Abstract
The existence of an immune based graft-versus-leukaemia (GvL) effect highlighted the prospect of managing relapsed leukaemias with T cell-based adoptive immunotherapy. Thus, various strategies have been explored for the in vitro expansion of acute myeloid leukaemia (AML)-specific T cells. In a popular approach, AML blasts have been genetically modified to express co-stimulatory molecules essential for effective T cell priming. One such tactic has been the modification of AML cells to express the B7/CD80 co-stimulatory molecule that binds to CD28 on T cells initiating events that culminate in enhanced cytokine production, proliferation and development of effector functions by T cells. The success of these strategies has been limited by difficulties in attaining sufficient transduction efficiencies and associated high levels of CD80 expression. We demonstrate that these problems can be circumvented by using anti-CD28 monoclonal antibody. Furthermore, we show that the synergistic relationship between CD80/CD28 pathway and interleukin 12 cytokine (IL-12), documented in the generation of cytotoxic T lymphocytes (CTL) for solid tumours, also applies to AML. CD28/IL-12 synergy facilitated the proliferation of allogeneic T cells in response to stimulation with primary AML blasts. The synergy also favoured generation of a Th1-type immune response, evidenced by gamma interferon (IFN-γ) secretion and facilitated naive and memory T cell proliferation. Unlike some methods of in vitro T cell expansion, use of CD28/IL-12 synergy left T cells in the physiologically appropriate CD45RA–/CCR7– subsets known to be associated with immediate cytotoxic functions.
Keywords: adoptive immunotherapy, CD28/IL-12 synergy, graft-versus-leukaemia
INTRODUCTION
In contrast to solid tumours, the immunotherapy of haematological malignancies has focused on the feasibility of generating tumour specific cytotoxic T lymphocytes (CTL) ex-vivo for the purpose of adoptive immunotherapy [1–3]. Such strategies depend largely on the use of the leukaemic blasts as source of antigen for the necessary in vitro T cell stimulations. It is therefore problematic that the antigen-presenting capacity of acute myeloid leukaemia (AML) cells is restricted by the fact that they generally lack expression of B7·1/CD80 [4], the most important co-stimulatory molecule essential for effective T cell priming [5–7]. B7/CD80 has also long been known to act in synergy with interleukin (IL)-12 cytokine in generating Th1 immune responses [8]. This synergistic relationship, in which the effect of the combined co-stimuli is greater than their additive effect, has been exploited with some success in the generation of CTL against solid tumours [9–11]. As such, various tactics have been deployed to genetically modify leukaemic blasts to express B7/CD80 and IL-12, including the use of retroviruses [12], and adeno-associated viruses [13]. However, these endeavours are hampered by the low levels of protein expression achieved in the AML cells. Therefore, in this study we have set out to initially explore an alternative method of attaining B7/CD80 co-stimulation for AML cells without the requirement for genetic modification and then ascertain whether the B7/CD80 and IL-12 synergy can also be applied to the generation of CTL for AML.
The CD80 co-stimulatory molecule is normally found on professional antigen-presenting cells (APCs) with its receptor, CD28, being expressed on CD4+ and CD8+ T cells [5–7]. On association with CD28, a sequence of events is initiated in the T cell that results in increased cytokine production, proliferation, clonal expansion and the development of effector functions [5–7]. Attempts to manipulate AML cells to express B7/CD80 thus aim to endow these cells with the antigen presenting capacity of the professional APCs. Work using murine models of AML has demonstrated that AML cells, transfected to express B7/CD80, are immunogenic and when used as vaccines can generate CTL able to eliminate established disease [14–16]. Indeed, it has been shown that B7/CD80 expression by human AML cells provides the required co-stimulation that permits them to stimulate allogeneic T cell proliferation [12,17].
As a result of their low proliferative rate [18] AML cells, in common with other cells of haematopoietic origin, are notoriously difficult to transduce [19], given that most vectors require replicating cells. As such, difficulties have been encountered in genetically modifying human AML cells to express acceptable and reproducible levels of B7/CD80 protein [12]. It has become imperative therefore to explore alternative ways of providing co-stimulation for AML stimulation of T cells without the requirement for gene modification. Notter and colleagues [4] have started on this exercise by creating a B7·1 IgG fusion protein that simply binds, through the Fc segment, to AML blasts via their Fcγ receptor I (CD64). In this manner, human AML cells acquired a B7/CD80 positive phenotype without any genetic manipulations and were proficient in stimulating proliferation of autologous T cells. In this study, we have explored another alternative of simply using a monoclonal antibody to CD28, the receptor for B7/CD80. We set out initially to determine whether use of this CD28 antibody would provide co-stimulation that would permit AML cells to stimulate T cell proliferation without the requirement for genetic manipulation. Secondly, we aimed to examine whether the synergistic relationship between B7/CD80 and IL-12 that has been well documented in the generation of CTL against solid tumours could also be utilized in a similar fashion for haematological malignancies such as AML.
