II. (a) IC were formed and then incubated with medium, normal or heat-inactivated (56°C, 30 min) human serum, for 1 h at 37°C. (b) IC were formed in the presence of medium, normal or heat-inactivated (56°C, 30 min) human serum, for 1 h at 37°C. Then, for both experimental conditions, human monocytes (0·5 × 106 cells/ml) were incubated with medium (control) or the IC preparations indicated above for 18 h. After this period, human monocytes were stained with an anti-HLA-DR antibody and the level of expression of MHC class II was evaluated by flow cytometry. Data are expressed as % MFI ± s.e.m. of control cells. Statistical significance was calculated using the Mann–Whitney test, two-tailed; n= 8. *P < 0·01 significantly different from control.
Effect of IC on the expression of MHC class II in a mouse model of chronic inflammation
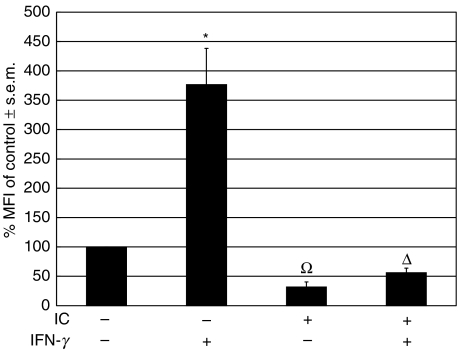
In order to investigate the in vivo effect of IC on the regulation of the expression of MHC class II, a glass cylinder was implanted subcutaneously into BALB/c mice [25]. After 20 days the cylinders induce a chronic inflammatory process. At that moment, the open ends of the cylinders were ‘closed’ by fibrotic tissue and were predominantly infiltrated by macrophages. Then, IC and/or murine IFN-γ were injected into the cylinder and 24 h later the cells that had been recruited into the cylinder were isolated and stained with an antibody against murine MHC class II (anti-I-Ad). Flow cytometry was performed and the macrophage population was studied. Figure 5 shows that IC exert a drastic inhibition of MHC class II up-regulation induced by IFN-γ on murine macrophages. The IC treatment also reduces the basal expression of MHC class II. These results are in agreement with our in vitro experiments using human monocytes. However, when IC were injected intravenously and 18 h later the mice peripheral blood cells were stained with an antibody against murine MHC class II (anti-I-Ad), they were not capable of reducing the basal expression of MHC class II (n = 5, not shown).
Fig. 5.
Effect of IC on basal and IFN-γ-induced MHC class II (I-Ad) expression in macrophages in a mouse model of chronic inflammation. A glass cylinder was introduced subcutaneously into BALB/c mice. After 20 days, the cylinders caused a chronic inflammatory process and pyrogen-free saline or 50 µl of IC were injected into the cylinder. Simultaneously, pyrogen-free saline or 50 µl of murine IFN-γ (3000 U/ml) were added to the cylinder. After 24 h, the cylinders were extracted from the mice and the cells that had been recruited were stained with an antibody against murine MHC class II (anti-I-Ad). Flow cytometry was performed and the macrophage population was analysed using macrophage-specific forward light scatter (FSC) and side light scatter (SSC) gates. Data are expressed as % MFI ± s.e.m. of control cells. Statistical significance was calculated using the unpaired t-test, two-tailed; n= 5. *P < 0·0001 significantly different from control. Ω, P < 0·0001 significantly different from control and IFN-γ-treated cells. Δ, P < 0·01 significantly different from control and P < 0·0001 significantly different from IFN-γ-treated cells.
DISCUSSION
In a previous report we found that IC down-regulate both basal and IFN-γ-induced surface expression of MHC class II on human monocytes [18]. This effect, at least in part, was attributed to the release of aspartic protease(s) and metalloprotease(s) by PBMC. Because a delay in the turnover of MHC class II molecules and the accumulation of internal MHC class II complex in intracellular vesicles could not be discarded [30], in this study we investigated the effect of IC on intracellular MHC class II.
