Abstract
In recent years, a pathophysiological role for T cells in immune thrombocytopenia (ITP) has been established. We applied cDNA size distribution analysis of the T cell receptor (TCR) β-variable (VB) complementarity-determining region 3 (CDR3) in order to investigate T cell repertoire diversity among immune thrombocytopenia patients who had either responded or not responded to splenectomy, and compared them to normal controls. ITP patients who had had a durable platelet response to splenectomy showed a mean 2·8 ± 2·1 abnormal CDR3 size patterns per patient, similar to healthy volunteers (2·9 ± 2·0 abnormal CDR3 size patterns). In contrast, patients unresponsive to splenectomy demonstrated evidence of significantly more clonal T cell expansions than patients who had responded to splenectomy or controls (11·3 ± 3·3 abnormal CDR3 size patterns per patient; P < 0·001). Of the VB subfamilies analysed, VB3 and VB15 correlated with response or non-response to splenectomy, each demonstrating oligoclonality in non-responding patients (P < 0·05). These findings suggest that removal of the spleen may lead directly or indirectly to reductions in T cell clonal expansions in responders, or that the extent of T cell clonality impacts responsiveness to splenectomy in patients with ITP.
Keywords: autoimmune disease, immune thrombocytopenia, platelets, splenectomy, T cells
INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired disorder in which antiplatelet antibodies result in platelet destruction. Antibodies against the platelet membrane glycoprotein (GP) receptors IIb/IIIa and/or Ib/IX are identifiable in approximately three-quarters of ITP patients; binding of autoantibody to platelets leads to their clearance by the mononuclear phagocyte system [1–4]. Although antiplatelet antibody production is B cell-mediated, numerous studies have demonstrated a concomitant T cell activation in patients with ITP [5–7]. Compared with control T cells, CD4+ T lymphocytes from patients with chronic ITP have been shown to exhibit accelerated proliferation in response to tryptic peptides of GPIIb and IIIa, enabling localization of putative immunodominant epitopes to the amino-terminal portions of the glycoprotein molecules [8,9]. The presence of reactive T cell populations in ITP has been thought to reflect immune stimulation by specific, as of yet unknown, antigens [10,11]. Inferential evidence of a pathogenic role of T cells in ITP is also derived from the clinical efficacy of agents that primarily block T cell proliferation and/or signalling, such as cyclosporin-A, tacrolimus, mycophenolate mofetil and anti-CD40 ligand [12,13], which is thought to react primarily with activated CD4+ T cells and block stimulation of the CD40 molecule on B cells [14].
Recently, molecular methodology has permitted size analysis of cDNAs for the complementarity-determining region 3 [CDR3] of the T cell receptor (TCR) β-variable (VB) region genes, a technique which enables assessment of complex populations of T cells for clonal restriction or dominance [15]. Using this approach, Shimomura et al. were able to demonstrate clonal peripheral blood T cell expansions in individuals with ITP versus normal controls [11]; however, the relationship of T cell repertoire diversity in ITP patients and response to treatment was not assessed. The present study extends these findings to ITP patients who had undergone splenectomy and either responded or did not respond, and includes comparison to healthy volunteers.
Methods
Study population
A total of 20 subjects were studied, including 12 patients with a diagnosis of ITP and eight age-matched normal controls; patient characteristics are summarized in Table 1. All patients had a platelet count of <30 × 109/l at diagnosis, with no other explanation for the thrombocytopenia than ITP. Individuals over 50 years of age underwent bone marrow aspiration and biopsy to document adequate or increased megakaryocytes and the absence of pathological lymphoid aggregates or myelodysplasia if they did not respond repeatedly to intravenous immune globulin (IVIg). Six patients underwent splenectomy and responded to it. Five patients achieved durable postsurgical platelet counts above 150 × 109/l off any ITP-specific therapy, while patient 3 exhibited a partial response, with a durable postsplenectomy platelet count of 60 × 109/l independent of any therapy. Patient 6, a complete responder to splenectomy, was taking stable, low doses of prednisone at the time of sample acquisition for an undifferentiated connective tissue disease whose primary symptom was fatigue. Six other patients had failed to respond to splenectomy, manifested by lack of response or relapse after the procedure. For the purpose of this analysis, the selection of these individuals was limited to those who were on no therapy (patient 8), who were receiving intermittent doses of IVIg (patients 7, 10, 11 and 12) or who were taking stable doses of prednisone (patient 9, for treatment of concurrent autoimmune haemolytic anaemia; i.e. Evans syndrome). When possible, samples for CDR3 size analysis were acquired at the time of platelet nadir off or between therapies.
