Abstract
CD30/CD30L is a member of tumour necrosis factor (TNF) receptor/TNF superfamily and has been implicated in immune-regulation. A genetic study has also suggested a possible implication of CD30 in spontaneous autoimmune diabetes in NOD mice. In this study, we investigated the involvement of CD30/CD30L in the development of diabetes in NOD mice. Flow cytometric analysis showed that CD30 and CD30L were highly expressed on CD4+ or CD8+ T cells in the spleen and pancreatic lymph node of younger NOD mice. In addition, islet-specific CD4+ or CD8+ T cell lines expressed CD30 and CD30L. Administration of a neutralizing anti-CD30L monoclonal antibody (mAb) from 2 to 10 week of age completely suppressed the development of spontaneous diabetes in NOD mice. In addition, the treatment with anti-CD30L mAb also inhibited the development of diabetes induced by adoptive transfer of spleen cells from diabetic NOD mice or islet-specific CD4+ or CD8+ T cell lines into NOD-SCID mice. Furthermore, anti-CD30L mAb inhibited T cell proliferation in response to islet antigens. These results suggested that CD30/CD30L interaction plays important roles in both induction and effector phases of autoimmune diabetes in NOD mice.
Keywords: immunotherapy, monoclonal antibody, NOD mouse, TNF superfamily, Type 1 diabetes
INTRODUCTION
Human type 1 diabetes (T1D) is a chronic autoimmune disease in which autoreactive T cells play a crucial role for pancreatic β-cell destruction [1–6]. Several autoantigens have been identified by autoantibodies in the sera of acutely diabetic or prediabetic subjects, including GAD, IA-2, and insulin [7–11]. However, the precise mechanism of pathogenesis remains largely unknown. Nonobese diabetic (NOD) mice, which spontaneously develop diabetes, have been useful as an experimental model of the human disease. The NOD mice develop insulitis from 3 to 4 week of age and most females develop diabetes by 30 weeks of age. The islet infiltrates consist of CD4+, CD8+ T cells, B cells, macrophages, and dendritic cells [12].
T cell activation by antigen-presenting cells (APC) involves not only the engagement of T cell receptor with antigen presented by major histocompatibility complex (MHC) but also costimulation through multiple receptor-ligand pairs including CD28/B7, LFA-1/ICAM-1, and the member of TNF receptor/TNF superfamily, such as CD40/CD40L, CD27/CD70, and CD134/CD134L [13–17]. CD30, a member of the TNF receptor superfamily, was originally identified as a cell surface antigen, Ki-1, on Hodgkin and Reed-Sternberg cells and widely used as a clinical marker for Hodgkin's lymphomas [18]. The ligand for CD30 (CD30L or CD153) is a type II membrane glycoprotein belonging to the TNF superfamily [19]. CD30 is expressed on activated T or B cells and CD30L is mainly expressed on activated T cells and inflamed tissues [20]. Like the other members of the TNF receptor superfamily, it has been demonstrated that signalling through CD30 could induce proliferation, differentiation, or apoptosis depending upon the cell type, stage of development, and presence of other stimuli [19,21–23]. Recent studies have implicated CD30 in regulation of inflammatory responses [24,25]
A recent report suggested a critical role of CD30 in protection against autoimmune diabetes, since islet-reactive CD8+ T cells from CD30-deficient mice were far more efficient than wild-type cells in inducing diabetes upon adoptive transfer [26]. However, this conclusion must be reconsidered, since the authors have made a correction that the capacity of low numbers of islet-reactive T cells to cause disease was segregated from their expression of CD30 by further backcrossing of the CD30-deficient mice, suggesting that CD30 was not responsible for the protection [27]. Nevertheless, a recent study has implicated CD30 as a possible candidate for a diabetes-susceptible gene (Idd 9) in NOD mice [28]. Thus, the possible role of CD30/CD30L interaction in the pathogenesis of NOD diabetes still remains to be determined properly.
To explore the role of CD30/CD30L interaction in NOD autoimmune diabetes without genetic modification, we used a functional blocking monoclonal antibody (mAb) against mouse CD30L. Administration of the anti-CD30L mAb markedly inhibited the development of spontaneous diabetes in NOD mice and also the adoptive transfer of diabetes by diabetogenic T cells, suggesting critical contributions of the CD30/CD30L interaction to both induction and effector phases of T1D development in NOD mice.
MATERIALS AND METHODS
Mice
NOD/Shi/Kbe and NOD/Caj mice were used in this study. In both NOD colonies, insulitis becomes apparent in most mice at 5–7 week of age. The cumulative incidence of diabetes is 85% in females at 30 week of age. 7–8-week-old-male CD1nu/nu mice were purchased from Charles River Japan (Atsugi, Japan) and NOD-scid/scid (NOD-SCID) mice were purchased from Clea Japan (Osaka, Japan). All experiments were conducted according to the guidelines for animal experiments of authorities.
