Abstract
Throughout history malaria has proved to be a significant threat to human health. Between 300 and 500 million clinical cases occur each year worldwide, approximately 2 million of which are fatal, primarily in children. The vast majority of malaria-related deaths are due to infection with Plasmodium falciparum; P. vivax causes severe febrile illness but is rarely fatal. Following repeated exposure to infection, people living in malaria endemic areas gradually acquire mechanisms to limit the inflammatory response to the parasite that causes the acute febrile symptoms (clinical immunity) as well as mechanisms to kill parasites or inhibit parasite replication (antiparasite immunity). Children, who have yet to develop protective immune mechanisms are thus at greater risk of clinical malaria, severe disease and death than adults. However, two epidemiological observations indicate that this is, perhaps, an oversimplified model. Firstly, cerebral malaria – a common manifestation of severe malaria – typically occurs in children who have already acquired a significant degree of antimalarial immunity, as evidenced by lower mean parasite densities and resistance to severe anaemia. One potential explanation is that cerebral malaria is, in part, an immune-mediated disease in which immunological priming occurs during first infection, eventually leading to immunopathology on re-infection. Secondly, among travelers from nonendemic areas, severe malaria is more common – and death rates are higher – in adults than in children. If severe malaria is an immune-mediated disease, what might be priming the immune system of adults from nonendemic areas to cause immunopathology during their first malaria infection, and how do adults from endemic areas avoid severe immunopathology? In this review we consider the role of innate and adaptive immune responses in terms of (i) protection from clinical malaria (ii) their potential role in immunopathology and (iii) the subsequent development of clinical immunity. We conclude by proposing a model of antimalarial immunity which integrates both the immunological and epidemiological data collected to date.
Keywords: parasitic-protozoa, human, malaria, immunoregulation
ANTI-MALARIAL EFFECTOR MECHANISMS
As many of the surface antigens of malaria parasites and the parasite proteins inserted into the plasma membrane of the infected red blood cell are polymorphic or exhibit clonal antigenic variation, it has been proposed that one may need to develop a diverse repertoire of antibodies capable of blocking parasite invasion and tissue adhesion in order to attain effective antiparasite immunity [1]. For example, P. falciparum erythrocyte membrane protein 1 (PfEMP1), an antigen involved in parasite sequestration [2] and possibly pathogenesis of cerebral malaria (CM) [3], is encoded by a family of var genes which undergo frequent nonhomologous recombination leading to heterologous expression of antigenic variants by different parasites. Infection with a parasite variant that is not recognized by the existing antibody repertoire may lead to uncontrolled parasite replication and therefore pathology. The gradual acquisition of clinical immunity (following repeated infection) parallels the development of a diverse antibody repertoire; these two observations may be causally linked (reviewed in [4]). Malaria-specific antibodies mediate a number of antiparasitic effector functions including inhibition of cytoadherence [5], inhibition of erythrocyte invasion [6] and antibody dependent cytotoxicity and cellular inhibition [7]. Cell-mediated immune effector mechanisms include macrophage activation by NK cell-, γδT cell- or Th1-derived interferon γ (IFN-γ) for enhanced phagocytosis and killing of parasitized erythrocytes [8], and inhibition of parasite growth and development inside hepatocytes by CD8+ cytotoxic and IFN-γ-producing T cells [9]. Nitric oxide (NO), produced by macrophages in response to parasitic components and T cell IFN-γ production, can have antiparasitic effects [10]. NO has been shown to kill P. falciparum and P. chabaudi parasites in vitro at high concentrations, while also having a cytostatic effect (whereby the parasites resume normal development following NO depletion) at lower concentrations [11]. Since NO can also interfere with neurotransmission, it has also been implicated in the pathogenesis of CM [12]. However, in vivo data are somewhat contradictory with some studies showing no role for NO in either parasite killing or onset of pathology [13,14].
IFN-γ And other inflammatory mediators of disease
Severe malaria has long been associated with high circulating levels of inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1) and IL-6; TNF-α levels, as measured by immunoassay, are substantially higher in plasma from children with CM or severe anaemia than in plasma from mild malaria cases [15–17]. However, recent evidence from mice indicates that it may be overproduction of lymphotoxin-α (LT-α) rather than TNF-α that leads to CM malaria [18]. Mice deficient in TNF-α were found to be just as susceptible to CM as controls, whereas LT-α deficient mice were resistant to CM pathology, dying from hyperparasitemia and severe anaemia instead. As most of the existing immunological reagents do not discriminate between TNF-α and LT-α, this study suggests that it may be time to re-evaluate the relative importance of these two cytokines in the pathogenesis of malaria. However a clear role for TNF-α has been shown in parasite killing. At physiological concentrations, recombinant TNF-α is antiparasitic, synergizing with IFN-γ to induce production of NO and other toxic radicals [19]. Thus, the successful resolution of a malaria infection and evasion of symptoms appears to depend on achieving an optimal level of TNF-α and other inflammatory cytokines.
