Abstract
The prevalence of type I allergy to Hevea brasiliensis latex is particularly high among individuals with frequent exposure to latex products, such as health-care workers (HCW) and patients with spina bifida (SB). Treatment of latex allergy seems problematic as preventive measures, such as allergen avoidance, are not always possible and conventional immunotherapy with standardized latex extracts is not performed routinely. Thus, the aim of the present study was to establish a mouse model of latex allergy using two major latex allergens for HCWs and SB patients, Hev b 1 and Hev b 3, for sensitization. Prophylactic measures on the basis of mucosal tolerance induction with the recombinant allergens were tested in this model. Female BALB/c mice immunized intraperitoneally with recombinant (r)Hev b 1 or rHev b 3 displayed strong immune responses in vivo and in vitro. Intranasal treatment with rHev b 1 and rHev b 3 prior to sensitization led to reduced allergen-specific IgG1/IgE levels and significantly suppressed allergen-induced basophil degranulation. Moreover, lymphocyte proliferation and cytokine production (IL-4, IL-5, IFN-γ) in vitro were significantly suppressed after pretreatment with both allergens. Suppressive cytokines, such as interleukin (IL)-10 and transforming growth factor (TGF)-β, remained unchanged after the intranasal pretreatment, indicating mechanism of anergy rather than active immunosuppression. Taken together, these results suggest that mucosal tolerance induction with recombinant allergens could present a promising prevention strategy against latex allergy.
Keywords: animal model, BALB/c, mucosal tolerance, recombinant allergens, type I latex allergy
INTRODUCTION
Natural rubber latex is used widely to manufacture medical devices and a variety of everyday articles. As a consequence, type I allergy to latex has become an important and increasing health problem worldwide. The clinical spectrum of the disease ranges from local contact urticaria, allergic rhinoconjunctivitis and asthma to life-threatening anaphylaxis [1–3]. Sensitization to latex allergens seems to occur with a higher frequency in individuals with a high degree of exposure to natural rubber latex, including health-care workers (HCWs), rubber industry workers and spina bifida (SB) patients, who have undergone multiple surgery during childhood [4,5]. Among the panel of latex allergens some, such as the rubber elongation factor Hev b 1, a 14·6-kDa protein, and Hev b 3, the small rubber particle protein, a 22·4-kDa molecule, have been characterized extensively with respect to immunological and molecular properties and were produced recombinantly [6–8]. These two proteins represent major allergens in SB patients with latex allergy and are also recognized frequently by IgE of latex-allergic HCWs [6–10]. Latex allergy prevention protocols have been proposed for people with occupational exposure to latex; however, the use of latex powder-free or synthetic gloves and latex-free material is not always possible [11,12]. In already sensitized individuals avoidance of natural rubber latex is even more critical, as only very small quantities of natural rubber latex suspended in the air can trigger allergic reactions [13]. Specific immunotherapy is the treatment of choice for many forms of type I allergy. However, concerning latex allergy this treatment is not performed routinely due to a lack of standardized latex extract preparations. In this respect it was shown that different batches of natural rubber latex can vary up to 25-fold in their total allergen content and composition [14,15]. In a recent clinical trial it was demonstrated that immunotherapy with latex allergens resulted in an increased prevalence of systemic reactions, which is in contrast to immunotherapy with common aeroallergens [13]. This might in part be related to the fact that natural latex allergen preparations include a series of biological active proteins that might trigger inflammatory reactions [13,15–17]. Moreover, as latex allergic patients, such as HCWs or SB patients, are sensitized to only few major latex allergens, immunotherapy with the natural extract preparation might yield the risk of sensitization to other allergen components present in the extract [18]. The use of recombinant allergens for immunotherapy would overcome these problems and provide a patient-tailored therapy [19]. Another improvement of treatment, resulting in an enhanced patient compliance, would be to change the conventional systemic route of antigen administration to the application of allergens via the mucosa. In this context it has been demonstrated in experimental animal models that mucosal administration of soluble antigen leads to a state of antigen-specific unresponsiveness termed oral or mucosal tolerance [20]. Therefore mucosal tolerance induction is suggested as a treatment strategy for diseases mediated by immunological hyperreactivity, such as autoimmune diseases or allergies [21–24]. We have previously established a mouse model of allergic sensitization to birch pollen and its major allergen Bet v 1 [24,25]. Using this model we demonstrated that intranasal application of rBet v 1 suppressed allergic sensitization to birch pollen in naive and in sensitized mice [25,26]. Based on these findings we now established a mouse model of latex sensitization using two latex allergens, Hev b 1 and Hev b 3, for sensitization. Using this model we aimed to induce tolerance by intranasal application of the recombinant allergens prior to sensitization. Our results indicate that mucosal tolerance induction with recombinant latex allergens may represent a promising prophylactic treatment strategy in patients with frequent exposure to latex allergens.
MATERIALS AND METHODS
Animals
Female 7-week-old BALB/c mice (n = 5 per group) were obtained from Charles River (Sulzfeld, Germany). All experiments were approved by the Animal Experimentation Committee of the University of Vienna and the Federal Ministry of Education, Science and Culture.