Cells
Islet-derived CD8+ T cell lines were the lymphocytes expanded by IL-2 from inflamed islet of 20-week-old NOD mice [29]. An islet-specific CD4+ T cell clone (YNK 7·3) was established from islet-infiltrating lymphocytes from 20-week-old female NOD mice as previously described [30].
mAb
The hybridoma secreting neutralizing antimouse CD30L mAb (RM153, rat IgG2b) has been described previously [31]. RM153-secreting hybridoma cells were injected into tetramethylpentadecane (Aldrich Chemical, Milwaukee, WI, USA) primed CD1 nu/nu mice and mAb was affinity-purified from ascites on protein G column. F(ab/)2 fragment was prepared by papain digestion. Biotin or fluorochrome-conjugated mAbs to CD30 and CD30L were purchased from BD pharmingen (San Diego, CA, USA). MAb to CD4 (GK1·5) or CD8 (TIB 105), kindly provided by Dr Janeway (Yale University), were also used for purification of CD4+ or CD8+ T cell populations.
Flow cytometric analysis
To better characterize the expression of CD30 and CD30L on CD4+ and CD8+ T cells, we used the enriched CD4+ and CD8+ T cell populations by negative selection using magnetic beads. After removing erythrocytes, freshly ex vivo splenocytes were divided into two aliquots and incubated with anti-CD4 (GK1·5; rat IgG2a) or anti-CD8 mAb (TIB-105; rat IgG2a), on ice for 30 min. The cells were then incubated with goat antimouse IgG/IgM and goat antirat IgG antibody-conjugated magnetic beads (Polysciences, Inc. Warrington, PA, USA) on ice with gentle agitation for 45 min. B cells and CD4+ or CD8+ cells were removed by using a magnetic plate (Polysciences, Inc.). Purity of the CD8+ and CD4+ population was approximately 70–90%. After the enrichment, the T cells were stained with PE-conjugated anti-CD30 or anti-CD30L mAb and FITC-conjugated anti-CD4 or anti-CD8 mAb and then analysed on flow cytometry. We also examined the expression of CD30 and CD30L on T cells from pancreatic lymph node (PLN) in a similar way. Islet-specific CD4+ (YNK7·3) and CD8+ T cells were also evaluated for CD30 and CD30L expression.
Pancreatic islets were isolated from 7 to 10-week-old NOD mice by collagen digestion and dispersed to a single cell suspension using 0·125% trypsin and 3 mm EGTA. These islet cells were incubated with murine recombinant interferon (rIFN)-γ (100 ng/ml, R & D Systems, Minneapolis, MN, USA) for 24 h at 37°C and then stained with biotin-conjugated anti-CD30L mAb followed by FITC-conjugated avidin. The stained cells were analysed on a FACS L-001 flow cytometer (Becton Dickinson, San Jose, CA, USA).
In vivo mAb treatment
Female NOD mice were intraperitoneally (i.p.) administrated with anti-CD30L mAb (RM153, 500 µg/dose) or control rat IgG (Chemicon International, Temecula, CA, USA, 500 µg/dose) twice a week for 2–10 week or 4–10 week of age. In some experiments, F(ab/)2 fragment of RM153 (500 µg/dose) was injected twice a week for 2–10 week of age. The mice were monitored for diabetes twice a week up to 30 week of age by urinary and blood glucose levels. All mice were sacrificed and pancreases were removed for histology at 30 week of age.
Adoptive T cell transfers
Splenocytes from diabetic NOD mice (3 × 107) or islet-derived CD8+ T cells (1 × 107) were injected i.p. into 7-day-old NOD-SCID mice. YNK7·3 cells (1 × 107) were injected i.p. at days 7 and 14 of age. Control rat IgG, or RM153 (500 µg) in 200 µl PBS were i.p. injected starting from one day before transfer and then twice a week for 3 week after the transfer. Mice were monitored for the onset of diabetes by testing blood glucose levels every other day. Mice were considered diabetic when they had blood glucose levels of > 16·7 mmol/l on two consecutive days. Pancreases were removed from each mouse for histology at 7 d after transfer of CD8+ CTL or 8 week after transfer of diabetogenic splenocytes or YNK7·3 CD4+ T cells.
Histology
Isolated pancreases were fixed in 10% buffered formalin, and then embedded in paraffin. Four-µm thin sections were stained with haematoxylin and eosin. At least 20 islets from each mouse were examined for insulitis. Insulitis was graded as 0 (–), no cellular infiltration; 1 (±), periinsulitis; 2 (+), intra-islet infiltration in less than 25% of islets; 3 (++), 25–50% intra-islet infiltration; 4 (+++), more than 50% intra-islet infiltration.
T cell proliferation assay
Splenocytes were prepared from 8 to 9-week-old female NOD mice. After haemolysis, T cells were isolated by removing B cells with goat antimouse IgG/IgM antibody-coated magnet beads. The isolated T cells (2 × 105/well) were cultured with mitomycin C-treated NOD islets (20/well) in 200 µl of RPMI 1640 with 0·5% NOD-SCID serum. After 3 days, 1 µCi[3H]-thymidine (Amersham, Tokyo, Japan) was added for the last 16 h. Cells were then harvested by Packard FilterMate harvester (Packard, Meriden, CT, USA) and [3H]-thymidine incorporation was measured on TopCount Microplate scintillation counter (Packard).