IFN-γ, on the other hand, has been clearly linked to the onset of pathology in mice as well as in humans. The detrimental effects of IFN-γ are believed to be due to its ability to activate macrophages which, in turn, produce endogenous pyrogens (TNF-α, IL-1 and IL-6) leading to an inflammatory cascade [20,21]. The mouse model has revealed a central role for IFN-γ, both in protection and pathogenesis; although IFN-γ is essential for the onset of CM (IFN-γ receptor deficient mice do not develop CM) [22], it is also absolutely essential for control of parasitemia [23]. C57BL/6 IFN-γ–/– mice infected with P. chabaudi AS produce lower amounts of other pro-inflammatory mediators, including TNF-α and NO, with significantly higher parasitemia and mortality in comparison to wild-type controls [23]. Likewise, in the same model system, blocking IFN-γ with a neutralizing MAb in otherwise normal mice exacerbated infection whereas IFN-γ treatment decreased and delayed parasitemia [24].
Similarly, in humans, a potent and timely IFN-γ response is indicative of an effective pro-inflammatory strike of the immune system upon the parasite [25–27]. In contrast to cells taken from children with severe disease, peripheral blood mononuclear cells (PBMC) taken from African children presenting with mild disease were more efficient producers of IFN-γ when stimulated with sporozoite or merozoite antigen peptides, thereby demonstrating an association between antigen-specific IFN-γ production and reduced pathology [25]. Furthermore, hyperparasitemia was associated with a lower frequency of IFN-γ producing CD4+ T cells in Gabonese patients presenting with acute P. falciparum malaria [28]. On the other hand, as in mice, high systemic levels of IFN-γ are correlated with severe pathology. Disease severity has been directly correlated with corresponding levels of IFN-γ, with high levels being found in symptomatic individuals and much lower levels both in plasma [29] and in cultured PBMC supernatants [30] where clinical pathology is not apparent. These data are supported by in vitro experiments where PBMC from clinically immune individuals produce lower levels of IFN-γ than those from unexposed donors [31,32], indicating that the control of clinical symptoms characteristic of immunity may hinge on the ability to regulate the inflammatory response.
Interestingly, in malaria-endemic populations, CM is uncommon in very young children experiencing their first malaria infection, but is common in slightly older children undergoing second or subsequent malaria infections. These children have already acquired a degree of antimalarial immunity as demonstrated by high levels of circulating antimalarial antibodies [33]. We have hypothesized that priming of malaria-specific αβ T cells during early life leads to excessive IFN-γ production on reinfection, predisposing an individual to the over-production of TNF-α and subsequent onset of severe pathology [34].
In addition to its ability to up-regulate TNF-α production, IFN-γ also promotes the quinolinic acid (QA) pathway of tryptophan metabolism by inducing the enzyme indoleamine 2,3 dioxygenase (IDO). Diversion of the pathway away from the production of kyurenic acid (KA) towards the production of neurotoxic QA may also contribute to the development of CM. IDO activity has been reported to be 5 fold higher in mice with cerebral malaria than in litter mates without cerebral symptoms; this is associated with a marked increase in the QA:KA ratio [35]. Raised QA:KA ratios have been reported in Vietnamese adults with cerebral malaria [36] with especially high ratios in those who died, who presented with mean QA concentrations well above the level at which neurotoxicity is known to occur.