Antigens
The complete coding sequence of Hev b 1 was amplified by reverse transcription-polymerase chain reaction (RT-PCR) from total latex RNA. Primers were designed according to the coding region of Hev b 1 (EMBL database, accession number X56535), flanked by BamHI and HindIII restriction sites. The PCR product was ligated into pMAL-c2x and cloned in TB 1 Escherichia coli cells [7]. Hev b 1 was expressed as a fusion protein with maltose-binding protein (MBP) in E. coli after induction with 2 mm IPTG, lysed in a French press and purified with an amylose column (New England Biolabs, Beverly, MA, USA). MBP-Hev b 1 was eluted with 10 mm maltose and dialysed against phosphate buffered saline (PBS) [7]. In the following, MBP-Hev b 1 will be referred to as rHev b 1. rHev b 3 was expressed in E. coli with a hexahistidyl affinity tag as described previously [8].
Sensitization
Sensitization was induced by three intraperitoneal (i.p.) injections of 5 µg rHev b 1 (group 1) or 5 µg rHev b 3 (group 2) adsorbed to aluminium hydroxide [Al(OH)3] (Serva, Heidelberg, Germany) in 14-day intervals. Sampling and analyses were performed 7 days after the last immunization.
Tolerance induction
For tolerance induction rHev b 1 (group 3) or rHev b 3 (group 4) were applied intranasally (i.n.) three times in 7-day intervals at a concentration of 10 µg in 30 µl 0·9% NaCl prior to sensitization. Control mice were i.n. sham-treated with 30 µl of 0·9% NaCl and 10 days after the last i.n. treatment all mice were sensitized as described. Sampling and analyses were performed 7 days after the last immunization.
Sampling
Blood samples were taken before and 7 days after sensitization or after i.n. treatment by tail bleeding. Serum was prepared and stored at −20°C until analysed. Spleens were removed under sterile conditions. The organs were homogenized and filtered through sterile filters. After lysation of erythrocytes, cells were washed and resuspended in complete medium [RPMI, 10% fetal calf serum (FCS), 0·1 mg/ml gentamycin, 2 mmol/l glutamine and 50 µmol/l 2-mercaptoethanol][24].
Detection of allergen-specific antibody levels in serum
Microtitre plates (Nunc) were coated with rHev b 1 (5 µg/ml) or rHev b 3 (5 µg/ml) overnight at 4°C. After washing and blocking with 1% bovine serum albumin (BSA)-PBS/Tween, serum samples were diluted 1/1000 for IgG1, 1/500 for IgG2a and 1/10 for IgE antibodies and incubated overnight at 4°C. Rat antimouse IgG1, IgG2a and IgE antibodies (1/500, Pharmingen, San Diego, CA, USA) followed by peroxidase-conjugated mouse antirat IgG antibodies (1/2000, Jackson Immuno Laboratory, West Grove, PA, USA) were used. Colour development was performed as described previously [24]. Results show the optical density (OD) values after subtraction of baseline levels from preimmune sera.
Rat basophil leukaemia cell mediator release assay (RBL assay)
RBL-2H3 cells were plated in 96-well cell-culture plates (4 × 104 cells/well). To determine allergenic serum activity, the RBL cells were sensitized passively for 2 h with sera obtained from Hev b 1/Hev b 3-sensitized or Hev b 1/Hev b 3-tolerized mice at dilutions of 1 : 10 and 1 : 100. Unbound antibodies were removed by washing the cell layer two times with Tyrode's buffer (137 mm NaCl, 2·7 mm KCl, 0·5 mm MgCl2, 1·8 mm CaCl2, 0·4 mm NaH2PO4, 5·6 mm d-glucose, 12 mm NaHCO3, 10 mm HEPES and 0·1% w/v BSA, pH 7·2). Degranulation of RBL cells was induced by adding 100 µl rHev b 1 or rHev b 3 diluted in Tyrode's buffer (0·3 µg/ml) for 30 min at 37°C. Supernatants were analysed for β-hexosaminidase activity by incubation with 80 mm 4-methylumbelliferyl-N-acetyl-β-dglucosamide (Sigma, St. Louis, MO, USA) in citrate buffer (0·1 mm, pH 4·5) for 1 h at 37°C. The reaction was stopped by addition of 100 µl glycine buffer (0·2 m glycine, 0·2 m NaCl, pH 10·7) and the fluorescence was measured at ëex: 360/λem: 465 nm using a fluorescence microplate reader (Spectrafluor, Tecan, Austria). Results are reported as percentages of total β-hexosaminidase released after addition of 1% Triton X-100 and are shown after subtraction of baseline release levels obtained with preimmune sera.
Lymphocyte proliferation assay
Spleen cell suspensions were plated into 96-well round-bottom plates (Nunc, Roshilde, Denmark) at a concentration of 2 × 105 cells/well and stimulated with or without concanavalin A (Con A; 0·5 µg/well; Sigma), rHev b 1 (3 µg/well), rHev b 3 (3 µg/well) and MBP (3 µg/well) for 4 days. Thereafter the cultures were pulsed with 0·5 µCi/well tritiated thymidine (Amersham, Buckinghamshire, UK) for 16 h and harvested. The proliferative responses were measured by scintillation counting. The ratio of the mean proliferation after antigen stimulation [counts per minute (cpm)] and medium control values (cpm), i.e. the stimulation index (SI), was calculated.