Table 1.
Patient characteristics
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Sex | Platelets (×109/l) | Months since diagnosis | Months since splenectomy | Prior | Current |
| Responders | |||||||
| 1 | 61 | F | 198 | 24 | 22 | Pr, IVIg | None |
| 2 | 31 | F | 383 | 201 | 177 | Pr, IVIg | None |
| 3 | 47 | M | 64 | 142 | 23 | Pr, IVIg, dan, anti-D, CSA, Vcr | None |
| 4 | 33 | F | 399 | 202 | 198 | Pr, IVIg | None |
| 5 | 22 | F | 209 | 95 | 83 | Pr, IVIg, MPr | None |
| 6 | 35 | F | 331 | 226 | 213 | Pr, IVIg, anti-D | None |
| Non-responders | |||||||
| 7 | 29 | F | 13 | 56 | 44 | Pr, IVIg | IVIg |
| 8 | 53 | F | 21 | 225 | 211 | Pr, IVIg, Vcr | None |
| 9 | 43 | F | 7 | 187 | 178 | Pr, IVIg, dan, CSA, Vcr | Pr |
| 10 | 38 | F | 6 | 10 | 7 | Pr, IVIg, dan, anti-D, Vcr, cytox | IVIg |
| 11 | 34 | F | 13 | 52 | 48 | Pr, IVIg, dan, anti-D, Vcr | IVIg |
| 12 | 54 | F | 5 | 58 | 55 | Pr, IVIg, dan, anti-D, Vcr | IVIg |
Pr = prednisone; IVIg = intravenous immune globulin; dan = danazol; CSA = cyclosporine; Vcr = vincristine; cytox = oral cyclophosphamide; MPr = methylprednisolone.
RNA isolation, complementary DNA synthesis and polymerase chain reaction (PCR)
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque sedimentation. Total RNA was extracted from PBMCs using Trizol reagent (Life Technologies Inc., Bethesda, MD, USA) and then subjected to reverse-transcription using the Geneamp RNA PCR Core Kit (Applied Biosystems, Foster City, CA, USA). Based on sequence homology, TCR VB genes are grouped into subfamilies designated VB1-24 (excluding VB10 and 19, which are pseudogenes) [16]. cDNA was amplified through 35 cycles with primers specific to each of the 22 VB subfamilies, and a fluorescent constant region primer [15].
CDR3 size distribution analysis
Details of the CDR3 size distribution assay have been reported elsewhere [15]. Briefly, 1 µl of amplified cDNA product was mixed with 12 µl 100% formamide (Sigma-Aldrich Inc., St Louis, MI, USA) and 0·5 µl size standard (Genescan-500 ROX, Applied Biosystems, Foster City, CA, USA), heated at 95°C for 2 min, chilled on ice and applied to an ABI 310 sequencer equipped with software capable of converting the fluorescent band intensity signal to a CDR3 size pattern. CDR3 patterns are characterized by a series of peaks that represent a collection of VB CDR3 transcripts of a given length; adjacent peaks signify transcripts occurring at three base-pair intervals. Because each T cell should have a unique TCR VB CDR3 DNA signature, and because polyclonality is the expectation in the normal scenario, CDR3 size analysis of PBMCs from healthy individuals should render a size pattern characterized by CDR3 fragments of many different lengths, producing a bell-shaped (Gaussian) curve. Dominant peaks are taken to represent accumulation of one or more clones (oligoclonality) within a specified VB subfamily. CDR3 size patterns were judged to be abnormal if a Gaussian distribution was absent, due to either a reduced (<5) peak number or the appearance of prominent peaks [17]. Three independent investigators experienced in the technique made this determination in a blinded fashion; CDR3 size patterns were reported as normal or abnormal only after consensus to the designation had been reached. The number of VB subfamilies displaying an abnormal CDR3 size pattern was determined for each subject.