Our results indicate that IC induce a depletion not only on the surface but also on the intracellular expression of MHC class II, leading us to discard the possibility that MHC class II were endocytosed upon IC stimulation. On the contrary, these data suggest that MHC class II are shed to the extracellular medium. In addition, the diminished intracellular content of MHC class II after IC treatment indicates that IC do not affect the translocation of these molecules to the monocyte surface. Moreover, the lack of replenishment of surface MHC class II at the expense of intracellular MHC class II depletion suggests that the kinetic of class II removal by proteolytic enzymes could be faster than or equal to that the kinetics of re-expression. However, our results can also be explained for an increased degradation and/or reduced production of class II molecules.
We observed that supernatants from IC-treated monocytes induce the down-regulation of surface MHC class II but not of the intracellular MHC class II. This result indicates that the translocation of intracellular MHC class II molecules is dependent on IC–FcγR interaction and is not caused merely by the lack of MHC class II molecules on the cell surface. We also studied the kinetics of the inhibition of MHC class II expression by IC. As can be seen in Fig. 1, a short period of incubation with IC (5 min) enhances MHC-II expression while 3 h of incubation with IC is necessary to observe the down-regulation of the basal expression of MHC class II induced by secreted proteases.
Previous reports have shown that IC inhibit IFN-γ-induced expression of FcγRI by preventing tyrosine phosphorylation of the transcription factor STAT-1 through the inhibition of the tyrosine kinases JAK1 and JAK2 [31]. This mechanism was also proposed for the inhibition of the transcription of many genes such as MHC class I and II, which are also dependent on STAT-1 activation [31]. Although our results obtained with monocytes treated first with IC and later with IFN-γ could support this interpretation [18], the results shown in Fig. 2 demonstrate that in our experimental conditions IC were not capable of modifying the IFN-γ-induced phosphorylation of STAT-1. Although the cause of this discrepancy is not known, it indicates that the effect of IC on surface MHC class II expression is not dependent on STAT-1 phosphorylation, and suggest that the secretion of enzymes and cleavage of MHC class II molecules could be the principal event in the regulation of surface MHC class II.
Another important issue was to determine the specificity of the effect of IC. For this purpose we evaluated the IC effect on other relevant molecules on the monocyte surface. The results shown in Fig. 3 indicate that the effect of IC, although not specific for MHC class II molecules, exerted a drastic and selective down-regulation of some inflammatory molecules including MHC class I, ICAM-1 and CD14 molecules, but not in others (i.e. CD11a and CD11b). These results suggest that a proinflammatory agent such as IC [3–8] could exert a modulation on inflammation by down-regulating molecules other than MHC class II.
Solubilization of IC by complement was described first by Miller and Nussenzweig in the 1970s [26] and it is known to occur by activation through the alternative pathway [26,28,32,33]. In the 1980s, Schifferli et al. [34–36] demonstrated the importance of the classical pathway in the inhibition of the formation of IC, and Isturiz et al. [19–21] showed the relevance of complement in the regulation of IC-FcγR-dependent effects. Taking these reports into account and the importance of complement activation on IC activity in vivo, we investigated the effect of complement on the IC-induced MHC class II down-regulation. For this purpose, preformed IC were incubated with normal or heat-inactivated human serum. Down-regulation of MHC class II was abrogated by normal human serum but not by heat-inactivated serum (Fig. 4a). These results are in agreement with the solubilization of IC by the activation of the complement system [26–28,32,33]. However, the formation of IC in circulation in the presence of complement seems to be more physiological. Then, we investigated the down-regulation of MHC class II by IC formed in the presence of complement. Our results indicate that IC formed in the presence of normal serum, but not in the presence of human heat-inactivated serum, are capable of abolishing the effect of IC on MHC class II expression (Fig. 4b). These results are supported by the inhibition of immune precipitation by complement observed by others [29,34–36]. Taken together, these results indicate that the activation of complement, probably by IC solubilization or by inhibition of immune precipitation, could be an crucial step on the regulation of the effect of IC on MHC class II expression. However, the demonstration of these effects in vivo using either complement-deficient mouse or cobra venom factor-treated animals was not possible, because intravenously injected IC are cleared rapidly by the reticulo-endothelial system from liver and spleen.