51Cr release assay
Islets were isolated from 7 to 10-week-old NOD-SCID mice. Single cell suspensions of islet cells were labelled with [51Cr]-sodium chromate for 90 min at 37°C, and resuspended in 100 µl RPMI 1640 containing 10% FCS at 1 × 104 cells per well of round-bottomed 96-well microculture plates for use as target cells. As effector cells, islet-derived CD8 CTL were added to each well in triplicate at different effector/target (E/T) ratios (2 : 1, 10 : 1, and 50 : 1). To block CD30/CD30L interactions, 100 µg/ml RM153 was added to the culture. The plates were incubated at 37°C for 8 h, and the supernatants were collected for determination of 51Cr release. Culture medium alone or 1% Triton X-100 was added to target cells for determination of spontaneous and total cell lysis, respectively. Specific 51Cr release was calculated as follows: specific lysis = 100 × (test cpm – spontaneous cpm)/(total cpm – spontaneous cpm).
Statistical analysis
Statistical analysis of diabetic incidence was performed by the Kaplan-Meier method. The insulitis scores and proliferation assay were analysed by the Mann–Whitney U-test. The diabetic incidence in the adoptive transfer experiments was analysed by χ2 test. P-values less than 0·05 were considered statistically significant.
RESULTS
Expression of CD30 and CD30L on T cells of NOD mice
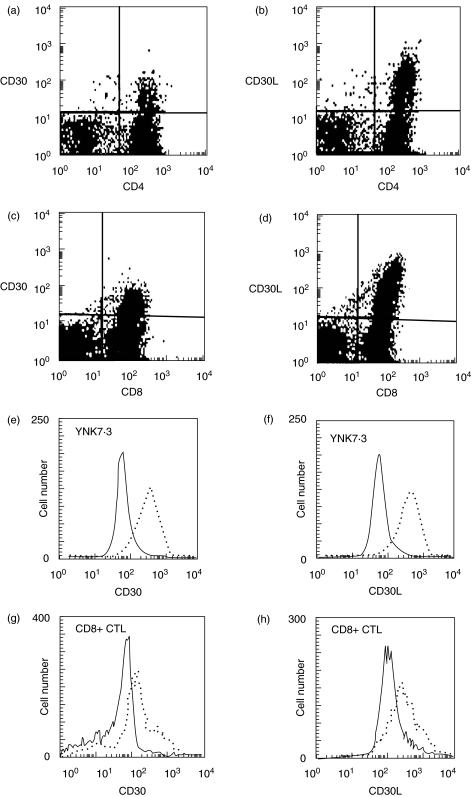
We first examined the expression of CD30 and CD30L on splenic and PLN T cells by flow cytometry. A substantial part of CD4+ or CD8+ splenic T cells from 6-week-old NOD mice showed the expression of CD30 and CD30L molecules (Fig. 1a). A similar expression of CD30 and CD30L was also observed on CD4+ or CD8+ T cells from PLN (not shown). Interestingly, islet-specific CD4+ T cell clone (YNK 7·3) and islet-derived CD8+ CTL also constitutively expressed both CD30 and CD30L (Fig. 1e).
Fig. 1.
Expression of CD30 and CD30L on NOD T cells. Semi-purified CD4+ (a, b) and CD8+ T cell (c, d) populations from 6-week-old NOD splenocytes were stained with PE-conjugated mAb to CD30 or CD30L. The cells were also costained with FITC-conjugated mAb to CD4 or CD8. Islet-specific CD4+ (YNK 7·3) (e, f) and islet-derived CD8+ CTL (g, h) were stained with biotin-conjugated anti-CD30 or CD30L mAb, followed by FITC-Avidin, and PE-conjugated mAbs to CD4 or CD8 molecules. Expression of CD30 and CD30L were indicated by dotted line. One of the three separate experiments was represented.
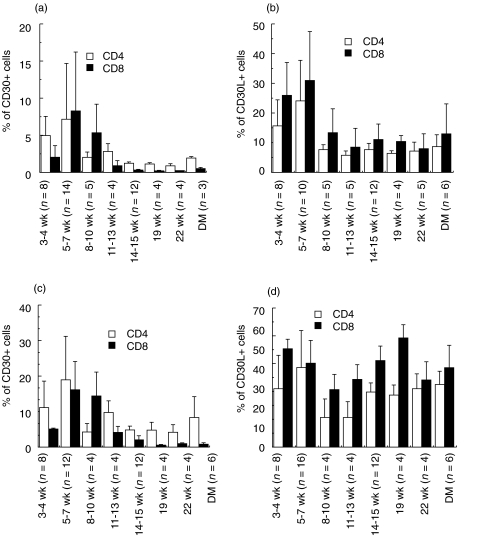
We next examined the kinetics of CD30 and CD30L expression on NOD T cells from the spleen and PLN at various ages (Fig. 2). Both CD4+ and CD8+ T cells in the spleen expressed CD30 at younger age (3–7 week), but mainly CD4+ T cells but not CD8+ T cells expressed CD30 after 11 week of age. In contrast, CD30L was most preferentially expressed on CD8+ T cells. The PLN contained higher percentages of CD30+ or CD30L+ T cells than the spleen. It is interesting to note that the number of CD30-expressing CD4+ T cells declined with age in both the spleen and PLN but the number of CD30L-expressing CD8+ T cells declined only in the spleen.
