Abstract
Triggering of the Fas receptor induces T cell apoptosis and is involved in shutting-off the immune response. Inherited defects impairing Fas function cause the autoimmune lymphoproliferative syndrome, and may play a role in other autoimmune diseases. The aim of this work was to analyse the Fas function in paediatric patients with thyroid autoimmunities. We found that T cells from 24/28 patients with Graves’ disease (GD) and 12/35 patients with Hashimoto's thyroiditis (HT) displayed defective Fas function. In HT, the defect was more frequent in patients requiring replacement therapy (11/20) than in those not requiring (1/15); moreover, in untreated HT the highest defect was displayed by patients with the highest levels of autoantibodies. Fas was always expressed at normal levels and no Fas mutations were detected. Analysis of the healthy parents of seven Fas-resistant patients showed that several of them were Fas-resistant, which suggests a genetic component. Fusion of Fas-resistant T cells with the Fas-sensitive HUT78 T cell line generated Fas-resistant hybrid cells, which suggests the presence of molecules exerting a dominant negative effect on Fas function. Analysis of Fas-induced activation of caspase-8 and -9 showed decreased activity of both caspases in HT, whereas activity of caspase-9 was increased and that of caspase-8 was decreased in GD. These data suggest that heterogeneous inherited defects impairing Fas function favour the development of thyroid autoimmunities.
Keywords: apoptosis, CD95, Fas, T cell
INTRODUCTION
Fas triggering by its ligand (FasL) induces programmed cell death by activating a caspase cascade [1]. Connection with the caspase pathway is mediated by the adaptor molecule FADD, which associates with both the cytoplasmic portion of Fas and caspase-8. Association with FADD activates caspase-8, which turns on a cascade composed of caspase-10, -7 and -3, and activates the apoptotic machinery. This extrinsic pathway also triggers a second pathway in which caspase-8 cleaves cytosolic bid, which translocates into mitochondria and induces release of cytocrome c, that binds to APAF-1 and activates caspase-9. This mitochondrial pathway can also be recruited by ceramide produced by an acidic sphingomyelinase activated upon Fas triggering [2]. The system is under the control of several inhibitors belonging to the bcl-2, FLIP and IAP families.
Fas plays a dual role in the immune response. Cytotoxic cells express FasL, whose interaction with Fas expressed by target cells is one of the mechanisms they use to exert their cytotoxic function. Moreover, lymphocytes can express Fas and be targets of cells expressing FasL. This Fas/FasL interaction is involved in shutting off the immune response and induction of peripheral tolerance.
Both Fas functions may be involved in autoimmunity. On one hand, the high levels of Fas and FasL found in autoimmune lesions of several cell-mediated autoimmune diseases suggest that Fas-mediated cytotoxicity plays a role in their tissue damage [3–7]. On the other hand, in both mice and humans, inherited deleterious mutations impairing Fas function perturb lymphocyte homeostasis and cause the autoimmune lymphoproliferative syndrome (ALPS), characterized by autoimmunities and polyclonal lymphocyte accumulation with lymphoadenopathy and/or splenomegaly and expansion of CD4/CD8 double-negative TCRαß+ T cells (DN T cells) [8–12]. Genetic defects causing ALPS are heterogeneous and a classification has applied the terms ALPS-Ia and -Ib to syndromes with mutations of Fas and FasL, respectively, and ALPS-II to those without these mutations and caused possibly by mutations hitting the Fas signalling pathway downstream from Fas [10,13]. The observation that families of ALPS patients display increased frequency of common autoimmune diseases suggests that the genetic alterations causing ALPS may also lead to other autoimmune diseases [11]. This possibility was confirmed by detection of defective Fas function in a proportion of patients with the multiple autoimmune syndrome (i.e. patients from families with more than one case of autoimmunity within first- or second-degree relatives), or multiple sclerosis (MS), or type 1 diabetes mellitus (T1DM) [14,15].
Defective Fas function may favour autoimmunity by two mechanisms. On one hand, it may alter the switching-off system and increase the risk of cross-reactions with self-antigens by ‘molecular mimicry’. On the other hand, it may slow down cell apoptosis and/or induce exposure of abnormal amounts of apoptosis-related antigens that may act as autoantigens in several autoimmune diseases [16–21].