Cross-reactive priming
In contrast to the pattern of severe malaria in endemic populations, when non-immune individuals move into a malaria endemic area the risk of severe pathology increases with age with adults being significantly more susceptible to clinical disease than children; conversely, adults develop antiparasitic immunity faster than children [37]. Importantly, T cells isolated from naïve adults, when cultured in vitro with P. falciparum antigens, respond in a classical MHC Class II-restricted manner, proliferating and secreting cytokines. T cell responses tend to peak after 6–7 days of activation in in vitro culture and the proliferating cells have been identified as either TCRαβ+ T cells, which respond to both live and dead parasite antigens, or TCRγδ+ T cells that respond preferentially to live parasites [38,39]; both cell types have been shown to secrete IFN-γ[40–42]. These cells express a memory phenotype [43], proliferate, and produce IFN-γ in amounts comparable to true malaria memory T-cells [38]. Cloned malaria-reactive memory Th1 cells from nonexposed donors respond to a wide range of antigens including Toxoplasma gondii, tetanus toxoid, adenovirus, mycobacterial, streptococcal, and fungal antigens [40]. Plasmodium antigens are thought to mimic antigens from other microbes thereby soliciting the reactivation of cross-reactively primed memory T cells [34]. As exposure to common pathogens, environmental or commensal organisms increases with age, the population of malaria cross-reactive memory Th1 cells is also likely to increase with age, thereby resulting in stronger inflammatory responses and higher risk of severe pathology in adults compared to children. In infants and young children the pool of cross-reactive memory cells is likely to be small therefore malaria infection is likely to be of limited pathogenicity, but will serve to prime T-cells for a pro-inflammatory role during second and subsequent infections. Thus, the higher frequency of cross-reactive primed T cells in older individuals may explain the higher risk of severe disease in nonimmune adults compared with nonimmune children.
Cross-reactively primed αβ T cells make significant amounts of IFN-γ but do not seem to protect naïve individuals from infection or disease; rather they seem to contribute to pathology. It is possible that exposure to malaria per se may induce qualitatively different T cell responses, for example, priming T cells that make IFN-γ in more appropriate amounts, or with more appropriate kinetics, or T cells with different homing characteristics allowing cytokine production to be effectively focused at sites of parasite sequestration and replication.
Thus, developing clinical immunity may hinge on the ability to down-regulate the nonprotective cross-reactive T cell response, leaving the innate response and protective T cells specifically primed by malaria infection to control parasitemia. Given the dual role of IFN-γ, its production needs to be tightly regulated in order to achieve clearance of infection whilst avoiding detrimental effects, a state which is characteristic of clinical immunity. This implies that the cellular sources of IFN-γ– and the balance between innate and adaptive sources of IFN-γ– may change depending on level of immunity, which may in turn influence the absolute levels that are produced.
Innate immune response
Whilst acquired immune responses eventually confer significant protection against malarial pathology, studies in mice undergoing a primary malaria infection have shown that the profile of cytokines, including IFN-γ, released in the first few hours of malaria infection predicts the course of infection and the final outcome [44,45]. Such rapid production of IFN-γ in naïve animals implies production either from pre-existing, cross-reactively primed, effector memory T cells or from cells of the innate immune system, e.g. phagocytic granulocytes, macrophages, NK cells or γδ T cells.
Glycoproteins and glycolipids (particularly the glycosylphosphatidylinositol (GPI) anchors of merozoite membrane proteins) released from rupturing malaria-infected erythrocytes directly induce low levels of TNF-α production from macrophages [46]. However, TNF-α production is significantly up-regulated in the presence of other mononuclear cells, including CD3+ T cells, indicating that a secondary stimulus, in addition to malarial GPI, is required for maximal activation of macrophages to produce TNF-α. Αn obvious candidate for this secondary stimulus is the macrophage activating factor, IFN-γ. In rodent models, early IFN-γ production is essential for controlling the initial wave of parasitaemia and much of this early cytokine response is NK cell-dependent [44,47].
We and others have shown that live P. falciparum can induce rapid (within 12 h) IFN-γ and TNF-α responses [48,49] and that TCRγδ+ T cells may contribute to the early IFN-γ response [50]. More recently, we have examined the kinetics of malaria-induced IFN-γ production by cells from malaria-naive and malaria-exposed donors. In many naïve donors, IFN-γ is produced within 18 h of in vitro stimulation of PBMC with intact parasitized erythrocytes; this early pulse of IFN-γ is derived principally from NK cells [49]. Subsequently, γδ T cells (at 24–48 h) and eventually αβ T cells (at 4–6 days) also begin to secrete IFN-γ.