Epitope mapping
Panels of 42 peptides for Hev b 1 [27] and 52 peptides for Hev b 3 [8] were used for epitope mapping experiments. The dodecapeptides were overlapping for three amino acids (neighbours shared nine residues) and spanned the whole amino acid sequence of Hev b 1 or Hev b 3. Spleen cell suspensions of mice i.p. immunized with rHev b 1 or rHev b 3 were cultured in 96-well round-bottom plates at a concentration of 2 × 105 cells/well and stimulated with the peptides (2 µg/well) for 4 days. [3H]-thymidine incorporation, scintillation counting and calculation of stimulation indices were performed as described above.
Measurement of cytokine production
For determination of interleukin (IL)-4, IL-5, IL-10 and interferon (IFN)-γ production spleen cell suspensions were cultured with or without Con A (2·5 µg/well), rHev b 1 (15 µg/well) or rHev b 3 (15 µg/well) in 48-well plates (Costar, Cambridge, MA, USA) at a concentration of 5 × 106 cells/well. After 40 h of antigen stimulation supernatants were taken and stored at −20°C until analysed. The levels of IL-4, IL-5 and IL-10 were measured using mouse ELISA kits (Endogen, Cambridge, MA, USA). The sensitivity of these assays was <5 pg/ml. IFN-γ levels were measured by ELISA as described previously [26]. Supernatants were applied undiluted on coated (rat antimouse IFN-γ; Endogen) 96-well plates (Nunc), thereafter biotin-labelled rat antimouse IFN-γ antibodies (0·1 µg/ml), followed by peroxidase-conjugated streptavidin (1 : 10 000 in PBS/4% BSA; Endogen) were applied. The sensitivity of the assay was <15 pg/ml. Results reflect the measured cytokine levels in pg/ml after subtraction of baseline levels of unstimulated cultures.
Detection of TGF-β mRNA
For quantification of TGF-β gene expression total RNA was isolated from pooled spleen cell suspensions at a concentration of 5 × 106 cells as described previously [24]. Total RNA was isolated using Rneasy Minikit (Quiagen, Valencia, CA, USA) according to the manufacturer's instructions, treated with DNase (Quiagen) and then reverse transcribed into cDNA using oligo dT primers (GeneAmp RT-PCR kit, PE, Boston, MA, USA). The DNA sequences of the TGF-β primers were as follows: 5′-TGF-β, ACC GCA ACA ACG CCA TCT AT; 3′ TGF-β, GTA ACG CCA GGA ATT GTT GC. Semiquantification of the RT-PCR results was achieved by amplification of β-actin using the following DNA sequences: 5′β-actin, TGG AAT CCT GTG GCA TCC ATG AAA C, 3′β-actin: TAA AAC GCA GTC CAG TAA CAG TCC G. Each PCR sample was electrophorized through a 1·2% agarose gel with ethidium bromide and scanned by a transilluminator and an image analyser system (Herolab; EASY, Wiesloch, Germany). Bands were assigned as densitrometric values and normalized to β-actin values.
Statistics
For statistical analysis, the Mann–Whitney U-test was used.
RESULTS
Characterization of immune responses in BALB/c mice after sensitization with rHev b 1 and rHev b 3
Antigen-specific antibody responses
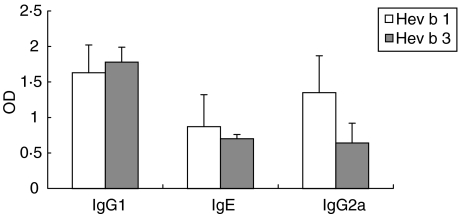
High Hev b 1-specific IgG1 and IgE levels were detected, which were similar to those of mice immunized with rHev b 3 (Fig. 1). Immunization with rHev b 1, but to a lesser extent with rHev b 3, led to strong allergen-specific IgG2a production (Fig. 1). As Hev b 1 has been expressed as a fusion protein with MBP, MBP-specific antibody levels in sera from Hev b 1-immunized mice were measured by ELISA, which were generally very low (IgG1: OD 0·37 ± 0·18; IgE: OD 0·115 ± 0·12; IgG2a: OD 0·46 ± 0·19).
Fig. 1.
Allergen-specific antibody levels in rHev b 1 and rHev b 3 sensitized mice. Allergen-specific serum IgG1, IgE and IgG2a antibody levels were detected by ELISA after immunization with rHev b 1/Al(OH)3 (white bars) or rHev b 3/Al(OH)3 (grey bars). Results are shown as mean values from three independent experiments with five animals per experiment. Baseline levels of preimmune sera were subtracted. Error bars indicate standard error of the means (s.e.m.). OD = optical density.