Fig. 2.
The kinetic expression of CD30/CD30L on enriched CD4+ and CD8+ T cells in NOD spleen and PLN cells. The CD4+ and CD8+ T cells were stained with FITC-conjugated mAbs to CD30 or CD30L and PE-conjugated anti-CD4 or anti-CD8 mAb. The percentages of (a, c) CD30-expressing or (b, d) CD30L-expressing cells in CD4+ (□) and CD8+ (▪) T cells from (a, b) spleen and (c, d) PLN at various ages are expressed as mean ± SD.
Effect of anti-CD30L mAb on spontaneous NOD diabetes
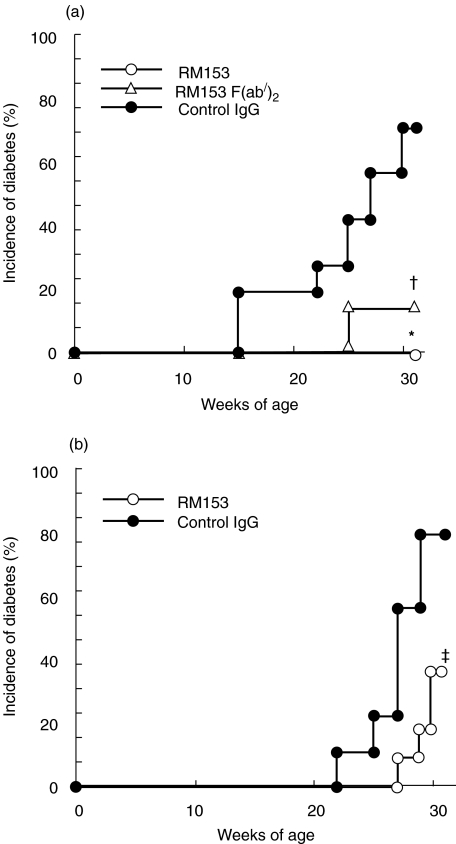
To explore whether the CD30/CD30L interaction plays a role in the development of autoimmune diabetes, female NOD mice were treated with a neutralizing anti-CD30L mAb (RM153) from 2 to 10 week of age. The mice were monitored until 30 week of age, when the cumulative incidence of diabetes reaches between 75 and 80% in our NOD colony. As shown in Fig. 3a, while > 70% (5/7) of the control IgG-treated NOD mice developed overt diabetes at 30 week of age, none (0/10) of the RM153-treated NOD mice developed spontaneous diabetes (P < 0·002). When the RM153 treatment was started from 4 week of age (Fig. 3b), only 36·4% (4/11) of the RM153-treated mice developed diabetes, while 77·7% (7/9) of the control mice developed diabetes at 30 week of age (P < 0·02). To avoid a possible deletion of CD30L-expressing T cells by the mAb, F(ab/)2 fragment of RM153 was injected in NOD mice for 2–10 week of age. Only 12·5% (1/8) of RM153 F(ab/)2-treated mice developed spontaneous diabetes (P < 0·02) at 30 week of age (Fig. 3a). These results suggest that the CD30/CD30L interaction plays a critical role in the early induction phase of NOD diabetes. Histological examination showed that the RM153 treatment significantly reduced the severity of insulitis but not completely abrogated periinsulitis or mild insulitis (Table 1). This suggested that anti-CD30L mAb might inhibit expansion of autoreactive T cells in the islets or PLN.
Fig. 3.
Effect of anti-CD30L mAb on spontaneous diabetes in NOD mice. Female NOD mice were injected i.p. with 500 µg RM153 (○, n = 10), RM153 F(ab/)2 (▵, n = 8), or control IgG (a) for 2–10 week of age (•, n = 7) or (b) 4–10 week of age (•, n= 11). Mice were monitored for development of diabetes until 30 week of age. *P < 0·0001 and †P < 0·02 versus control IgG group (a) and ‡P < 0·02 versus control IgG group (b) (Kaplan-Meier).
Table 1.