Statistical analysis
Standard analysis of variance (anova) was used to compare the number of abnormal CDR3 size profiles in the three subject groups (patients who failed splenectomy, patients who responded to splenectomy and healthy volunteers), followed by the Games–Howell post-hoc tests to assess intergroup differences. The Kruskal–Wallis test for trend in contingency tables was used to calculate nominal P-values. To compare the proportions in the three groups demonstrating oligoclonality in individual VB subfamilies, Bonferroni's inequality (accounting for multiple comparisons) was used to adjust P-values. The Wilcoxon non-parametric test or Kruskal–Wallis non-parametric anova procedure was used to assess whether the groups differed on various baseline parameters (such as age, months postdiagnosis, months postsplenectomy and time from diagnosis to splenectomy). All P-values are two-tailed.
RESULTS
There was no significant difference in median age among the three subject groups (responders to splenectomy, non-responders to splenectomy and healthy volunteers). All patients, except for one individual in the splenectomy-responder group, were female. Patients who responded to splenectomy and those who did not showed no significant difference in median months since diagnosis, months since splenectomy or time from diagnosis to splenectomy.
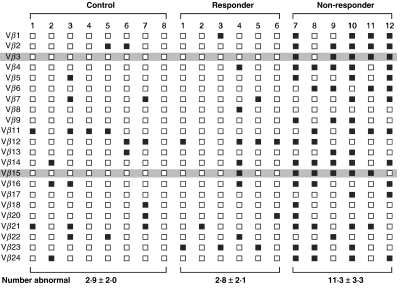
Representative CDR3 profiles from splenectomy-responder, splenectomy non-responder and healthy individuals are shown in Fig. 1. Responders to splenectomy showed 1, 2, 2, 2, 3 and 7 abnormal CDR3 size patterns per patient, compared to 7, 9, 10, 12, 15 and 15 abnormal CDR3 size patterns in non-responding patients (Fig. 2). ITP patients who had undergone splenectomy and responded showed a mean 2·8 ± 2·1 abnormal CDR3 size patterns per patient, a number identical to that of healthy volunteers (2·9 ± 2·0 oligoclonal CDR3 size profiles). In contrast, patients who had failed splenectomy demonstrated 11·3 ± 3·3 abnormal CDR3 size patterns per patient, a difference that was highly statistically significant (P < 0·001). Of the 22 VB subfamilies analysed, VB3 and VB15 correlated with response or non-response to splenectomy, with six of six and five of six splenectomy responders showing normal VB3 and VB15 size profiles, respectively, and five of six splenectomy non-responders manifesting abnormal VB3 and VB15 size profiles (P < 0·05) (Fig. 2).
Fig. 1.
Representative CDR3 size profiles from (a) a normal individual, (b) a patient who responded to splenectomy and (c) a patient who did not respond to splenectomy. Fluorescence intensity of CDR3 fragments is recorded on the y axis versus molecular weight on the x axis. In the normal scenario, CDR3 size profiles should exhibit a bell shape (for example, panel (a), Vβ4, Vβ17), indicating a balanced (Gaussian) distribution of variously sized cDNA fragments, and thus polyclonality. A reduced peak number (panel (b), Vβ21) or dominant peaks (panel (c), Vβ5) disrupt the Gaussian shape and signify oligoclonality within a given Vβ subfamily.
Fig. 2.
Summary of CDR3 size patterns in healthy controls (individuals 1–8) and ITP patients who had either responded (patients 1–6) or not responded (patients 7–12) to splenectomy. Open squares (□) indicate a normal CDR3 size profile, while closed squares (▪) indicate an abnormal CDR3 size profile. Shaded areas highlight subfamilies Vβ3 and Vβ15, in which CDR3 size pattern characteristics correlated with responsiveness to splenectomy in ITP patients. The mean number of abnormal CRD3 size patterns per group is given below the columns representative of each cohort.
DISCUSSION
Aberrant T cell repertoires assessed by the CDR3 size determination method have been demonstrated in a variety of disorders characterized by immune dysregulation. These include rheumatoid arthritis [18–20] mixed connective tissue disease [21], multiple sclerosis [22,23] and others [17,24–26]. Use of this methodology enables rapid determination of T cell repertoire diversity without requiring the more labourious tasks of cloning and sequencing and is a more sensitive method than flow cytometry for the detection of restricted TCR expression [15]. Few studies have shown a correlation between T cell repertoire diversity and responsiveness to therapy, but aplastic anaemia patients who achieved unmaintained remission after immunosuppressive treatment exhibited normalization of a previously abnormal T cell repertoire [17].