Finally, although under restricted conditions, we investigated the IC effect on MHC class II expression in vivo. Thus, in a closed inflammatory focus (glass cylinder), we observed that IC were capable of inducing the down-regulation of basal and IFN-γ-induced expression of MHC class II in macrophages. However, it has to be taken into account that at the subcutaneous level the concentration of complement component is not the same as that observed in serum.
Our results demonstrate that IC, at the site of inflammation, could be relevant in the regulation of molecules involved in the immune response as well as in the development of the inflammatory phenomena in vivo.
Acknowledgments
Blood samples were kindly provided by the Servicio de Hemoterapia, CEMIC, Buenos Aires, Argentina. We thank Sonia Gomez for critical reading of the manuscript, Fundación de la Hemofilia for the FACScan Flow Cytometer and Carolina Schere, Sergio Fridman, Viviana Pressiani and Antonio Fernández for technical assistence. This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica, Fundación Antorchas and Fundación Alberto J. Roemmers (Buenos Aires, Argentina).
REFERENCES
- 1.Schifferli JA, Taylor RP. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- 2.Mannik M. Pathophysiology of circulating immune complexes. Arthritis Rheumat. 1982;25:783–7. doi: 10.1002/art.1780250713. [DOI] [PubMed] [Google Scholar]
- 3.Kasai S, Akaike T, Kunimoto T, Nitta K. Superoxide anion production from mouse peritoneal macrophages stimulated with surface-bound IgG and immune complexes: adsorption characteristics of IgG in relationship to biological activity. Eur J Immunol. 1982;12:1054–7. doi: 10.1002/eji.1830121213. [DOI] [PubMed] [Google Scholar]
- 4.Bonney RJ, Naruns P, Davies P, Humes JL. Antigen–antibody complexes stimulate the synthesis and release of prostaglandins by mouse peritoneal macrophages. Prostaglandins. 1979;18:605–16. doi: 10.1016/0090-6980(79)90027-3. [DOI] [PubMed] [Google Scholar]
- 5.Rouzer CA, Scott WA, Hamill AL, Liu FT, Katz DH, Cohn ZA. Secretion of leukotriene C and other arachidonic acid metabolites by macrophages challenged with immunoglobulin E immune complexes. J Exp Med. 1982;156:1077–86. doi: 10.1084/jem.156.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polat GL, Laufer J, Fabian I, Passwell JH. Cross-linking of monocyte plasma membrane Fc alpha, Fc gamma or mannose receptors induces TNF production. Immunology. 1993;80:287–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Berger S, Balló H, Stutte HJ. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes. a network of pro- and anti-inflammatory cytokines dependent on the antigen : antibody ratio. Eur J Immunol. 1996;26:1297–301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 8.Nitta T, Suzuki T. Biochemical signals transmitted by Fc-gamma receptors. triggering mechanisms of the increased synthesis of adenosine-3′, 5′-cyclic monophosphate mediated by Fc-gamma2a- and Fc-gamma2b-receptors of a murine macrophage-like cell line (P388D1) J Immunol. 1982;129:2708–14. [PubMed] [Google Scholar]
- 9.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 10.Clynes R, Ravetch JV. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995;3:21–6. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 11.Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 12.Virgin HW, Wittenberg GF, Unanue ER. Immune complex effects on murine macrophages. I. Immune complexes suppress interferon-γ induction of Ia expression. J Immunol. 1985;135:3735–43. [PubMed] [Google Scholar]
- 13.Revel M, Chebath J. Interferon-activated genes. Trends Biochem Sci. 1986;11:166–70. [Google Scholar]
- 14.Hanaumi K, Gray P, Suzuki T. Fcγ receptor-mediated supression of γ-interferon-induced Ia antigen expression on a murine macrophage-like cell line (P338D1) J Immunol. 1984;133:2852–6. [PubMed] [Google Scholar]
- 15.Farraf MA, Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 16.Rosa FM, Cochet MM, Fellous M. Interferon and major histocompatibility complex genes: a model to analyse eukaryotic gene regulation? Interferon. 1986;7:48–87. [PubMed] [Google Scholar]