MATERIALS AND METHODS
Primary AML cells
Samples of peripheral blood and/or bone marrow were obtained, subject to local ethical committee approval, from consenting adult AML patients attending the Department of Haematology, Royal Free Hospital and other collaborating centres in the United Kingdom. Cells were isolated on a LymphoPrep (Amersham, UK) gradient and cryopreserved in RPMI-1640 (10%) fetal calf serum (FCS) (80%) with 10% DMSO. In all cases, AML blasts were phenotyped for expression of HLA class I antigens, HLA-DR, CD80 and CD86. All samples expressed normal levels of HLA class I (W632 monoclonal antibody) compared to normal donor mononuclear cells. HLA-DR was also expressed by all samples, although the intensity of expression varied between donors (data not shown). CD80 was absent from all AML samples tested and CD86 was expressed on more than 5% of blasts in all 24 samples tested. The 5% threshold was used because our group has shown previously that AML cells can stimulate allogeneic T cells when as few as 5% express CD86 in the absence of CD80 [20]. Density of expression of HLA-DR does not influence significantly the allogeneic response to AML [20].
Isolation of T cells
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of normal donors or AML patients in complete remission by density gradient separation (LymphoPrep, Amersham, UK). T cells (purity greater than 95% CD3+) were then obtained by immunomagnetic depletion of other lymphocyte populations using anti CD14, CD19 and CD56 PE conjugated antibodies and anti-PE microbeads (Miltenyi Biotec, Germany). Sorted T cells were maintained in RPMI-1640, 10% FCS, 2 mm l-glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin.
Stimulation of T cells with phytohaemaglutinnin (PHA)
Purified T cells (2 × 105cells/well) were stimulated with PHA (0·5–16 µg/ml) in 96-well plates. After 48 h, cells were pulsed with [3H]thymidine (1 µCi/well) for 17 h and then harvested. [3H]thymidine uptake was determined using a scintillation counter.
T cell proliferation in response to patient-derived AML blasts
For allogeneic studies T cells were selected from peripheral blood of healthy volunteers and tested against a variety of allogeneic AML blasts. For autologous experiments T cells were isolated from AML patients in remission and tested against AML blasts from the same patient taken at the time of disease presentation. In all cases, 2 × 105 T cells were stimulated with irradiated (10 000 rad) AML blasts (2 × 104) for 5 days in 96-well plates. To assess anti-CD28/IL-12 synergy, IL-12 (10 ng/ml) and anti-CD28 antibody (5 µg/ml) were added as supplements to the stimulations. The specificity of CD28 antibody was assessed by using an irrelevant goat antimouse antibody (5 µg/ml) as a negative control. On day 5, cells were pulsed with [3H]thymidine (1 µCi/well) for 17 h prior to harvesting. [3H]thymidine uptake was determined using a scintillation counter.
Detection of IFN-γ secreting and perforin positive T cells
Allogeneic T cells were isolated from peripheral blood donated by healthy volunteers as described previously; 2 × 105 T cells were then stimulated with 4 × 104 irradiated (10 000 rad) AML blasts for 48 h in 96-well plates. The cells were then harvested and divided. The percentage of IFN-γ secreting cells was determined using the MACs IFN-γ secretion assay (Miltenyi Biotec, Germany) before flow cytometric analysis. Intracellular perforin expression was determined by surface labelling with anti-CD3 FITC and anti-CD8 PerCP followed by fixation and permeabilization (Dako Intrastain, Ely, UK) and labelling with antiperforin PE (PharMingen, Oxford, UK) and subsequent flow cytometric analysis.
Expression of CCR7/CD45RA
T cells, 2 × 105, isolated as described previously from normal volunteers, were stimulated with 2 × 104 irradiated AML blasts (10 000 rad) for 5 days with IL-12 (10 ng/ml) and varying concentrations of CD28 antibody (0·5–16·0 µg/ml); 2 × 105 T cells were also stimulated with PHA (0·5–16 µg/ml). On day 5, the level of proliferation was assessed by [3H]thymidine uptake and a concentration of CD28/IL-12 was determined that gave the same level of proliferation as PHA. The cells were then analysed for the expression of CCR7/CD45RA using the following antibodies; CD45RA APC (Pharmingen), CCR7 IgM (Pharmingen), IgM FITC secondary antibody (Pharmigen), CD8 PE (BD), CD3 PerCP (BD).