Proliferative responses of spleen cells and cytokine production in vitro
Splenocytes from rHev b 1-, as well as from rHev b 3-immunized mice proliferated vigorously upon in vitro stimulation with the respective antigens (rHev b 1-immunized mice: background medium value 4129·66 ± 874·77 cpm, Hev b 1-value 40095·67 ± 6123·33 cpm; rHev b 3-immunized mice: background medium value 5058·58 ± 2660·93 cpm, Hev b 3-value 18329·11 ± 7085·12 cpm; Table 1). Additionally, high IL-4, IL-5 and IFN-γ levels were measured in supernatants of antigen-stimulated spleen cell cultures. The IFN-γ production was more pronounced in rHev b 3 sensitized mice, whereas IL-4 and IL-5 levels were higher in rHev b 1 sensitized animals (Table 1). Proliferative responses or cytokine levels from naive splenocytes stimulated with rHev b 1 and rHev b 3 or from Hev b 1 immunized splenocytes stimulated with MBP did not exceed control values of spleen cells cultured in medium alone, assuring the specificity of the responses of the spleen cell cultures from immunized mice (data not shown).
Table 1.
T cell proliferation and cytokine production in spleen cell cultures from mice sensitized with rHev b 1/Al(OH)3 or rHev b 3/Al(OH)3
| SI | IFN-γ (pg/ml) | IL-4 (pg/ml) | IL-5 (pg/ml) | |
|---|---|---|---|---|
| Hev b 1 | 6·85 ± 2·96 | 683·3 ± 550·7 | 104·3 ± 71·3 | 448·3 ± 171·3 |
| Hev b 3 | 3·32 ± 0·31 | 2050·1 ± 1719·7 | 28·26 ± 22·1 | 173·3 ± 77·5 |
Spleen cells from mice immunized with rHev b 1 or rHev b 3 were cultured with the respective antigens. Proliferative responses were measured by [3H]-incorporation and indicated as stimulation indices (SI). IFN-γ, IL-4 and IL-5 levels were determined in supernatants after 40 h of antigen-specific stimulation by ELISA. All results are mean values (± s.d.) from three independent experiments with five animals per experiment.
Epitope mapping
For T cell epitope mapping experiments pooled spleen cell suspensions from rHev b 1 or rHev b 3 immunized mice were used. After lymphocyte stimulation with 42 overlapping peptides, covering the whole sequence of Hev b 1 (accession number P15252 [6]), one immunodominant T cell epitope, PPIVKDASIQVV, near the C-terminus corresponding to the amino acid sequence positions 94–105 was identified. This epitope has also been described as a T cell-reactive region in human in vitro studies with peripheral blood mononuclear cells from latex-sensitized patients [27]. In the case of Hev b 3 (accession number DNA AJ223388 [8]) epitope mapping was performed with 52 overlapping peptides and the immunodominant T cell epitope PRIVLDVASSVF was located at amino acid sequence positions 104–115. The same sequence has been identified previously in human in vitro studies with Hev b 3-specific T cell lines and T cell clones from SB patients [28].
Induction of mucosal tolerance with rHev b 1 and rHev b 3
Antibody responses in tolerized vs. sensitized mice
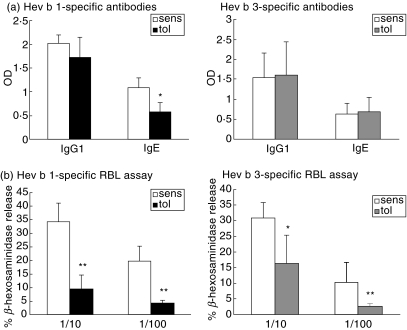
The pretreatment with rHev b 1 led to a slight reduction of Hev b 1-specific IgG1 and a significant reduction of Hev b 1-specific IgE levels compared to the sensitized control group (Fig. 2a). IgG2a antibody levels did not differ significantly between these groups (tolerized OD 2·38 ± 0·12 versus sensitized OD 1·97 ± 0·46, n.s.). Pretreatment with rHev b 3 had no significant effect on Hev b 3-specific IgG1 and IgE levels (Fig. 2a). However, in this group a 12-fold reduction of IgG2a antibodies was detected compared to the sensitized controls (tolerized OD 0·046 ± 0·06 versus sensitized OD 0·61 ± 0·51, n.s.).
Fig. 2.
Allergen-specific antibody responses and RBL cell mediator release in sensitized and tolerized mice. (a) Serum IgG1 and IgE antibody levels against rHev b 1 and rHev b 3 were detected by ELISA. Open bars (sens) indicate mean OD of five mice sensitized with rHev b 1/Al(OH)3 or rHev b 3/Al(OH)3. Black bars (tol Hev b 1) or grey bars (tol Hev b 3) show mean OD of five mice i.n. treated with rHev b 1 or rHev b 3 prior to sensitization. (b) Allergen-induced β-hexosaminidase release from RBL cells after incubation with sera from five Hev b 1/Hev b 3 (white bars) sensitized mice or five Hev b 1 (black bars)/Hev b 3 (grey bars) pretreated mice. Error bars indicate s.e.m. *P < 0·05, **P < 0·01 as determined by the Mann–Whitney U-test.