Interestingly, we found that the T cell repertoires of patients who failed to respond to splenectomy showed significantly more evidence of T lymphocyte clonal expansions than did those who responded to splenectomy. This expansion is not merely a result of splenectomy per se; responders to splenectomy showed no greater number of abnormal CDR3 size profiles per patient than did healthy volunteers, who are understood to manifest evidence of low-level T cell clonal expansion in the normal scenario [27]. The degree of baseline T cell oligoclonality in our normal control group was, in fact, comparable to that noted in healthy subjects by other investigators [21]. One might consider whether the therapies (intravenous immune globulin and prednisone) that some non-responding patients were receiving could have accounted for the difference. Only one patient (a splenectomy non-responder), however, was taking prednisone in substantial doses at the time of CDR3 size determination. Intravenous immune globulin is certainly immunomodulatory, but it is unclear how such a treatment could facilitate the expansion of T lymphocytes.
In ITP, the spleen serves as an organ for clearance of sensitized platelets [4] and may be a primary site for production of antiplatelet antibodies [28,29]. Nevertheless, only two-thirds of patients with ITP will respond durably to the procedure [30]. An attractive hypothesis for the lack of response in some patients is that a population of pathogenic T cells, which has either accumulated in the periphery or spread to it after initial expansion in the spleen, continues to drive antibody production postsplenectomy. In contrast, when expansion of T lymphocyte clones occurs to a lesser extent or is confined to the spleen, splenectomy may be sufficiently immunomodulatory to produce a clinical remission. This premise supposes, in effect, that the extent of T cell oligoclonality at baseline varies among ITP patients, and that this difference impacts the likelihood of response to splenectomy. In a preliminary effort to address this issue, we have now analysed the pre- and postsplenectomy CDR3 profiles of two additional patients with ITP, and observed a surprising lack of accumulation of oligoclonal T cells in the peripheral blood at all time-points (data not shown). Of even greater interest, both patients responded to splenectomy, suggesting that a persistence of an initially diverse T cell repertoire influenced the likelihood of a response.
The true prevalence of clonal T cell derangements among ITP patients who have not yet undergone splenectomy is not known. Five of seven patients with ITP studied by Shimomora et al. had not had splenectomy, and all five manifested oligoclonal accumulations of T cells [11]. Taken with our results, these data could suggest that removal of the spleen (which contains a large reservoir of lymphocytes, including T cells) may affect peripheral blood T cell repertoires in some patients, and if return to a normal repertoire is achieved a clinical response might be observed. Changes in peripheral blood lymphocyte populations after splenectomy have been documented in patients whose spleens were removed following trauma in whom lymphocyte phenotyping has revealed, for example, a sustained reduction in CD4+ effector cells [31]. Extrapolation of findings such as these to patients who require splenectomy due to autoimmunity may not be possible. However, Ware et al. [32] demonstrated regression of an accumulation of gamma-delta T cells in a paediatric patient with chronic ITP following splenectomy, a change that was associated with normalization of the platelet count. (Notably, however, a similar decline in an over-representation of gamma-delta T cells was observed in another child from the same series whose ITP resolved spontaneously.) Whether these results indicate that clearance of pathogenic T cell populations (by surgical removal or other, but as yet undefined, spontaneous processes) is a necessary precursor for resolution of disease, or that normalization in T cell expansions serves merely as a marker for termination of autoimmunity, has not been elucidated.
Finally, it is not known if T cell repertoire expansions as measured by CDR3 size analysis using peripheral blood lymphocytes are reflective of expansions that may occur in the spleen, or if synchronous accumulations occurring in these (or other locations) are likely to be similar or distinct. Studies designed to assess a potential pathogenic involvement of T cell clones in other disorders of immune dysregulation have led to the interesting observation that oligoclonal T cell populations may be more or less confined to an anatomical site of disease. For instance, studies of T cell repertoires in patients with melanoma, graft-versus-host disease and rheumatoid arthritis have demonstrated that lymphocytes from the affected tissue (e.g. skin or synovium) may exhibit evidence of oligoclonality that is either attenuated or absent when lymphocytes from the peripheral blood are examined [15,18,33]. Whether theoretical splenic clones could spread throughout the body in ITP patients and persist after splenectomy, or if two or more anatomically separate clonal populations co-exist prior to removal of the spleen, is unknown.