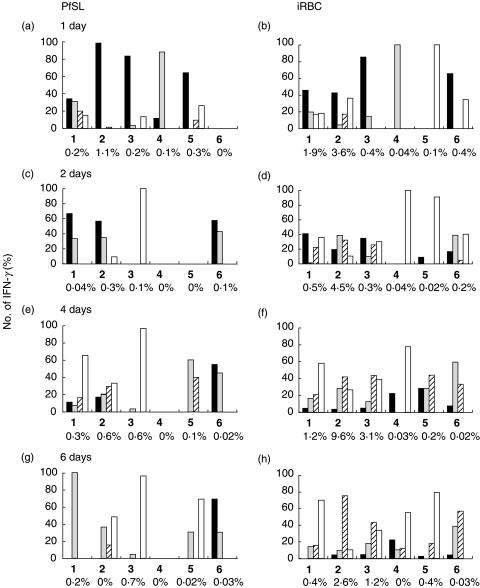
Interestingly, there is considerable variation between donors in their early NK-mediated IFN-γ production with some donors showing little if any NK activation [49]. Individuals who do not mount a strong NK response at 24 h also fail to mount a robust IFN-γ response at later time points, with reduced IFN-γ production by γδ and αβ T cells (Fig. 1). These data suggest that NK cells may be necessary to ‘kick-start’ the inflammatory response. This hypothesis is supported by experiments by Carnaud et al. [51] who demonstrated that after blocking early IFN-γ production from NK-T and NK cells, the secondary wave of IFN-γ release was delayed or entirely eliminated. The clinical consequences of this inherent variation in the innate immune response are not yet known, but we can speculate that the ability of NK cells to produce a strong, early IFN-γ response may correlate either with better containment of parasite replication and thus avoidance of hyper-parasitaemia and ensuing severe pathology or, conversely, rapid production of high levels of IFN-γ may lead to excessive inflammatory reactions and therefore contribute to pathology. Clinical studies in endemic populations are required to answer this question.
Fig. 1.
Percentage of IFN-γ+ cells that are αβ (□), γδ ( ), NK (▪) or CD56+CD3+* (
), NK (▪) or CD56+CD3+* ( ) cells. PBMC (106/ml) from 6 donors (numbered 1–6) were cultured with P. falciparum schizont lysate (PfSL), intact P. falciparum infected red blood cells (iRBC) or uninfected red blood cells (uRBC) (3 × 106/ml) for 1, 2, 4 or 6 days. Cells were stained for presence of αβ, γδ or NK surface phenotypes in combination with intracellular IFN-γ. The percentage of all IFN-γ+ cells belonging to each surface phenotype are shown for each donor at each time-point in response to PfSL (a, c, e, g) or iRBC (b, d, f, h) with uRBC control values already subtracted. Each bar represents a different cell phenotype. The percentage of all events that were IFN-γ+ are shown along the x-axes for each time point. *CD56+CD3+ cells may represent a population of NK-T cells, but this has not been formally demonstrated.
) cells. PBMC (106/ml) from 6 donors (numbered 1–6) were cultured with P. falciparum schizont lysate (PfSL), intact P. falciparum infected red blood cells (iRBC) or uninfected red blood cells (uRBC) (3 × 106/ml) for 1, 2, 4 or 6 days. Cells were stained for presence of αβ, γδ or NK surface phenotypes in combination with intracellular IFN-γ. The percentage of all IFN-γ+ cells belonging to each surface phenotype are shown for each donor at each time-point in response to PfSL (a, c, e, g) or iRBC (b, d, f, h) with uRBC control values already subtracted. Each bar represents a different cell phenotype. The percentage of all events that were IFN-γ+ are shown along the x-axes for each time point. *CD56+CD3+ cells may represent a population of NK-T cells, but this has not been formally demonstrated.
Activation of NK cells by P. falciparum is highly dependent on IL-12 and, to a lesser extent, IL-18 [49], presumably coming from monocyte-macrophages or dendritic cells (DC). There is evidence that P. falciparum parasitized erythrocytes bind to immature DC leading to inhibition of DC maturation, abrogation of IL-12 production, a switch to IL-10 production, and reduction of the subsequent T cell proliferative response [52]. These data imply that innate and/or adaptive inflammatory cytokine responses may be directly inhibited by the parasite in order, perhaps, to facilitate its own survival. Whilst similar data have recently been published for the rodent parasite P. yoelii[53], bone marrow-derived murine DC stimulated in vitro with P. chabaudi produce IL-12 within 30 min and subsequently mature into efficient antigen-presenting cells [54]. Similarly, lack of NK responses to P. falciparum is not due to failure to produce IL-12/IL-18 [49] and we have identified populations of IL-12 producing cells following short-term in vitro stimulation of human PBMC with P. falciparum-infected erythrocytes (R. M. Walther and E. M. Riley, unpublished observation).