Allergen-specific basophil degranulation in tolerized vs. sensitized mice
The allergenic activity of serum IgE antibodies was investigated using a rat basophil degranulation assay. The percentage of β-hexosaminidase release from RBL cells treated with sera from rHev b 1-tolerized mice was reduced significantly compared to rHev b 1-immunized mice (Fig. 2b). Similar results were seen with sera from rHev b 3 sensitized and rHev b 3 pretreated mice (Fig. 2b).
Proliferative responses of spleen cells and cytokine production in vitro
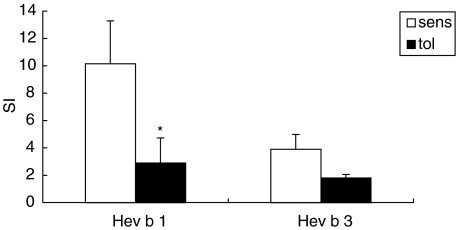
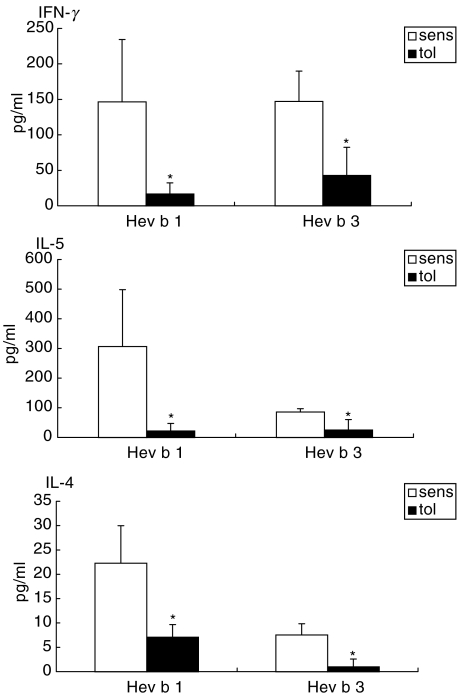
Intranasal pretreatment with the recombinant latex allergens resulted in a marked reduction of lymphoproliferative responses (rHev b 1 pretreated mice: background medium value 7677·00 ± 2097·79 cpm, Hev b 1-value 19581·57 ± 5475·43 cpm; rHev b 3 pretreated mice: background medium value 4866·08 ± 2053·43 cpm, Hev b 3-value 8983·42 ± 2357·56 cpm; Fig. 3) together with a significant suppression of IL-4, IL-5 and IFN-γ levels in both rHev b 1 and rHev b 3 pretreated mice compared to the sensitized controls (Fig. 4). IL-10 production was either decreased (Hev b 1 pretreatment: tolerized 29·79 ± 21·78 pg/ml versus sensitized 103·54 ± 60·37 pg/ml) or remained unchanged (Hev b 3 pretreatment: tolerized 65·94 ± 18·79 pg/ml versus sensitized 46·23 ± 27·47 pg/ml) in the tolerized animals.
Fig. 3.
Lymphoproliferation in spleen cell cultures from sensitized and tolerized mice. T cell proliferation was measured in spleen cell cultures after in vitro stimulation with rHev b 1 or rHev b 3 for 4 days. Open bars (sens) represent mean SI of five mice sensitized with rHev b 1/Al(OH)3 or rHev b 3/Al(OH)3. Black bars (tol) show mean SI of five mice i.n. treated with rHev b 1 or rHev b prior to sensitization. Error bars indicate s.e.m. *P < 0·05 as determined by the Mann–Whitney U-test. SI = stimulation index.
Fig. 4.
Cytokine production in spleen cell cultures from sensitized and tolerized mice. Cytokine production (pg/ml) in spleen cell cultures was determined by ELISA after in vitro stimulation with rHev b 1 or rHev b 3 for 40 h. Open bars (sens) indicate mean values of five mice sensitized with rHev b 1/Al(OH)3 or rHev b 3/Al(OH)3. Black bars (tol) represent mean values of five mice i.n. treated with rHev b 1 or rHev b 3 prior to sensitization. The values represent cytokine concentrations after subtraction of baseline values. Error bars indicate s.e.m. *P < 0·05 as determined by the Mann–Whitney U-test.
TGF-β mRNA expression
The expression of TGF-β mRNA in pooled spleen cells remained unchanged after pretreatment with rHev b 1 (TGF-β/β-actin ratio tolerized 0·90 versus sensitized 0·97) as well as after pretreatment with rHev b 3 (TGF-β/β-actin ratio tolerized 0·84 versus sensitized 0·89) compared to the sensitized controls.
DISCUSSION
In this study a mouse model of latex sensitization, meeting important immunological parameters of the human disease, was established using rHev b 1 and rHev b 3 for sensitization. Prevention of latex allergic immune responses was achieved by intranasal application of rHev b 1 and rHev b 3.