Our study is the first to show an association between response to therapy and T cell repertoire characteristics as assessed by the CDR3 size determination method in patients with ITP. Lacking presplenectomy data, and with appropriate caution that recalls the failure of other initially promising measures to predict responsiveness to splenectomy in ITP patients [34], a causal relationship cannot be assumed. Long-term studies that assess T cell repertoire diversity and span the pre- and postsplenectomy periods are required to define further the role of oligoclonal T cell expansions in ITP and their potential usefulness in predicting clinical outcomes.
Acknowledgments
The authors thank Robert Wesley PhD for assistance with statistical analysis.
REFERENCES
- 1.Berchtold P, Wenger M. Autoantibodies against platelet glycoproteins in autoimmune thrombocytopenic purpura: their clinical significance and response to treatment. Blood. 1993;81:1246–50. [PubMed] [Google Scholar]
- 2.He R, Reid DM, Jones CE, Shulman NR. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood. 1994;83:1024–32. [PubMed] [Google Scholar]
- 3.Kiefel V, Freitag E, Kroll H, Santoso S, Mueller-Eckhardt C. Platelet autoantibodies (IgG, IgM, IgA) against glycoproteins IIb/IIIa and Ib/IX in patients with thrombocytopenia. Ann Hematol. 1996;72:280–5. doi: 10.1007/s002770050173. [DOI] [PubMed] [Google Scholar]
- 4.Stratton JR, Ballem PJ, Gernsheimer T, Cerqueira M, Slichter SJ. Platelet destruction in autoimmune thrombocytopenic purpura: kinetics and clearance of indium-111-labeled autologous platelets. J Nucl Med. 1989;30:629–37. [PubMed] [Google Scholar]
- 5.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78:2619–25. [PubMed] [Google Scholar]
- 6.Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–54. [PubMed] [Google Scholar]
- 7.Ware RE, Howard TA. Phenotypic and clonal analysis of T lymphocytes in childhood immune thrombocytopenic purpura. Blood. 1993;82:2137–42. [PubMed] [Google Scholar]
- 8.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb–IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwana M, Kaburaki J, Kitasato H, et al. Immunodominant epitopes on glycoprotein IIb–IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98:130–9. doi: 10.1182/blood.v98.1.130. [DOI] [PubMed] [Google Scholar]
- 10.Posnett DN, Gottlieb A, Bussel JB, et al. T cell antigen receptors in autoimmunity. J Immunol. 1988;141:1963–9. [PubMed] [Google Scholar]
- 11.Shimomura T, Fujimura K, Takafuta T, et al. Oligoclonal accumulation of T cells in peripheral blood from patients with idiopathic thrombocytopenic purpura. Br J Haematol. 1996;95:732–7. doi: 10.1046/j.1365-2141.1996.d01-1967.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuwana M, Kawakami Y, Ikeda Y. Suppression of autoreactive T-cell response to glycoprotein IIb/IIIa by blockade of CD40/CD154 interaction: implications for treatment of immune thrombocytopenic purpura. Blood. 2003;101:621–3. doi: 10.1182/blood-2002-07-2157. [DOI] [PubMed] [Google Scholar]
- 13.Bussel JB. Overview of idiopathic thrombocytopenic purpura: new approach to refractory patients. Semin Oncol. 2000;27:91–8. [PubMed] [Google Scholar]
- 14.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 15.Even J, Lim A, Puisieux I, et al. T-cell repertoires in healthy and diseased human tissues analysed by T-cell receptor beta-chain CDR3 size determination: evidence for oligoclonal expansions in tumours and inflammatory diseases. Res Immunol. 1995;146:65–80. doi: 10.1016/0923-2494(96)80240-9. [DOI] [PubMed] [Google Scholar]
- 16.Currier JR, Deulofeut H, Barron KS, Kehn PJ, Robinson MA. Mitogens, superantigens, and nominal antigens elicit distinctive patterns of TCRB CDR3 diversity. Hum Immunol. 1996;48:39–51. doi: 10.1016/0198-8859(96)00076-6. [DOI] [PubMed] [Google Scholar]
- 17.Zeng W, Nakao S, Takamatsu H, et al. Characterization of T-cell repertoire of the bone marrow in immune-mediated aplastic anemia: evidence for the involvement of antigen-driven T-cell response in cyclosporine-dependent aplastic anemia. Blood. 1999;93:3008–16. [PubMed] [Google Scholar]