Statistical analysis
All T cell proliferation data were distributed normally. Paired distributions were tested for comparability of variance by F-test. Differences between means of datasets with comparable variance (F-test, P > 0·05) were tested by conventional non-paired Student's t-test. Welch's correction was used to determine the significance of differences between the means of distributions with significantly different variance as determined by F-test (P < 0·05).
RESULTS
Anti-CD28/IL-12 synergy drives the proliferation and blastogenesis of allogeneic T cells to primary AML blasts
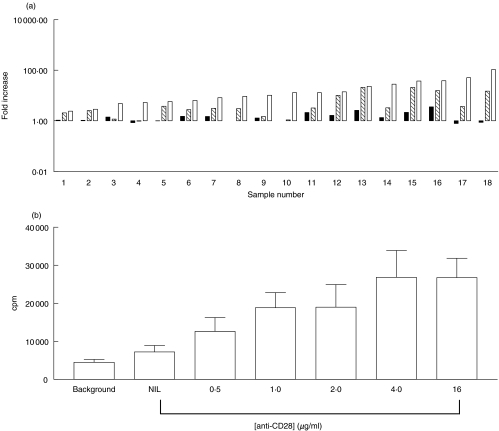
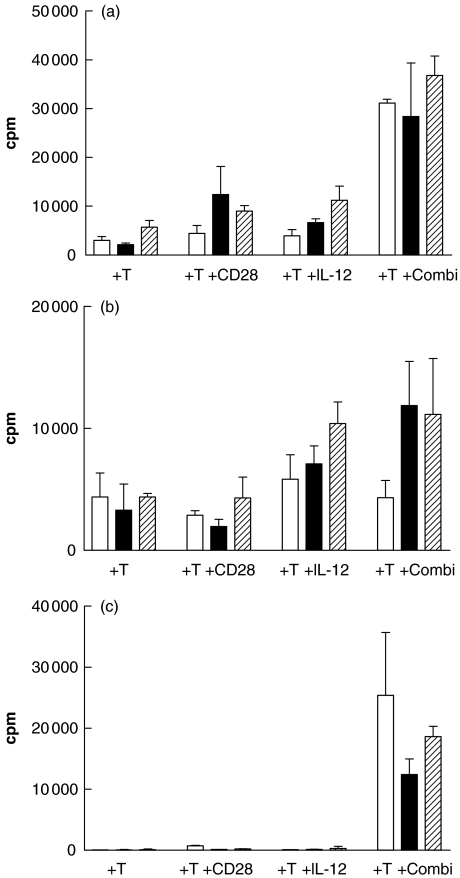
The ability of CD28/IL-12 in combination to drive the proliferation of T cells in response to stimulation by AML blasts was assessed initially using allogeneic T cells obtained from normal donors. T cells were stimulated with irradiated patient AML blasts in the presence of anti-CD28 antibody, IL-12 cytokine or the two agents combined. Figure 1a shows that, when compared to the proliferation of allogeneic T cells stimulated with blasts alone, CD28 was able to induce a modest level of proliferation in 15/18 cases; however, the degree of allogeneic T cell proliferation attained was enhanced significantly (P = 0·0017, 14/18 experiments) when CD28 antibody was used in conjunction with IL-12 cytokine. Generally, IL-12 cytokine used alone did not enhance the level of proliferation when compared to that observed with allogeneic T cells stimulated only with blasts. However, Fig. 1a shows that proliferation observed when IL-12 was used in combination with CD28 was always significantly better than that observed with IL-12 alone (P = 0·0035). In 11/18 cases the combination of IL-12 plus anti-CD28 was synergistic rather than simply additive. Addition of an irrelevant goat antimouse antibody did not affect proliferation of allogeneic T cells stimulated with AML blasts (data not shown).
Fig. 1.
Anti-CD28/IL-12 synergy drives the proliferation of allogeneic T cells to primary AML blasts. (a) Proliferation, as determined by [3H] thymidine uptake, of allogeneic T cells (2 × 105 cells/well) stimulated with primary AML blasts (2 × 104cells/well) in the presence of anti-CD28 antibody  , IL-12 cytokine (▪) or anti-CD28 in combination with IL-12 cytokine (□). Fold increase was calculated relative to the proliferation of T cells stimulated with AML blasts alone. (b) The effect of anti-CD28 on allogeneic T cell response to AML blasts plus IL-12.