Several experimental studies have been performed to characterize the immune responses to latex allergens or to study pathophysiological mechanisms leading to the development of latex allergy. The use of a complete latex extract or soluble latex proteins for immunization led to elevated levels of serum IgE and eosinophilia in the blood and lung of mice [29,30] or increased airway reactivity upon metacholine challenge [31]. It has been reported, however, that the number and the amount of proteins significantly vary in various latex extracts [15]. This might influence the degree of sensitization or the outcome of treatment strategies in an experimental model in dependence of the applied extract preparation. Therefore, we used recombinant allergens for sensitization of BALB/c mice, a mouse strain suited to induce preferentially Th-2-like immune responses. Similarly, as it has been described for latex allergic patients, the mice displayed high allergen-specific IgE/IgG1 antibody levels, positive type I skin test reactivity, as well as IL-4/IL-5 production upon allergen-challenge in vitro. Additionally, T cell epitope mapping experiments identified two immunodominant peptides in rHev b 1 and rHev b 3 immunized mice that are also major T cell epitopes in latex-sensitized HCWs and SB patients [27,28]. Thus, this model represents immunological characteristics comparable to those of human latex allergy.
Based on the fact that allergen avoidance as a preventive measure is often not feasible − in particular in patients with occupational allergen exposure – and conventional latex immunotherapy as a curative treatment is not routinely available, there is a need for developing new measures against latex allergy. Treatment via the mucosa seems attractive due to the ease of application leading to better compliance of the patient. In this respect, we have described previously in a murine model of birch pollen allergy that mucosal application of the recombinant major allergen of birch pollen, Bet v 1, prevented allergic immune responses in naive and in sensitized mice [24,26]. Similarly, in the present study, we demonstrated that the intranasal low dose treatment with only 10 µg of rHev b 1 led to significant reduction of allergen-specific IgE antibody levels and allergen-induced basophil degranulation (Fig. 2), along with a strong suppression of allergen-specific lymphocyte proliferation (Fig. 3) and cytokine production in vitro (Fig. 4). The dose of antigen application seems to be crucial for successful tolerance induction [32], as pretreatment with fivefold higher antigen concentrations (and more) could not increase the immunosuppressive effects (data not shown). It has also been shown that tolerance induction is induced more easily at the T than at the B cell level [33], showing that delayed type hypersensitivity (DTH) reaction and IL-2 production can be suppressed while antibody levels often remain unchanged [34]. Pretreatment with rHev b 3 led to successful reduction of lymphocyte proliferation and cytokine production, i.e. IL-4, IL-5 and IFN-γ (Fig. 4), but at the B cell level only IgG2a antibody levels seemed to be affected by the pretreatment. Nevertheless, allergen-induced degranulation of RBL cells was reduced significantly after pretreatment with rHev b 3. Thus, besides IgE measurement by ELISA − which might have limitations at low detection levels − the basophil degranulation assay represents a very sensitive method for evaluation of allergen-induced immediate type reactions. Moreover, the successful reduction of IL-4, known to be the switching factor for IgE production [35], and IL-5, a crucial cytokine for eosinophil recruitment and development of airway inflammation [36], might further guarantee an adequate clinical effect.
Immunological responses after tolerance induction with different antigens are variable. Apart from the antigen dose and the frequency of antigen application [32,37], the nature of the antigen per se, characterized by certain intrinsic activities such as enzymatic activities or structural/molecular features [38,39], may play an important role in the efficacy of tolerance induction. In this context we have shown previously that intranasal application of low doses of the inhalant allergen Bet v 1 led to prevention of allergen-specific antibody levels and cytokine production, whereas intranasal treatment with equivalent doses of the dietary antigen ovalbumin did not, or only marginally, influence subsequent sensitization with the same antigen [40]. Moreover, pollen allergens other than Bet v 1, such as Phl p 1 and Phl p 5 − major grass pollen allergens − appeared to be less tolerogenic than Bet v 1 in the same concentrations [41], demonstrating that allergens might have different tolerogenic properties. Other studies using ovalbumin or bee venom allergens for tolerance induction demonstrated a reduction of IgE antibody levels, whereas IgG2a and IFN-γ production were increased after intranasal or inhalative allergen application [42,43]. As the pretreatment with rHev b 1 and rHev b 3 led to suppressed Th1- and Th2-like cytokines, which in both cases were not associated with increased IgG2a production, indicated that in our case the effects on the immune system were not due to a shift towards Th-1 like immune responses but due rather to general immunosuppression. The fact that suppressive/regulatory cytokines, such as IL-10 [44] and TGF-β[45], were either decreased or remained unchanged in the tolerized animals suggested that immunosuppression was mediated rather by anergy and/or cell-cell contact dependent mechanisms.
Taken together, we have established a mouse model of latex sensitization representing immunological features comparable to those of a defined collective of latex allergic patients, such as HCWs and SB patients. Our results indicate that recombinant latex allergens offer promising tools for the development of patient-tailored treatment strategies based on the induction of mucosal tolerance. In particular people with expected exposure to latex products, such as medical and paramedical personnel, rubber industry workers or patients, who have to undergo multiple surgery, could benefit from prophylactic treatment protocols as we have established in the present study.
Acknowledgments
This study was supported by grants from the Austrian Science Fund (P14634-PAT, P12838-GEN) and the SBF grant F018.