- 18.Lim A, Toubert A, Pannetier C, et al. Spread of clonal T-cell expansions in rheumatoid arthritis patients. Hum Immunol. 1996;48:77–83. doi: 10.1016/0198-8859(96)00089-4. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Sun GR, Tumang JR, Crow MK, Friedman SM. CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest. 1994;94:2525–31. doi: 10.1172/JCI117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goronzy JJ, Bartz-Bazzanella P, Hu W, Jendro MC, Walser-Kuntz DR, Weyand CM. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest. 1994;94:2068–76. doi: 10.1172/JCI117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okubo M, Kurokawa M, Ohto H, et al. Clonotype analysis of peripheral blood T cells and autoantigen-reactive T cells from patients with mixed connective tissue disease. J Immunol. 1994;153:3784–90. [PubMed] [Google Scholar]
- 22.Oksenberg JR, Panzara MA, Begovich AB, et al. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362:68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 23.Musette P, Bequet D, Delarbre C, Gachelin G, Kourilsky P, Dormont D. Expansion of a recurrent V beta 5.3+ T-cell population in newly diagnosed and untreated HLA-DR2 multiple sclerosis patients. Proc Natl Acad Sci USA. 1996;93:12461–6. doi: 10.1073/pnas.93.22.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karadimitris A, Manavalan JS, Thaler HT, et al. Abnormal T-cell repertoire is consistent with immune process underlying the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood. 2000;96:2613–20. [PubMed] [Google Scholar]
- 25.Yassai M, McFarland JG, Newton-Nash D, Newman PJ, Eckels DD, Gorski J. T cell receptor and alloimmune thrombocytopenias: a model for autoimmune diseases? Ann Med Interne (Paris) 1992;143:365–70. [PubMed] [Google Scholar]
- 26.Alatrakchi N, Farace F, Frau E, Carde P, Munck JN, Triebel F. T-cell clonal expansion in patients with B-cell lymphoproliferative disorders. J Immunother. 1998;21:363–70. doi: 10.1097/00002371-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Gorski J, Yassai M, Zhu X, et al. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–19. [PubMed] [Google Scholar]
- 28.McMillan R, Longmire RL, Yelenosky R, Donnell RL, Armstrong S. Quantitation of platelet-binding IgG produced in vitro by spleens from patients with idiopathic thrombocytopenic purpura. N Engl J Med. 1974;291:812–7. doi: 10.1056/NEJM197410172911602. [DOI] [PubMed] [Google Scholar]
- 29.Karpatkin S, Strick N, Siskind GW. Detection of splenic anti-platelet antibody synthesis in idiopathic autoimmune thrombocytopenic purpura (ATP) Br J Haematol. 1972;23:167–76. doi: 10.1111/j.1365-2141.1972.tb03470.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Leber MD, Gillis S, Giunta A, Eldor A, Bussel JB. Long term follow-up after splenectomy performed for immune thrombocytopenic purpura (ITP) Am J Hematol. 2003;72:94–8. doi: 10.1002/ajh.10253. [DOI] [PubMed] [Google Scholar]
- 31.Wolf HM, Eibl MM, Georgi E, Samstag A, Spatz M, Uranus S, Passl R. Long-term decrease of CD4+CD45RA+ T cells and impaired primary immune response after post-traumatic splenectomy. Br J Haematol. 1999;107:55–68. doi: 10.1046/j.1365-2141.1999.01686.x. [DOI] [PubMed] [Google Scholar]
- 32.Ware RE, Howard TA. Elevated numbers of gamma-delta (gamma delta+) T lymphocytes in children with immune thrombocytopenic purpura. J Clin Immunol. 1994;14:237–47. doi: 10.1007/BF01552310. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto K, Sakoda H, Nakajima T, et al. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol. 1992;4:1219–23. doi: 10.1093/intimm/4.11.1219. [DOI] [PubMed] [Google Scholar]
- 34.Ruivard M, Caulier MT, Vantelon JM, et al. The response to high-dose intravenous immunoglobulin or steroids is not predictive of outcome after splenectomy in adults with autoimmune thrombocytopenic purpura. Br J Haematol. 1999;105:1130–2. doi: 10.1046/j.1365-2141.1999.01464.x. [DOI] [PubMed] [Google Scholar]