, IL-12 cytokine (▪) or anti-CD28 in combination with IL-12 cytokine (□). Fold increase was calculated relative to the proliferation of T cells stimulated with AML blasts alone. (b) The effect of anti-CD28 on allogeneic T cell response to AML blasts plus IL-12.
To determine the optimum concentration of anti-CD28 for the co-stimulation of T cells to AML blasts, we conducted a dose–response experiment with T cells from three donors. Figure 1b shows the proliferation (mean ± s.d.) of the donor T cells in response to a single AML sample. Background T cell proliferation is compared to proliferation of purified T cells to irradiated AML blasts in the presence of IL-12, as described above. Concentrations of anti-CD28 MoAb ranged from nil to 16 µg per well. The lowest concentration of anti-CD28 showed significant increases in proliferation compared with IL-12 alone (P < 0·05), whereas concentrations of 4 µg or greater were associated with greater increases in proliferation which were also significant (P < 0·01).
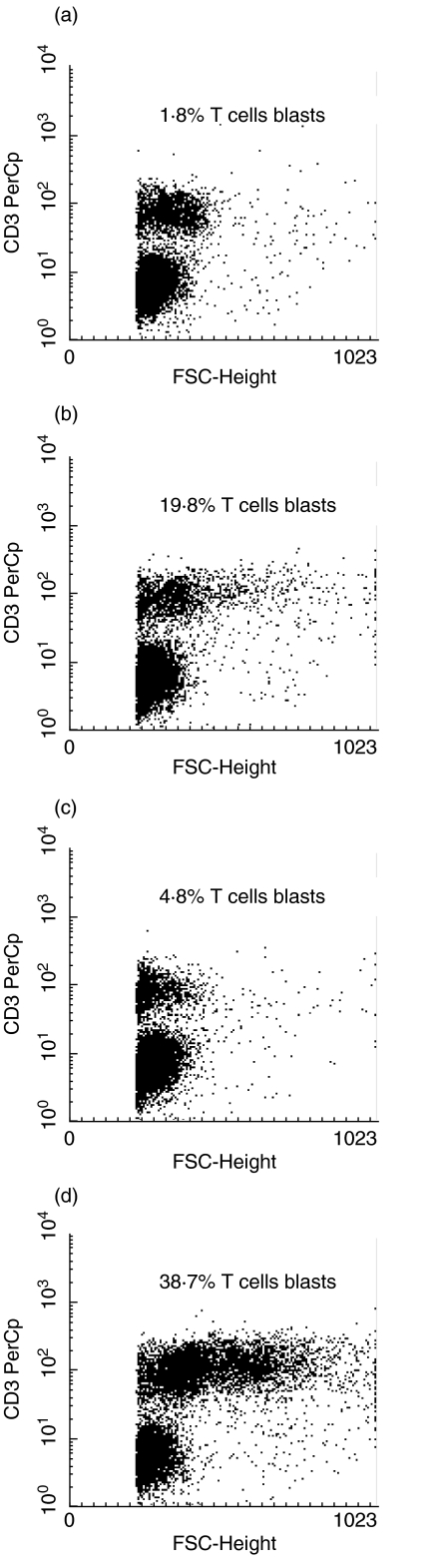
Blastogenesis of the T cell population in these cultures was also observed on day 5 and the synergy between IL-12 and anti-CD28 was confirmed. Addition of anti-CD28 to the cultures increased the proportion of blastoid T cells by an average of ninefold (mean 8·7; s.d. 1·45) while IL-12 alone had signficantly less effect (2·53; 0·89). In contrast, the use of the combined IL-12/CD28 led to increases of up to 20-fold (19·7; 3·21) (Fig. 2).
Fig. 2.
Blastogenesis of T cells in response to (a) AML blasts alone; (b) AML blasts plus anti-CD28; (c) AML blasts plus IL-12; (d) AML blasts plus anti-CD28 and IL-12. The data are presented as flow cytometric dot plots of forward angle light scatter (indicative of cell size) versus CD3 expression. The percentages represent the CD3+ blast cells as a proportion of total CD3+ cells. This is representative of five experiments.