REFERENCES
- 1.Turjanmaa K. Incidence of immediate allergy to latex gloves in hospital personnel. Contact Derm. 1987;17:270–5. doi: 10.1111/j.1600-0536.1987.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 2.Arellano R, Bradley J, Sussman G. Prevalence of latex sensitization among hospital physicians occupationally exposed to latex gloves. Anesthesiology. 1992;77:905–8. doi: 10.1097/00000542-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Slater JE. Rubber anaphylaxis. N Engl J Med. 1989;320:1126–30. doi: 10.1056/NEJM198904273201707. [DOI] [PubMed] [Google Scholar]
- 4.Slater JE. Latex allergy. J Allergy Clin Immunol. 1994;94:139–49. doi: 10.1016/0091-6749(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 5.Turjanmaa K, Alenius H, Makinen-Kiljunen S, Reunala T, Palosuo T. Natural rubber latex allergy. Allergy. 1996;51:593–602. doi: 10.1111/j.1398-9995.1996.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 6.Czuppon AB, Chen Z, Rennert S, et al. The rubber elongation factor of rubber trees (Hevea brasiliensis) is major allergen latex. J Allergy Clin Immunol. 1993;92:690–7. doi: 10.1016/0091-6749(93)90012-5. [DOI] [PubMed] [Google Scholar]
- 7.Yeang HY, Cheong KF, Sunderasan E, et al. The 14·6 kD rubber elongation factor (Hev b 1) and 24 kD (Hev b 3) rubber particle proteins are recognized by IgE from patients with spina bifida and latex allergy. J Allergy Clin Immunol. 1996;98:628–39. doi: 10.1016/s0091-6749(96)70097-0. [DOI] [PubMed] [Google Scholar]
- 8.Wagner B, Krebitz M, Buck D, et al. Cloning, expression, and characterization of recombinant Hev b 3, a Hevea brasiliensis protein associated with latex allergy in patients with spina bifida. J Allergy Clin Immunol. 1999;104:1084–92. doi: 10.1016/s0091-6749(99)70093-x. [DOI] [PubMed] [Google Scholar]
- 9.Alenius H, Palosuo T, Kelly K, et al. IgE reactivity to 14-kD and 27-kD natural rubber proteins in latex-allergic children with spina bifida and other congenital anomalies. Int Arch Allergy Immunol. 1993;102:61–6. doi: 10.1159/000236551. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Cremer R, Posch A, Raulf-Heimsoth M, Rihs HP, Baur X. On the allergenicity of Hev b 1 among health care workers and patients with spina bifida allergic to natural rubber latex. J Allergy Clin Immunol. 1997;100:684–93. doi: 10.1016/s0091-6749(97)70174-x. [DOI] [PubMed] [Google Scholar]
- 11.Allmers H, Brehler R, Chen Z, Raulf-Heimsoth M, Fels H, Baur X. Reduction of latex aeroallergens and latex-specific IgE antibodies in sensitized workers after removal of powdered natural latex gloves in a hospital. J Allergy Clin Immunol. 1998;102:841–6. doi: 10.1016/s0091-6749(98)70026-0. [DOI] [PubMed] [Google Scholar]
- 12.Baur X, Ammon J, Chen Z, Beckmann U, Czuppon AB. Health risk in hospitals trough airborne allergens for patients presensitized to latex. Lancet. 1993;342:1148–9. doi: 10.1016/0140-6736(93)92127-f. [DOI] [PubMed] [Google Scholar]
- 13.Leynadier F, Herman D, Vervloet D, Andre C. Specific immunotherapy with a standardized latex extract versus placebo in allergic healthcare workers. J Allergy Clin Immunol. 2000;106:585–90. doi: 10.1067/mai.2000.109173. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton RG, Adkinson NF. Natural rubber latex skin testing reagents: safety and diagnostic accuracy of nonammoniated latex, ammoniated latex, and latex rubber glove extracts. J Allergy Clin Immunol. 1996;98(5 Part 1):872–83. doi: 10.1016/s0091-6749(96)80003-0. [DOI] [PubMed] [Google Scholar]
- 15.Chambeyron C, Dry J, Leynadier F, Pecquet C, Tran Xaan Thao. Study of the allergenic fractions of latex. Allergy. 1992;47:92–7. doi: 10.1111/j.1398-9995.1992.tb05094.x. [DOI] [PubMed] [Google Scholar]
- 16.Breiteneder H. The allergens of Hevea brasiliensis. ACI Int. 1998;10:101–9. [Google Scholar]
- 17.Breiteneder H, Scheiner O. Molecular and immunological characteristics of latex allergens. Int Arch Allergy Immunol. 1998;116:83–92. doi: 10.1159/000023930. [DOI] [PubMed] [Google Scholar]
- 18.Scheiner O, Kraft D. Basic and practical aspects of recombinant allergens. Allergy. 1995;50:384–91. doi: 10.1111/j.1398-9995.1995.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 19.Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Gronlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT) Clin Exp All. 1999;29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 20.Garside P, Mowat AM, Khoruts A. Oral tolerance in disease. Gut. 1999;44:137–42. doi: 10.1136/gut.44.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:1625–34. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 22.Shi FD, Li H, Wang H, et al. Mechanisms of nasal tolerance induction in experimental autoimmune myasthenia gravis: identification of regulatory cells. J Immunol. 1999;162:5757–63. [PubMed] [Google Scholar]