Resolving inflammation
The severity of disease in malaria infection has been correlated with high systemic levels of IFN-γ and TNF-α. We hypothesize that clinical immunity is associated with the ability to regulate the production of pro-inflammatory cytokines to an intermediate level, which mediates parasite clearance while simultaneously avoiding severe pathology. It has been proposed that antibodies to malarial GPI and other glycolipids block induction of TNF-α from macrophages thereby down-regulating the inflammatory cascade and preventing immunopathology. Whilst there is now good evidence that such protective responses can be induced in rodents by vaccination [55], two recent immuno-epidemiological studies provide only very limited evidence that naturally acquired anti-GPI antibody responses contribute to clinical immunity [56,57]. Alternatively, the initial pro-inflammatory (Th1/IFN-γ) immune response may switch to a predominantly anti-inflammatory response mediated by cytokines such as TGF-β or IL-10 [58]. This hypothesis is supported by data from murine malaria studies where IL-10–/– mice demonstrated higher mortality as compared to wild-type controls when infected with P. chabaudi AS [59]. Similarly, when comparing mice infected with lethal or nonlethal strains of malaria parasites, a strong and sustained TGF-β response, beginning at 5–6 days post infection when the peak of parasite replication has been reached, was associated with abrogation of mortality and resolution of infection [60]. Neutralization of TGF-β leads to 100% mortality in BALB/c mice infected with normally nonlethal P. chabaudi A/J [60] and exacerbates pathology and accelerates mortality in IL-10–/– C57BL/6 mice (Li, Omer, Riley and Langhorne, unpublished observation). However, in some mouse-parasite combinations, a very early burst of TGF-β production (within the first 24 h of infection) totally suppresses the inflammatory cytokine response leading to more rapid parasite growth and early death from anaemia (Omer, de Souza and Riley, unpublished observation); administration of high doses of exogenous TGF-β has similar effects [61]. Thus, the timing of the TGF-β (and IL-10) response seems to be critical, with high levels too early in infection compromising cell-mediated effector mechanisms and low levels later in infection leading to a failure to control the inflammatory cytokine cascade with subsequent development of severe pathology.
Data is beginning to emerge in humans supporting the notion that cytokine balance is important for resolving malaria infections without severe pathology. Severe malarial anaemia has been linked to high TNF-α levels and low IL-10 levels with the ratio between these two opposing cytokines being the clearest predictor of clinical presentation [17,62]. Immuno-histopathology performed on brain tissue from patients who died with cerebral malaria has shown localized build-up of TNF-α, IFN-γ and IL-1β[63] associated with up-regulation of adhesion molecules such as ICAM-1 and sequestration of parasitized erythrocytes and/or lymphocytes and monocytes [64–66]. In Vietnamese adults, severe malaria associated with acute multiorgan failure is characterized by elevated plasma concentrations of inflammatory cytokines; patients who eventually died had significantly lower plasma IL-10 concentrations than those who survived [21]. Finally, in a longitudinal study of the relationship between pro- and anti-inflammatory cytokine production and clinical immunity to malaria in Ghana [67], we have recently shown that high ratio's of IFN-γ, IL-12 or TNF-α to TGF-β are associated with reduced risk of parasitemia but increased risk of febrile illness. These data support the notion that anti-inflammatory cytokines are required to down-regulate the pathological effects of high concentrations of pro-inflammatory cytokines. A dynamic equilibrium seems to be required with pro-inflammatory effector mechanisms targeting and controlling the parasite, and anti-inflammatory cytokines suppressing immunopathology.