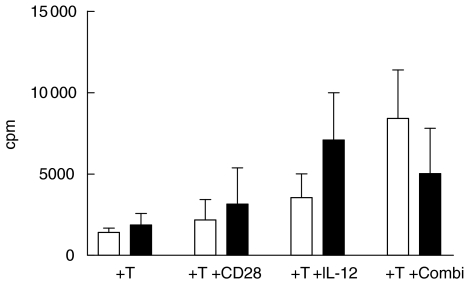
Anti-CD28/IL-12 synergy drives the proliferation of autologous T cells to primary AML blasts. In two cases we were able to obtain freshly isolated T cells from AML patients in complete haematological remission from whom we had cryopreserved their AML blasts at time of disease presentation. The addition of anti-CD28 alone was unable to enhance the proliferative response in either case while addition of IL-12 plus anti-CD28 led to significantly increased proliferation in both cases (P < 0·05 and P < 0·03, respectively) compared to autologous T cells without either co-stimulant (Fig. 3).
Fig. 3.
Two cases of autologous T cell proliferation to AML blasts. AML 55 (▪) AML 65 (□).
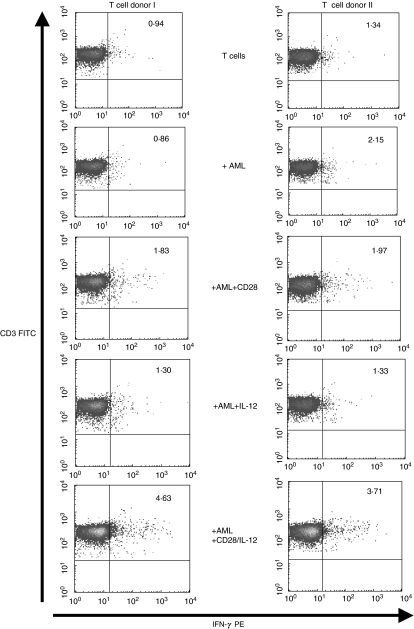
Anti-CD28/IL-12 synergy stimulates allogeneic T cells to produce IFN-γ and perforin in response to primary AML blasts
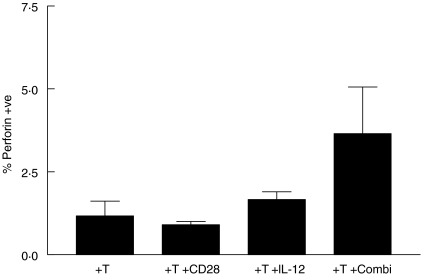
Having demonstrated the ability of CD28/IL-12 synergy to drive the proliferation of allogeneic T cells in response to AML blasts, we set out to determine if this synergy pushed T cells through a Th1 pathway. To this end, we stimulated allogeneic T cells with AML blasts in the presence of CD28 alone, IL-12 alone, and the two agents in combination. After 48 h, we tested the ability of the stimulated T cells to secrete IFN-γ, a ThI cytokine. Figure 4 shows that T cells stimulated with IL-12 and CD28 alone do produce low levels IFN-γ; however, T cells stimulated with AML blasts in the presence of the two agents combined show, on average, a twofold increase in the frequency of IFN-γ-producing T cells. The frequency of CD3+/CD8+ T cells expressing intracellular perforin increased by nearly threefold during the same culture period (mean 2·86; s.d. 0·3) (Fig. 5).
Fig. 4.
Anti-CD28/IL-12 synergy stimulates T cells to produce IFN-γ in response to primary AML blasts. IFN-γ production by CD3+ selected T cells, stimulated with AML blasts for 5 days in the presence of anti-CD28 alone, IL-12 alone or the two agents in combination. Percentage of cytokine-producing cells determined using MACs IFN-γ secretion assay and measured by flow cytometry.
Fig. 5.
Intracellular perforin expression in CD3+/CD8+ T cells after stimulation with anti-CD28, IL-12 or a combination of both.
IL-12/CD28 synergy initiates proliferation of CD45RA+ T cells
Normal donor T cells from three donors were isolated and sorted subsequently into CD45RA+ and CD45RO+ fractions to>95% purity and co-cultured with allogeneic AML blasts in the presence and absence of IL-12 ± anti-CD28. In common with the 18 samples shown in Fig. 1b, the three AML samples all showed increased allostimulatory capacity in the presence of anti-CD28/IL-12 than with either co-stimulant alone (Fig. 6a). CD45RO T cells showed an enhanced proliferative response following addition of IL-12 but anti-CD28 had no additive effect either in the presence or absence of IL-12 (Fig. 6b). In marked contrast, the CD45RA+ T cells responded only to allogeneic AML blasts in the presence of both anti-CD28 and IL-12 (Fig. 6c). It was apparent that over 60% of the T cell proliferation in the cultures supplemented with anti-CD28 plus IL-12 was in the CD45RA subset.
Fig. 6.