- 17.Van Weyenbergh J, Lipinski P, Abadie A, Chabas D, Blank U, Liblau R, Wietzerbin J. Antagonistic action of IFN-β and IFN-γ on high affinity Fcγ receptor expression in healthy controls and multiple sclerosis patients. J Immunol. 1998;161:1568–74. [PubMed] [Google Scholar]
- 18.Barrionuevo P, Beigier-Bompadre M, de la Barrera S, et al. Immune complexes (IC) dowregulate the basal and interferon-γ-induced expression of MHC class II on human monocytes. Clin Exp Immunol. 2001;125(2):251–7. doi: 10.1046/j.1365-2249.2001.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isturiz MA, Fink S, Bracco MME. Reversal of immune complex inhibition of antibody dependent cell mediated cytotoxicity by normal human serum. Clin Exp Immunol. 1982;48:685–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Geffner J, Giordano M, Serebrinsky G, Palermo M, Isturiz MA. Inhibition of lymphocyte and monocyte ADCC by immune complexes: effect of normal human serum. Immunol Lett. 1987;15:255–9. doi: 10.1016/0165-2478(87)90033-2. [DOI] [PubMed] [Google Scholar]
- 21.De la Barrera S, Sasiain MC, Geffner J, Isturiz MA, Segal-Eiras A, Bracco MME. Inhibition of antibody-dependent cell-mediated cytotoxicity by serum from lepromatous leprosy patients. Int J Lepr Other Mycobact Dis. 1985;53:218–24. [PubMed] [Google Scholar]
- 22.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 23.Tosi MF, Zakem H. Surface expression of Fc gamma receptor III (CD16) on chemoattractant-stimulated neutrophils is determined by both surface shedding and translocation from intracellular storage compartments. J Clin Invest. 1992;90:462–70. doi: 10.1172/JCI115882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleisher TA, Dorman SE, Anderson JA, Vail M, Brown MR, Holland SM. Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin Immunol. 1999;90:425–30. doi: 10.1006/clim.1998.4654. [DOI] [PubMed] [Google Scholar]
- 25.Lanari C, Molinolo AA, Pasqualini CD. Inhibitory effect of medroxyprogesterone acetate on foreign body tumorigenesis in mice. J Natl Cancer Inst. 1986;77:157–64. [PubMed] [Google Scholar]
- 26.Miller GW, Nussenzweig V. A new complement function: solubilization of antigen–antibody aggregates. Proc Natl Acad Sci USA. 1975;72:418–22. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czop J, Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976;143:615–30. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Tack BF, Nussenzweig V. Requirements for the solubilization of immune aggregates by complement: assembly of a factor B-dependent C3-convertase on the immune complexes. J Exp Med. 1977;145:86–100. doi: 10.1084/jem.145.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong K, Takata Y, Sayama K, et al. Inhibition of immune precipitation by complement. J Immunol. 1984;133:1464–70. [PubMed] [Google Scholar]
- 30.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting and recycling. Immunity. 1997;7:861–71. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 31.Feldman GM, Chuang EJ, Finbloom DS. IgG immune complexes inhibit IFN-γ -induced transcription of the Fcγ RI gene in human monocytes by preventing the tyrosine phosphorylation of the p91 (Stat1) transcription factor. J Immunol. 1995;154:318–25. [PubMed] [Google Scholar]
- 32.Takahashi M, Czop J, Ferreira A, Nussenzweig V. Mechanism of solubilization of immune aggregates by complement. Implication for immunopathology. Transplant Rev. 1976;32:121–39. doi: 10.1111/j.1600-065x.1976.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi M, Takahashi S, Hirose S. Solubilization of antigen–antibody complexes: a new function of complement as a regulator of immune reactions. Prog Allergy. 1980;27:134–66. [PubMed] [Google Scholar]
- 34.Schifferli JA, Bartolotti SR, Peters DK. Inhibition of precipitation by complement. Clin Exp Immunol. 1980;42:387–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Schifferli JA, Woo P, Peters DK. Complement-mediated inhibition of immune precipitation. I. Role of the classical and alternative pathways. Clin Exp Immunol. 1982;47(3):555–62. [PMC free article] [PubMed] [Google Scholar]
- 36.Schifferli JA, Peters DK. Complement-mediated inhibition of immune precipitation. II. Analysis by sucrose density gradient ultracentrifugation. Clin Exp Immunol. 1982;47:563–9. [PMC free article] [PubMed] [Google Scholar]