A MODEL OF ANTIMALARIAL IMMUNITY
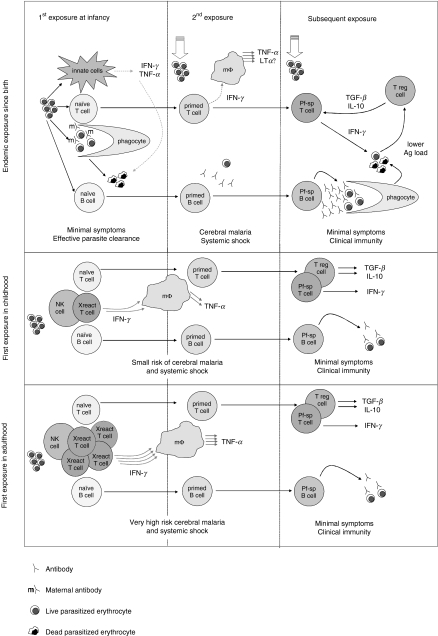
The following model (Fig. 2) fits both the immunological and epidemiological data. The hypothesis is that, in endemic populations, primary malaria infections in infants induce low levels of IFN-γ and TNF-α via an innate pathway and, at the same time, antigen-specific T cells are primed. The infection induces minimal clinical symptoms and parasites are cleared, either immunologically (via opsonization by maternal antibody or cytokine-mediated parasite killing) or because parasites fail to establish themselves in the face of physiological barriers (e.g. fetal haemoglobin and dietary deficiencies) [68]. On re-infection, the malaria primed T cells produce greatly increased amounts of IFN-γ which synergise with malarial GPI to up-regulate the production of TNF-α (and/or lymphotoxin) leading to an increased risk of cerebral malaria or systemic shock. Further infections induce effective antiparasitic immunity which reduces the parasite load and thus the concomitant level of antigenic stimulation, thereby dampening the pro-inflammatory cytokine cascade. Falling antigen concentrations or other factors lead to a switch in the predominant T cell phenotype from Th1 (IFN-γ producing) to a regulatory T (Treg) cell phenotype (IL-10 and TGF-β producing). The clinically immune individual can now clear the infection without running the risk of overproducing dangerous inflammatory mediators. The risk of severe disease may thus depend on the relative speed with which the different components of the antimalarial immune response develop.
Fig. 2.
A proposed model for the development of clinical immunity to P. falciparum malaria, dependent on age at first exposure. For details, see text.
By contrast, nonimmune adults who contract malaria during travel to an endemic area, or during an epidemic in a previously malaria-free region, have no protective immunity and are unable to control their infections. Innate responses may provide a degree of protection, as has been described for some individuals with experimental malaria infections [69], but cross-reactively primed T cells appear to contribute to development of severe disease. Non-immune children on the other hand may have fewer cross-reactively primed cells (due to lower levels of exposure to cross-reacting microbes) and are, subsequently, at lower risk of cerebral or severe malaria.
This model of antimalarial immunity has obvious implications for vaccine development; Th1-like cellular responses are clearly required for parasite clearance, but need to be induced in a controlled, site- or organ-specific manner in order to avoid systemic disease.
REFERENCES
- 1.Molineaux L. Plasmodium falciparum malaria. some epidemiological implications of parasite and host diversity. Ann Trop Med Parasitol. 1996;90:379–93. doi: 10.1080/00034983.1996.11813067. [DOI] [PubMed] [Google Scholar]
- 2.Yipp BG, Baruch DI, Brady C, et al. Recombinant PfEMP1 peptide inhibits and reverses cytoadherence of clinical Plasmodium falciparum isolates in vivo. Blood. 2003;101:331–7. doi: 10.1182/blood-2002-06-1725. [DOI] [PubMed] [Google Scholar]
- 3.Smith JD, Craig AG, Kriek N, et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci USA. 2000;97:1766–71. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull PC, Marsh K. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 2002;10:55–8. doi: 10.1016/s0966-842x(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 5.Newbold CI, Pinches R, Roberts DJ, Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992;75:281–92. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 6.Guevara Patino JA, Holder AA, McBride JS, Blackman MJ. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1689–99. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, et al. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–602. [PubMed] [Google Scholar]
- 8.Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J Infect Dis. 2001;183:1388–94. doi: 10.1086/319860. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji M, Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003;19:88–93. doi: 10.1016/s1471-4922(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 10.Brunet LR. Nitric oxide in parasitic infections. Int Immunopharmacol. 2001;1:1457–67. doi: 10.1016/s1567-5769(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 11.Balmer P, Phillips HM, Maestre AE, McMonagle FA, Phillips RS. The effect of nitric oxide on the growth of Plasmodium falciparum. P. chabaudi and P. berghei in vitro. Parasite Immunol. 2000;22:97–106. doi: 10.1046/j.1365-3024.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 12.Al Yaman FM, Mokela D, Genton B, Rockett KA, Alpers MP, Clark IA. Association between serum levels of reactive nitrogen intermediates and coma in children with cerebral malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1996;90:270–3. doi: 10.1016/s0035-9203(96)90243-6. [DOI] [PubMed] [Google Scholar]
- 13.Favre N, Ryffel B, Rudin W. Parasite killing in murine malaria does not require nitric oxide production. Parasitology. 1999;118:139–43. doi: 10.1017/s0031182098003618. [DOI] [PubMed] [Google Scholar]