Differential proliferative responses of CD45RA versus CD45RO T cells to allogeneic AML blasts: non-sorted T cells; (b) CD45RO+ T cells; (c) CD45RA+ T cells. Three separate AML blasts were tested, represented by the variously shaded bars.
The CD45RA−/CCR7− T cell subset proliferates following stimulation with AML blasts using the anti-CD28/IL-12 synergy
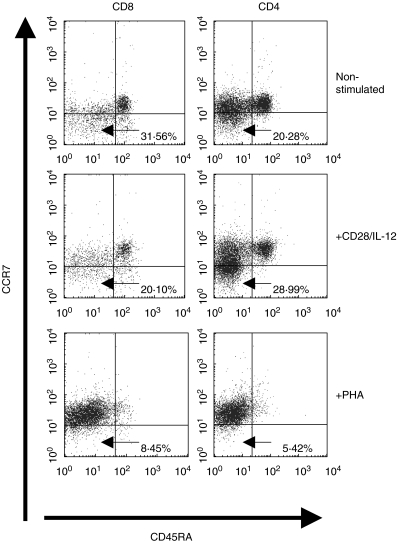
It has been observed that ex-vivo expanded T cells often have impaired function when used for adoptive immunotherapy [21–23]. This has been attributed largely to the methods used for ex-vivo expansion such as phytohaemagglutinin (PHA), concavalin A and high levels of CD3 stimulation [21–23]. Recent work [24] has shown that the method of ex-vivo expansion can affect the distribution of CD45RA/CCR7 subsets that describe the functionality of T cells. Of particular interest in the field of tumour immunology are the CD45RA−/CCR7− double negative cells which migrate to inflamed areas and have immediate cytolytic functions [25]. This subset is lost when T cells are expanded polyclonally with non-physiological stimuli such as PHA [24]. Therefore, having shown that CD28/IL-12 synergy could drive T cells to proliferate in response to AML blasts and also secrete IFN-γ, we set out to see whether this method of ex-vivo T cell expansion preserved the CD45RA−/CCR7− subset. [3H]thymidine uptake assays were carried out initially to determine the concentrations of CD28/IL-12 and PHA that gave comparable levels of T cell proliferation. Figure 7 shows the expression of CD45RA/CCR7 on T cells after 5-day stimulation with AML blasts and CD28/IL-12 compared to that of T cells that were stimulated with PHA (4 µg/ml) for the same duration of time. Cells stimulated with CD28/IL-12 synergy remain in the physiologically relevant CD45RA/CCR7 subsets, as shown by the profile of unstimulated cells, whereas T cells stimulated with PHA lose the CCR7−/CD45RA− double negative population that is associated with immediate effector functions. The scenario holds true for CD4+ and CD8+ T cells.
Fig. 7.
The CD45RA−/CCR7− T cell subset proliferates following stimulation with AML blasts using the anti-CD28/IL-12 synergy. Expression of CD45RA and CCR7 by sorted CD3+ allogeneic T cells, stimulated for 5 days with AML blasts and anti-CD28/IL-12 (5 µg/ml anti-CD28) or PHA only (4 µg/ml).
DISCUSSION
Evidence of a graft-versus-leukaemia [26,27] effect showed that haematological malignancies such as AML could be responsive to T cell-based immunotherapy. Subsequent attempts to use AML blasts in vitro for the ex-vivo generation of CTL that could be used for adoptive immunotherapy were thwarted by the fact that AML cells rarely express B7/CD80 co-stimulatory molecules [4] and thus make poor antigen presenters. As attempts to modify these cells genetically to express B7/CD80 as well as cytokines essential for competent antigen presentation have experienced technical difficulties in transducing these cells, our study has now established that CD28 monoclonal antibody can provide primary AML blasts with the co-stimulation required to drive the proliferation of allogeneic and autologous T cells, thus circumventing the need for genetic manipulation. Furthermore, the co-stimulation provided by CD28 antibody could be enhanced in a synergistic fashion by the exogenous addition of IL-12 cytokine. This combination also facilitated the generation of a proliferative response within the naive CD45RA+ T cell subsets.