Degree of insulitis in RM 153-treated NOD mice
| Degree of insulitis (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Duration | n | 0 | 1 | 2 | 3 | 4 | Insulitis score |
| Ctr IgG | 2–10 week | 5 | 4·6 | 3·1 | 14·1 | 22·7 | 55·7 | 3·2 ± 0·06 |
| RM153 | 2–10 week | 5 | 30·5 | 23 | 20 | 20 | 6·4 | 1·5 ± 0·43* |
| RM153 (Fab/)2 | 2–10 week | 5 | 27 | 16·6 | 20·9 | 22·9 | 12·42 | 1·8 ± 0·22* |
| RM153 | 4–10 week | 5 | 21·4 | 17·6 | 11 | 23·5 | 26·3 | 2·2 ± 0·52* |
RM153 mAb or control IgG was i.p. injected twice a week during 2–10 and 4–10 week of age. Five nondiabetic mice of each group mAb or control IgG (Ctr IgG) were killed at 30 week of age for histological examination. At least 20 islets per mice were examined for degree of insulitis as described in Materials and Methods. Insulitis score is expressed as mean ± SD.
P < 0·01 versus control IgG group (Mann–Whitney U-test).
Effect of anti-CD30L mAb on adoptive transfer of diabetes by islet-reactive CD4+ or CD8+ T cells
To specifically explore whether the CD30/CD30L interaction may be also involved in the effector phase of diabetes, we examined the effect of RM153 on the development of diabetes induced by adoptive transfer of diabetogenic T cells into NOD-SCID mice (Table 2). When splenocytes from diabetic NOD mice were transferred to 7-day-old NOD-SCID mice, 62% (10/16) of the recipients became diabetic within 4 week. Administration of RM153 inhibited the incidence of diabetes (4/16). When islet-derived CD8+ CTL were injected into NOD-SCID mice, all recipient mice became diabetic within 7 days. Administration of RM153 completely abrogated the development of overt diabetes (0/5, P <0·001). On the other hand, adoptive transfer of islet-specific CD4+ T cell clone (YNK 7·3) induced severe insulitis but not overt diabetes in the control IgG-treated mice. Administration of RM153 significantly inhibited the development of insulitis. These results suggest that the CD30/CD30L interaction also contributes to the effector phase of NOD diabetes, including reactivation of autoreactive CD4+ or CD8+ T cells in the islets and/or PLN.
Table 2.
Effect of anti-CD30L mAb in adoptive transfer experiments
| Control IgG | RM153 | |||
|---|---|---|---|---|
| Donor | Diabetes | Insulitis | Diabetes | Insulitis |
| Diabetogenic splenocyte | 10/16 (62%) | + + + | 4/16 (25%)* | + + ∼ + + + |
| Islet-derived CD8 CTL | 10/10 (100%) | + + + | 0/5 (0%)† | ± ∼ + |
| YNK 7·3 | 0/4 | + + ∼ + + + | 0/4 | ± ∼ + |
Splenocytes from diabetic NOD mice (3 × 107), islet-derived CD8+ CTL (1 × 107), or YNK7·3 (1 × 107) were i.p. injected into 7-day-old NOD-SCID mice. RM153 or control IgG (500 µg) were i.p. injected from one day before transfer and twice a week for three week after transfer. Pancreases from each group were removed at 8 week after transfer of with diabetic splenocytes, 7 d after transfer of CD8+ CTL, or 8 week after transfer of YNK7·3.
P < 0·05 versus control IgG
P < 0·001 versus control IgG group.
Co-stimulatory effect of CD30/CD30L interaction on T cell proliferation
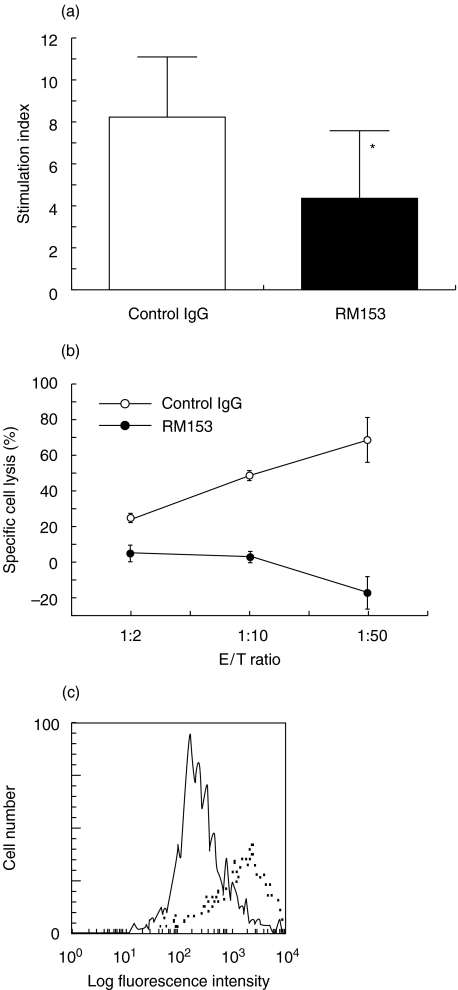
To determine whether the CD30/CD30L interaction has a costimulatory effect on autoreactive T cell proliferation, splenic T cells from 8 to 9-week-old female NOD mice were cocultured with NOD islets in presence of control rat IgG or RM153. As shown in Fig. 4(a), islet autoantigens induced vigorous proliferation of NOD T cells, which was significantly inhibited by RM153 (P < 0·05). This suggests that the CD30/CD30L interaction has a costimulatory effect on islet-reactive T cell proliferation.