- 14.Favre N, Ryffel B, Rudin W. The development of murine cerebral malaria does not require nitric oxide production. Parasitology. 1999;118:135–8. doi: 10.1017/s0031182098003606. [DOI] [PubMed] [Google Scholar]
- 15.Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–91. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski D, Hill AV, Sambou I, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–4. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzhals JA, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–72. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 18.Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–7. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria. inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern P, Hemmer CJ, Van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–43. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 21.Day NP, Hien TT, Schollaardt T, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 22.Amani V, Vigario AM, Belnoue E, et al. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur J Immunol. 2000;30:1646–55. doi: 10.1002/1521-4141(200006)30:6<1646::AID-IMMU1646>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun. 2000;68:4399–406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meding SJ, Cheng SC, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–8. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–8. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 26.Torre D, Giola M, Speranza F, Matteelli A, Basilico C, Biondi G. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur Cytokine Netw. 2001;12:361–4. [PubMed] [Google Scholar]
- 27.Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin Diagn Laboratory Immunol. 2002;9:348–51. doi: 10.1128/CDLI.9.2.348-351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler S, Willheim M, Baier K, et al. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J Infect Dis. 1999;179:209–16. doi: 10.1086/314571. [DOI] [PubMed] [Google Scholar]
- 29.Riley EM, Jakobsen PH, Allen SJ, et al. Immune response to soluble exoantigens of Plasmodium falciparum may contribute to both pathogenesis and protection in clinical malaria: evidence from a longitudinal, prospective study of semi-immune African children. Eur J Immunol. 1991;21:1019–25. doi: 10.1002/eji.1830210424. [DOI] [PubMed] [Google Scholar]
- 30.Mshana RN, Boulandi J, Mshana NM, Mayombo J, Mendome G. Cytokines in the pathogenesis of malaria: levels of IL-I beta, IL-4, IL- 6, TNF-alpha and IFN-gamma in plasma of healthy individuals and malaria patients in a holoendemic area. J Clin Laboratory Immunol. 1991;34:131–9. [PubMed] [Google Scholar]
- 31.Rhee MS, Akanmori BD, Waterfall M, Riley EM. Changes in cytokine production associated with acquired immunity to Plasmodium falciparum malaria. Clin Exp Immunol. 2001;126:503–10. doi: 10.1046/j.1365-2249.2001.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chizzolini C, Grau GE, Geinoz A, Schrijvers D. T lymphocyte interferon-gamma production induced by Plasmodium falciparum antigen is high in recently infected non-immune and low in immune subjects. Clin Exp Immunol. 1990;79:95–9. doi: 10.1111/j.1365-2249.1990.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erunkulu OA, Hill AV, Kwiatkowski DP, et al. Severe malaria in Gambian children is not due to lack of previous exposure to malaria. Clin Exp Immunol. 1992;89:296–300. doi: 10.1111/j.1365-2249.1992.tb06948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley EM. Is T-cell priming required for initiation of pathology in malaria infections? Immunol Today. 1999;20:228–33. doi: 10.1016/s0167-5699(99)01456-5. [DOI] [PubMed] [Google Scholar]
- 35.Sanni LA, Thomas SR, Tattam BN, et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152:611–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Medana IM, Hien TT, Day NP, et al. The clinical significance of cerebrospinal fluid levels of kynurenine pathway metabolites and lactate in severe malaria. J Infect Dis. 2002;185:650–6. doi: 10.1086/339009. [DOI] [PubMed] [Google Scholar]
- 37.Baird JK, Masbar S, Basri H, Tirtokusumo S, Subianto B, Hoffman SL. Age-dependent susceptibility to severe disease with primary exposure to Plasmodium falciparum. J Infect Dis. 1998;178:592–5. doi: 10.1086/517482. [DOI] [PubMed] [Google Scholar]
- 38.Dick S, Waterfall M, Currie J, Maddy A, Riley E. Naive human alpha beta T cells respond to membrane-associated components of malaria-infected erythrocytes by proliferation and production of interferon-gamma. Immunology. 1996;88:412–20. doi: 10.1046/j.1365-2567.1996.d01-661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterfall M, Black A, Riley E. Gamma delta+ T cells preferentially respond to live rather than killed malaria parasites. Infect Immun. 1998;66:2393–8. doi: 10.1128/iai.66.5.2393-2398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currier J, Sattabongkot J, Good MF. ‘Natural’ T cells responsive to malaria. evidence implicating immunological cross-reactivity in the maintenance of TCR alpha beta+ malaria-specific responses from non-exposed donors. Int Immunol. 1992;4:985–94. doi: 10.1093/intimm/4.9.985. [DOI] [PubMed] [Google Scholar]