The quest to find alternative ways of providing AML cells with the co-stimulation required for T cell stimulation without genetic modification was already begun by Notter and coworkers [4]. This group of workers showed that a B7·1 Ig fusion protein, capable of binding to AML cells via the CD64 receptor, could provide the co-stimulation required by human AML blasts to stimulate the proliferation of allogeneic and autologous T cells. Our data now provide another alternative method for the provision of co-stimulation without genetic manipulation. In addition to circumventing the technical difficulties encountered when transducing AML cells, the use of CD28 monoclonal antibody in this manner will be more practical than AML cells modified genetically with viral vectors due to safety issues for both the individual patients as well as the public at large [28]. Notter and colleagues [4] found that the co-stimulation provided by the use of the B7·1 Ig fusion protein could drive the proliferation only of preactivated autologous T cells and not resting ones. Our data show that use of CD28 alone induces a modest level of proliferation in resting autologous T cells, which is enhanced further by the addition of IL-12.
It has been documented for some time that IL-12 cytokine acts in synergy with B7/CD80 in the generation of Th1 immune response [8]. Indeed, this synergistic relationship had already been utilized successfully in the generation of CTL for the eradication of solid tumours [9–11]. Using a murine model of leukaemia, Dunussi-Joannopoulos and colleagues [29] had also shown that AML cells transduced to express IL-12 were effective in stimulating therapeutic immunity. However, the synergistic relationship existing between B7/CD80 and IL-12 was yet to be evaluated with respect to AML or indeed any other haematological malignancy. Our data therefore extend this area of research by showing that this synergy also applies to AML stimulation of both allogeneic and autologous T cells and could thus be exploited in the ex-vivo generation of CTL for adoptive immunotherapy. Furthermore, such AML-reactive T cells appear to retain functional activity with respect to interferon-γ synthesis and intracellular perforin and, when expanded in vitro, retain the CD45RA–/CCR7– phenotype associated with immediate cytolytic activity.
There is now a body of opinion that suggests that allogeneic CTL would be suitable for the immunotherapy of leukaemia [30] in patients from whom functional autologous CTL cannot be derived. Such patients would receive an allogeneic transplant from an HLA-identical donor which was depleted of T cells, thus reducing the mortality associated with graft-versus-host disease. They would then receive AML-specific CTL generated ex-vivo from the same HLA-matched donor. This anti-CD28/IL-12 strategy is readily translatable to clinical practice and we believe that it represents a pragmatic alternative to the use of genetically modified tumour cells. As such, it is suited to a broad range of haematological and solid tumours alike.
Acknowledgments
We would like to thank our collaborators at the Royal United Hospital, Bath, Queen Alexandra Hospital, Portsmouth and the William Harvey Hospital, Ashford for provision of additional AML samples. This work was undertaken at the Royal Free Hampstead NHS Trust which received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was sponsored by the Leukaemia Research Fund.
REFERENCES
- 1.Faber LM, van Luxemburg-Heijs SA, Willemze R, Falkenburgh JHF. Generation of leukaemia reactive cytotoxic T lymphocyte clones from the HLA-identical bone marrow donor of a patient with leukaemia. J Exp Med. 1992;176:1283–9. doi: 10.1084/jem.176.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer MW, Vallera DA, Taylor PA, et al. The role of B7 co-stimulation by murine acute myeloid leukaemia in the generation and function of CD8+ T-cell line with potent in vivo graft-versus-leukaemia properties. Blood. 1997;89:3477–85. [PubMed] [Google Scholar]
- 3.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relasped leukaemia with ex vivo – generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–41. [PubMed] [Google Scholar]
- 4.Notter M, Willinger T, Erben U, Thiel E. Targeting of a B7-1 (CD80) immunoglobulin G fusion protein to acute myeloid leukemia blasts increases their co-stimulatory activity for autologous remission T cells. Blood. 2001;97:3138–45. doi: 10.1182/blood.v97.10.3138. [DOI] [PubMed] [Google Scholar]
- 5.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol. 1996;14:233. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 6.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 7.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7: CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–82. [PubMed] [Google Scholar]