Fig. 4.
Co-stimulatory activity of CD30/CD30L interaction in T cell proliferation and β cell destruction. (a) Splenic T cells were cultured with MMC-treated NOD islets in the presence of control IgG (□) or RM153 (▪). After 3 days, 1 µCi 3H-thymidine was added 16 h before harvesting. Data are presented as mean ± SD stimulation index of triplicated samples. *P < 0·05 versus control. (b) Specific lysis by islet-derived CD8+ CTL in the presence of anti-CD30L mAb (•) or control IgG (○) was assessed by 8 h 51Cr-release assay at the indicated E/T ratios. One representative of three separate experiments was shown (mean ± SE). (c) Expression of CD30L on NOD islet cells was evaluated after exposure to IFN-γ. Freshly isolated islet cells from NOD mice were cultured with 100 ng/ml IFN-γ for 24 h and then were stained with biotin-conjugated anti-CD30L mAb (………) and FITC-conjugated-avidin (——).
CD30/CD30L interaction on b cell destruction
CD8+ CTL-mediated cytotoxic activity against islet cells was tested by the 51Cr-release assay. As shown in Fig. 4b, islet-derived CD8+ CTL is highly toxic to pancreatic β cells. The addition of anti-CD30L antibody significantly suppressed specific lysis by CD8+ CTL. This result indicates that CD30/CD30L interaction plays an important role in CD8+ T cell mediated β cell destruction.
To examine a possibility of the CD30/CD30L interaction between islet cells and NOD T cells, CD30L expression on islet cells was evaluated by flow cytometry. Although freshly isolated NOD islet cells did not express CD30L (not shown), CD30L expression was induced by exposure to IFN-γ (Fig. 4c). In addition, CD30 and CD30L expression was also detectable on islet-infiltrating lymphocytes from 20-week-old NOD mice. 12·5 ± 2·1% (n = 3) and 5·2 ± 0·9% (n = 3) of islet-infiltrating CD4+ and CD8+ T cells expressed CD30, respectively. This suggests that the interaction between CD30 on islet-infiltrating T cells and CD30L on inflamed islet cells may contribute to the effector phase of NOD diabetes.
DISCUSSION
CD30 has been implicated in various autoimmune diseases including type 1 diabetes [32–34]. In this study, we first characterized the expression of CD30 and CD30L on CD4+ or CD8+ T cells in the spleen or PLN of NOD mice at various ages (Fig. 2). Both CD4+ and CD8+ T cells in spleen and PLN expressed CD30 at younger age, along with age, the CD30-positive T cells were decreased. Similarly to CD30 expression, T cells in the spleen expressed CD30L at younger age and CD30L-positive T cells were also decreased, suggesting a possible contribution of the CD30/CD30L interaction to the initial phase of autoimmune diabetes. However, in the regional lymph nodes, high proportion of T cells expressed CD30L regardless of age, suggesting that CD30L-positive T cells may play an important role in the development of insulitis.
To examine the role of CD30/CD30L in autoimmune diabetes in vivo, we found that the administration of anti-CD30L mAb for 2–10 week of age prevented the development of overt diabetes completely. However, when the mAb was administered from 4 week of age, the protection was not complete in spite of the marked delay and reduced incidence of diabetes. These results suggested that the CD30/CD30L interaction plays an essential role at the early stage of NOD diabetes development. A similar complete prevention of autoimmune diabetes by blockade of some other costimulatory pathways has been also observed only at younger age of NOD mice [13–15]. Furthermore, increasing evidences have been shown that CD8+ T cells are important in the initial phase of autoimmune diabetes development [35–38]. It is interesting that over 40% of CD8+ T cells in the PLN and about 20% of CD8+ T cells in the spleen of young NOD mice express CD30L. These findings suggest that CD30L-positive CD8+ T cells might play a critical role in the initial phase of autoimmune diabetes. However, at the same time, these results do not exclude the potential role of CD30L also in the later phase of diabetes development and then we addressed this point using adoptive transfer models.
In the adoptive transfer models, the anti-CD30L mAb treatment blocked rapid induction of diabetes by islet-derived CD8+ T cells, though significant insulitis still remained in these mice (Table 2). As we and others have previously reported, CD8+ effector T cells induce overt diabetes without CD4+ T cells help [29,37]. Thus, this result suggested that the CD30/CD30L interaction contributes to the CD8+ T cell-mediated β cell destruction. In fact, in vitro cytotoxic assay revealed that the blockade of CD30/CD30L interaction results in the abrogation of CD8+ T cell-mediated cytotoxicity against islet cells (Fig. 4b).