- 23.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–600. [PubMed] [Google Scholar]
- 24.Wiedermann U, Jahn-Schmid B, Bohle B, et al. Suppression of antigen-specific T and B-cell responses by intranasal or oral administration of recombinant bet v 1, the major birch pollen allergen, in a murine model of type I allergy. J Allergy Clin Immunol. 1999;103:1202–10. doi: 10.1016/s0091-6749(99)70200-9. [DOI] [PubMed] [Google Scholar]
- 25.Wiedermann U, Herz U, Baier K, et al. Intranasal treatment with a recombinant hypoallergenic derivative of the major birch pollen allergen Bet v 1 prevents allergic sensitization and airway inflammation in mice. Int Arch Allergy Immunol. 2001;126:68–77. doi: 10.1159/000049496. [DOI] [PubMed] [Google Scholar]
- 26.Winkler B, Baier K, Wagner S, et al. Mucosal tolerance as therapy of type I allergy: intranasal application of recombinant Bet v 1, the major birch pollen allergen, leads to the suppression of allergic immune responses and airway inflammation in sensitized mice. Clin Exp Allergy. 2002:32:30–6. doi: 10.1046/j.0022-0477.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 27.Raulf-Heimsoth M, Chen Z, Rihs HP, Kalbacher H, Liebers V, Baur X. Analysis of T cell reactive regions and HLA-DR4 binding motifs on the latex allergen Hev b 1 (rubber elongation factor) Clin Exp Allergy. 1998;28:339–48. doi: 10.1046/j.1365-2222.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 28.Bohle B, Wagner B, Vollmann U, et al. Characterization of T cell responses to Hev b 3, an allergen associated with latex allergy in spina bifida patients. J Immunol. 2000;164:4393–8. doi: 10.4049/jimmunol.164.8.4393. [DOI] [PubMed] [Google Scholar]
- 29.Kurup VP, Kumar A, Choi H, et al. Latex antigens induce IgE and eosinophils in mice. Int Arch Allergy Immunol. 1994;103:370–7. doi: 10.1159/000236656. [DOI] [PubMed] [Google Scholar]
- 30.Woolhiser MR, Munson AE, Meade BJ. Immunological responses of mice following administration of natural rubber latex proteins by different routes of exposure. Toxicol Sci. 2000;55:343–51. doi: 10.1093/toxsci/55.2.343. [DOI] [PubMed] [Google Scholar]
- 31.Thakker JC, Xia JQ, Rickaby DA, et al. A murine model of latex allergy-induced airway hyperreactivity. Lung. 1999;177:89–100. doi: 10.1007/pl00007633. [DOI] [PubMed] [Google Scholar]
- 32.Peng HJ, Turner MW, Strobel S. The kinetics of oral hyposensitization to a protein antigen are determined by immune status and the timing, dose and frequency of antigen administration. Immunology. 1989;67:425–30. [PMC free article] [PubMed] [Google Scholar]
- 33.Mowat AM. The regulation of immune responses to dietary protein antigens. Immunol Today. 1987;8:93–8. doi: 10.1016/0167-5699(87)90853-X. [DOI] [PubMed] [Google Scholar]
- 34.Leishman AJ, Garside P, Mowat AM. Induction of oral tolerance in the primed immune system: influence of antigen persistence and adjuvant form. Cell Immunol. 2000;202:71–8. doi: 10.1006/cimm.2000.1665. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 36.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402(Suppl.):B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 37.Chung Y, Chang SY, Kang CY. Kinetic analysis of oral tolerance: memory lymphocytes are refractory to oral tolerance. J Immunol. 1999;163:3692–8. [PubMed] [Google Scholar]
- 38.Dudler T, Machado D, Kolbe L, et al. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J Immunol. 1995;155:2605–13. [PubMed] [Google Scholar]
- 39.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187:271–5. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiedermann U, Jahn-Schmid B, Lindblad M, et al. Suppressive versus stimulatory effects of allergen/cholera toxoid (CTB) conjugates depending on the nature of the allergen in a murine model of type I allergy. Int Immunol. 1999;11:1717–24. doi: 10.1093/intimm/11.10.1717. [DOI] [PubMed] [Google Scholar]
- 41.Hufnagl K, Winkler B, Baier K, Valenta R, Kraft D, Wiedermann U. Induction of mucosal tolerance by co-application of recombinant Bet v 1, the major birch pollen allergen, recombinant Phl p 1 and Phl p 5, major grass pollen allergens, in poly-sensitized mice. Allergy. 2002;57(Suppl.):49. [Google Scholar]
- 42.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 43.Astori M, von Garnier C, Kettner A, Dufour N, Corradin G, Spertini F. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J mice. J Immunol. 2000;165:3497–505. doi: 10.4049/jimmunol.165.6.3497. [DOI] [PubMed] [Google Scholar]
- 44.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 45.Weiner HL. Oral tolerance. immune mechanisms and the generation of Th3-type TGF-β-secreting regulatory cells. Microbes Infect. 2001;3:947–54. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]