- 41.Goodier MR, Lundqvist C, Hammarstrom ML, Troye-Blomberg M, Langhorne J. Cytokine profiles for human V gamma 9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol. 1995;17:413–23. doi: 10.1111/j.1365-3024.1995.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 42.Zevering Y, Amante F, Smillie A, et al. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992;22:689–96. doi: 10.1002/eji.1830220311. [DOI] [PubMed] [Google Scholar]
- 43.Fell AH, Currier J, Good MF. Inhibition of Plasmodium falciparum growth in vitro by CD4+ and CD8+ T cells from non-exposed donors. Parasite Immunol. 1994;16:579–86. doi: 10.1111/j.1365-3024.1994.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 44.De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–8. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choudhury HR, Sheikh NA, Bancroft GJ, Katz DR, De Souza JB. Early nonspecific immune responses and immunity to blood-stage nonlethal Plasmodium yoelii malaria. Infect Immun. 2000;68:6127–32. doi: 10.1128/iai.68.11.6127-6132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–53. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohan K, Moulin P, Stevenson MM. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–8. [PubMed] [Google Scholar]
- 48.Scragg IG, Hensmann M, Bate CA, Kwiatkowski D. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur J Immunol. 1999;29:2636–44. doi: 10.1002/(SICI)1521-4141(199908)29:08<2636::AID-IMMU2636>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria. rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–63. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 50.Hensmann M, Kwiatkowski D. Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun. 2001;69:2364–71. doi: 10.1128/IAI.69.4.2364-2371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carnaud C, Lee D, Donnars O, et al. Cutting edge. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 52.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA. 2001;98:8750–5. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ocana-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197:143–51. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seixas E, Cross C, Quin S, Langhorne J. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur J Immunol. 2001;31:2970–8. doi: 10.1002/1521-4141(2001010)31:10<2970::aid-immu2970>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 55.Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 2002;418:785–9. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 56.Naik RS, Branch OH, Woods AS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192:1563–76. doi: 10.1084/jem.192.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Souza JB, Todd J, Krishegowda G, Gowda DC, Kwiatkowski D, Riley EM. Prevalence and boosting of antibodies to Plasmodium falciparum glycosylphosphatidylinositols and evaluation of their association with protection from mild and severe clinical malaria. Infect Immun. 2002;70:5045–51. doi: 10.1128/IAI.70.9.5045-5051.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 59.Linke A, Kuhn R, Muller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi. differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–63. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 60.Omer FM, Riley EM. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsutsui N, Kamiyama T. Transforming growth factor beta-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect Immun. 1999;67:2306–11. doi: 10.1128/iai.67.5.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–82. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 63.Udomsangpetch R, Chivapat S, Viriyavejakul P, et al. Involvement of cytokines in the histopathology of cerebral malaria. Am J Trop Med Hyg. 1997;57:501–6. doi: 10.4269/ajtmh.1997.57.501. [DOI] [PubMed] [Google Scholar]
- 64.Aikawa M, Iseki M, Barnwell JW, Taylor D, Oo MM, Howard RJ. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43:30–7. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- 65.Tongren JE, Yang C, Collins WE, Sullivan JS, Lal AA, Xiao L. Expression of proinflammatory cytokines in four regions of the brain in Macaque mulatta (rhesus) monkeys infected with Plasmodium coatneyi. Am J Trop Med Hyg. 2000;62:530–4. doi: 10.4269/ajtmh.2000.62.530. [DOI] [PubMed] [Google Scholar]
- 66.Rest JR. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–5. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 67.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185:971–9. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 68.Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 2001;23:51–9. doi: 10.1046/j.1365-3024.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- 69.Collins WE, Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum in patients previously infected with heterologous species of Plasmodium: effect on development of parasitologic and clinical immunity. Am J Trop Med Hyg. 1999;61:36–43. doi: 10.4269/tropmed.1999.61-036. [DOI] [PubMed] [Google Scholar]