We have previously reported that the islet-specific CD4+ T cell clone (YNK 7·3) could induce overt diabetes in 50–60% of nonirradiated young NOD recipients [30]. However, if young NOD-SCID mice were used as recipients, YNK7·3 cells could induce only insulitis, but not diabetes. It is possible that YNK 7·3 cells require host-derived CD8+ T cells for developing overt diabetes. In this study, we evaluated the degree of insulitis in the NOD-SCID recipients after transfer of YNK 7·3 cells with or without anti-CD30L mAb treatment (Table 2). A significant suppression of insulitis by anti-CD30L mAb suggest that CD30/CD30L interaction is also involved in the activation of effector CD4+ T cells in the islets.
The effect of anti-CD30L mAb was also tested in the adoptive transfer model using total splenocytes derived from diabetic NOD mice (Table 2). Also in this model, we found that the induction of diabetes was significantly suppressed in the anti-CD30L-mAb treated recipients. Thus in three different adoptive transfer models, we have demonstrated that CD30L plays a critical role in the effector phase of diabetes development.
The critical contributions of the CD30/CD30L interaction to both induction and effector phases of NOD diabetes development were supported by the in vitro data, indicating a significant inhibition by anti-CD30L mAb of the proliferative response of NOD splenic T cells to islet antigens (Fig. 4a). In murine system, it has been reported that the engagement of CD30 by CD30L enhances the proliferation of anti-CD3-stimulated T cells [31]. Furthermore, Opat & Gaston showed a diminished proliferation of CD4+ T cell clones following TCR ligation in the presence of a blocking mAb against CD30L [39]. Our present results suggest a critical role of the CD30/CD30L interactions in the costimulation of autoreactive T cell responses.
We also demonstrated that NOD islet cells express CD30L on their surface upon exposure to IFN-γ (Fig. 4c), as we previously observed with cardiac myoblasts [20], and substantial parts of islet-infiltrating CD4+ or CD8+ T cells expressed CD30 on their surface. Therefore, the interaction between CD30 expressed on infiltrating T cells and CD30L on islet cells induced by infiltrating T cell-derived IFN-γ may contribute to the activation of effector T cells in the islet.
In summary, our present results have provided a strong evidence that the CD30/CD30L interaction plays a critical role in both induction and effector phases of autoimmune diabetes in NOD mice. The blockade of interaction can disrupt the disease process and delay or prevent diabetes onset. This suggests that the interruption of CD30/CD30L interaction may be a novel strategy for the prevention and treatment of type 1 diabetes in humans.
Acknowledgments
This work was funded by Insulin Study Foundation, Japan and in part by NIH grants (AI-44427), DPPG core B (DK-53015), USA. S.C. and H.Y. were supported by Yoneyama Rotary Foundation, and Uehara Memoriral Foundation, Japan. L.W. is a recipient of JDRF CDA award (298210). We also thank Ms. Atsumi Katsuta for her excellent technical assistance.
REFERENCES
- 1.Eisenbarth GS. Type I diabetes: a chronic autoimmune disease. N Eng J Med. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Stiller CR, Dupre J, Gent M, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223:1362–7. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 4.Roep BO, Arden SD, de Vries RR, et al. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990;345:632–4. doi: 10.1038/345632a0. [DOI] [PubMed] [Google Scholar]
- 5.Roep BO, Kallan AA, Hazenbos WL, et al. T-cell reactivity to 38 kD insulin-secretory-granule protein in patients with recent-onset type 1 diabetes. Lancet. 1991;337:1439–41. doi: 10.1016/0140-6736(91)93127-u. [DOI] [PubMed] [Google Scholar]
- 6.Panina-Bordignon P, Lang R, van Endert PM, et al. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995;181:1923–7. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 8.Rabin DU, Pleasic SM, Shapiro JA, et al. Islet cell antigen 512 is a diabetic-specific islet autoantigen related to protein tyrosin phosphateses. J Immunol. 1994;152:3183–91. [PubMed] [Google Scholar]
- 9.Dean BM, Pujol-Borrel R, Botazzo GF. Determination of islet cell antibodies by immunofluorescence. Lancet. 1982;2:1343–7. doi: 10.1016/s0140-6736(82)91547-1. [DOI] [PubMed] [Google Scholar]
- 10.Lan MS, Lu J, Goto Y, et al. Molecular cloning and identification of a relation type protein tyrosin phosphatase, IA-2 from human insulinoma. DNA Cell Biol. 1994;13:505–14. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- 11.Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetes before insulin treatment. Science. 1983;222:1337–9. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 12.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–38. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 13.Lenschow DJ, Ho SC, Sattar H, et al. Differential effects of anti-B7–1 and anti-B7–2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mice. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriyama H, Yokono K, Amano K, et al. Induction of tolerance in murine autoimmune diabetes by transient blockade of leukocyte function-associated antigen-1/ intercellular adhesion molecule-1 pathway. J Immunol. 1996;157:3737–43. [PubMed] [Google Scholar]