- 8.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–22. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putzer BM, Hitt M, Muller WJ, Emtage P, Gauldie J, Graham FL. Interleukin 12 and B7-1 co-stimulatory molecule expressed by an adenovirus synergistically to facilitate tumor regression. Proc Natl Acad Sci USA. 1997;94:10889–94. doi: 10.1073/pnas.94.20.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Robbins PD, Storkus WJ, et al. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of efficient? immunity and therapy of established tumors. Eur J Immunol. 1996;26:1335–41. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin CM, Wysocka M, Kurzawa HL, Lee WM, Trinchieri G, Eck SL. B7-1 and interleukin 12 synergistically induce effective antitumor immunity. Cancer Res. 1995;55:4980–7. [PubMed] [Google Scholar]
- 12.Hirst WJA, Buggins A, Darling D, Gaken J, Farzaneh F, Mufti GJ. Enhanced immune co-stimulatory activity of primary acute myeloid leukaemia blasts after retrovirus-mediated gene transfer of B7.1. Gene Ther. 1997;4:691. doi: 10.1038/sj.gt.3300437. [DOI] [PubMed] [Google Scholar]
- 13.Anderson R, Macdonald I, Corbett T, Hacking G, Lowdell MW, Pretince HG. Construction and biological characterisation of an interleukin-12 fusion protein (Flexi-12): delivered to AML blasts using adeno-associated virus. Human Gene Ther. 1997;8:1125–35. doi: 10.1089/hum.1997.8.9-1125. [DOI] [PubMed] [Google Scholar]
- 14.Matulonis UA, Dosiou C, Lamont C, et al. Role of B7-1 in mediating an immune response to myeloid leukemia cells. Blood. 1995;85:2507–15. [PubMed] [Google Scholar]
- 15.Dunussi-Joannopoulos K, Krenger W, Weinstein HJ, Ferrara JLM, Croop JM. CD8+ T cells activated during the course of murine acute myelogenous leukemia elicit therapeutic responses to late B7 vaccines after cytoreductive treatment. Blood. 1997;89:2915–24. [PubMed] [Google Scholar]
- 16.Boyer MW, Vallera DA, Taylor PA, et al. The role of B7 co-stimulation by murine acute myeloid leukemia in the generation and function of a CD8+ T cell line with potent in vivo graft-versus-leukemia properties. Blood. 1997;89:3477–85. [PubMed] [Google Scholar]
- 17.Mutis T, Schrama E, Melief CJ, Goulmy E. CD80 transfected acute myeloid leukemia cells induce primary allogeneic T-cell responses directed at patient specific minor histocompatibility antigens and leukemia-associated antigens. Blood. 1998;92:1677. [PubMed] [Google Scholar]
- 18.Arlin ZA, Fried J, Clarkson BD. Therapeutic role of cell kinetics in acute leukaemia. Clin Haematol. 1978;7:339–62. doi: 10.1016/s0308-2261(78)80009-5. [DOI] [PubMed] [Google Scholar]
- 19.Wattel E, Vanrumbeke M, Abina MA, et al. Differential efficacy of adenovirus mediated gene transfer into cells from hematological cell lines and fresh hematological malignancies. Leukemia. 1996;10:171–4. [PubMed] [Google Scholar]
- 20.Whiteway A, Corbett T, Anderson R, Macdonald M, Prentice HG. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may affect duration of first remission. Br J Haematol. 2003;120:442–51. doi: 10.1046/j.1365-2141.2003.04085.x. [DOI] [PubMed] [Google Scholar]
- 21.Contassot E, Murphy W, Angonin R, et al. In vivo alloreactive potential of ex-vivo expanded primary T lymphocytes. Transplantation. 1998;65:1365–70. doi: 10.1097/00007890-199805270-00014. [DOI] [PubMed] [Google Scholar]
- 22.Perruche S, Angonin R, Ferrand C, et al. Ex vivo expanded donor T cells have a reduced ability to facilitate the engraftment of an allogeneic bone marrow graft as compared to fresh donor T cells [Abstract] Blood. 2001;98:381a. [Google Scholar]
- 23.Drobyski WR, Majewski D, Ozker K, Hanson G. Ex vivo anti-CD3 antibody-activated donor T cells have a reduced ability to cause lethal murine graft-verus-host disease but retain their ability to facilitate alloengraftment. J Immunol. 1998;161:2610–9. [PubMed] [Google Scholar]
- 24.Duarte RF, Chen FE, Lowdell MW, et al. Functional impairment of human T-lymphocytes following polyclonal stimulation for retroviral transduction: implications for immunotherapy. Gene Ther. 2002;9:1359–68. doi: 10.1038/sj.gt.3301807. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 27.Kolb H, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–50. [PubMed] [Google Scholar]
- 28.Miller AD. Human gene therapy comes of age. Nature. 1992;357:455–60. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- 29.Dunussi-Joannopoulos K, Runyon K, Erickson J, Schaub RG, Hawley RG, Leonard JP. Vaccines with interleukin-12 transduced acute myeloid leukemia cells elicit very potent therapeutic and long-lasting immunity. Blood. 1999;94:4263–73. [PubMed] [Google Scholar]
- 30.Stauss HJ. Immunotherapy with CTLs restricted by nonself MHC. Immunol Today. 1999;20:180–3. doi: 10.1016/s0167-5699(99)01443-7. [DOI] [PubMed] [Google Scholar]