- 15.Balasa B, Krahl T, Patstone G, et al. CD40 ligand-CD40 interactioins are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–7. [PubMed] [Google Scholar]
- 16.Arens R, Tesselaar K, Baars PA, et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–12. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto S, Kanno Y, Tanaka Y, et al. CD134L engagement enhances human B cell Ig production. CD154/CD40, CD70/CD27, and CD134/CD134L interactions coordinately regulate T cell-dependent B cell responses. J Immunol. 2000;164:4097–104. doi: 10.4049/jimmunol.164.8.4097. [DOI] [PubMed] [Google Scholar]
- 18.Schwab U, Stein H, Gerdes J, et al. Production of monoclonal antiboby specific for Hodge and Reed-Sternberg cells of Hodgekin disease and a subset of normal lymphoid cells. Nature. 1982;299:65–71. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 19.Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgekin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1999;73:1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 20.Seko Y, Takahashi N, Oshima H, et al. Expression of tumour necrosis factor (TNF) receptor/ligand superfamily co-stimulatory molecules CD40, CD30L, CD27L, and OX40L in murine hearts with chronic ongoing myocarditis caused by coxsackie virus B3. J Pathol. 1999;188:423–30. doi: 10.1002/(SICI)1096-9896(199908)188:4<423::AID-PATH373>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Shanebeck KD, Maliszewski CR, Kennedy MK, et al. Regulation of murine B cell growth and differtiation by CD30 ligand. Eur J Immunol. 1995;25:2147–53. doi: 10.1002/eji.1830250805. [DOI] [PubMed] [Google Scholar]
- 22.Gruss HJ, Boiani N, Williams DE, et al. Pleotropic effects of the CD30 ligand on CD30 expressing cells and lymphoma cell lines. Blood. 1994;83:2045–56. [PubMed] [Google Scholar]
- 23.Bowen MA, Olsen KJ, Cheng L, et al. Functional effect of CD30 on a large granular lymphoma cell line, YT. Inhibition of cytotoxicity, regulation of CD28 and IL-2R and induction of homotypic aggregation. J Immunol. 1993;151:5896–906. [PubMed] [Google Scholar]
- 24.Gerli R, Pitzalis C, Bistoni O, et al. CD30+ T cells in rheumatoid synovitis. mechanisms of recruitment and functional role. J Immunol. 2000;164:4399–407. doi: 10.4049/jimmunol.164.8.4399. [DOI] [PubMed] [Google Scholar]
- 25.Saraiva M, Smith P, Fallon PG, et al. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med. 2002;196:829–39. doi: 10.1084/jem.20020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurts C, Carbone FR, Krummel MF, et al. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398:341–4. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- 27.Kurts C, Carbone FR, Krummel MF, et al. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 2000;407:413. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- 28.Seigmund T, Armitage N, Wicker LS, et al. Analysis of the mouse CD30 gene; A candidate for the NOD mouse type 1 diabetes locus IDD9. 2 Diabetes. 2000;49:1612–6. doi: 10.2337/diabetes.49.9.1612. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda R, Yokono K, Nagata M, et al. CD8 cytotoxic T-cell clone rapidly transfers autoimmune diabetes in very young NOD and MHC class 1- compatible scid mice. Diabetologia. 1997;40:1044–52. doi: 10.1007/s001250050786. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama M, Nagata M, Yasuda H, et al. Fas/Fas ligand interactions play an essential role in the initiation of murine autoimmune diabetes. Diabetes. 2002;51:1391–7. doi: 10.2337/diabetes.51.5.1391. [DOI] [PubMed] [Google Scholar]
- 31.Shimozato O, Takeda K, Yagita H, et al. Expression of CD30 ligand (CD153) on murine activated T cells. Biochem Biophys Res Commun. 1999;256:519–26. doi: 10.1006/bbrc.1999.0336. [DOI] [PubMed] [Google Scholar]
- 32.Caligaris-Cappio F, Bertero MT, Converso M, et al. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with SLE and correlate with disease activity. Clin Exp Rheumatol. 1995;13:339–43. [PubMed] [Google Scholar]
- 33.Gerli R, Muscat C, Bistoni O, et al. High level of the soluble form of CD30 molecules in rheumatoid arthritis are expression of CD30+ T cell involvement in the inflamed joints. Clin Exp Immunol. 1995;102:547–50. doi: 10.1111/j.1365-2249.1995.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okumura M, Hidaka Y, Kuroda S, et al. Increased serum concentration of soluble CD30 in patients with Graves’ disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1997;82:1757–60. doi: 10.1210/jcem.82.6.4000. [DOI] [PubMed] [Google Scholar]
- 35.Wicker LS, Leiter EH, Todd JA, et al. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–4. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 36.Serreze DV, Chapman HD, Varnum DS, et al. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol. 1997;158:3978–86. [PubMed] [Google Scholar]
- 37.Wong FS, Visintin I, Wen L, et al. CD8 T-cell clones from young nonobese diabetic (NOD) can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong FS, Karttunen J, Dumont C, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–31. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 39.Opat S, Gaston JS. CD30:CD30L interactions in the immune response. Autoimmunity. 2001;33:45–60. doi: 10.3109/08916930108994109. [DOI] [PubMed] [Google Scholar]