The aim of this work was to extend the analysis of Fas function to thyroid autoimmune diseases, as a small proportion of patients with autoimmune thyroiditis used as a control group in a previous study displayed defective Fas function [15]. Therefore, we assessed Fas function in activated T cells derived from patients with Hashimoto's thyroiditis (HT) or Graves’ disease (GD) and we detected decreased function of Fas in both groups.
MATERIALS AND METHODS
Patients
We evaluated 63 children and young adults with thyroid-specific autoimmune diseases: 35 with HT (30 females, five males; median age in years: 14, range: 7–23; median age at onset in years: 11, range: 3–20; median disease duration in years: 3) and 28 with GD (23 females, 5 males; median age: 13, range: 5–22; median age at onset: 10, range: 3–17; median disease duration: 3).
All GD patients displayed serum antithyroid stimulating hormone (TSH) receptor antibodies and goitre and were in therapy with methimazole. Two of them displayed ophthalmopathy. All patients with HT displayed serum antithyroglobulin (TG) or antithyroperoxidase (TPO) antibodies, and ultrasonographic features of thyroiditis (Sostre grading 2–3) [22]. Twenty of them were treated with l-thyroxine because of low FT4 levels (free thyroxine) (<0·80 ng/dl; normal range 0·80–1·90) or high TSH levels (>8 UI/ml; normal range 0·4–4) (17 females, three males; median age: 13, range: 7–18; median age at onset: 11, range: 3–18; median disease duration: 3). Fifteen HT patients displayed normal FT4 and TSH levels and never received therapy (13 females, two males; median age: 14, range: 8–23; median age at onset: 11, range: 4–20; median disease duration: 3). Patients with multiple autoimmune diseases were excluded.
Sixty-five age- and sex-matched controls without autoimmune diseases were recruited from our out-patient clinic.
Informed consent was obtained from patients or their parents and the study was planned according to the guidelines of the local ethical committee.
Patient analysis
Immunophenotype analysis.
Expression of surface molecules was evaluated by direct immunofluorescence and flow cytofluorimetry (FACScan, Becton Dickinson, San Jose, CA, USA) using monoclonal antibodies (MoAb) to CD3, CD4, CD8, TCRαβ (Becton Dickinson) and Fas (Immunotech, Marseilles, France) on fresh and activated peripheral blood lymphocytes. CD4/CD8 DN T cells were detected using FITC-conjugated anti-TCRαβ MoAb and PE-conjugated anti-CD4 and -CD8 MoAbs.
Analysis of Fas-induced cell death.
Fas-induced cell death was evaluated as reported previously [11–15] on activated T cells obtained by treating peripheral blood mononuclear cells (PBMC) with PHA at days 0 (1 µg/ml) and 12 (0·1 µg/ml), followed by their culture in RPMI-1640 + 10% FCS + rIL-2 (2 U/ml) (Biogen, Geneva, Switzerland). Fas function was assessed 6 days after the second stimulation (18-day cells) by incubating cells with control medium or anti-Fas MoAb (CH11, IgM isotype) (1 µg/ml) (UBI, Lake Placid, NY, USA) in the presence of rIL-2 (1 U/ml) to minimize spontaneous cell death. Cell survival was evaluated after 18 h by counting live cells in each well by the trypan blue exclusion test. The same conditions were used to measure cell death induced by methyl-prednisolone (100 µm) (PDN) (Upjohn, Puurs, Belgium) or C2-ceramide (50 µm) (N-acetyl-D-sphingosine) (Sigma, St Louis, MO, USA). Assays were performed in triplicate and analysed by a blind observer. Cells from two normal donors were included in each experiment as a positive control. Results were expressed as relative cell survival percentage, calculated as follows: (total live cell count in the well with the apoptotic stimulus/total live cell count in the well without the apoptotic stimulus) × 100. This protocol was chosen because it was found to give the most reproducible results in previous studies [12]. It evaluates the overall cell survival at each time-point, and was found to be more sensitive than other techniques (such as staining with annexin V), detecting the instantaneous proportion of dying cells at each time.
In Fas-resistant patients, Fas-resistance was confirmed by staining apoptotic cells with annexin V (Annexin-V-Fluos kit, Boehringer Mannhein, GmBh, Germany). In the control wells (i.e. in the absence of apoptotic stimuli), spontaneous cell loss was always < 10% of the seeded cells and similar in cultures from the patients and normal donors.
The upper limit of the normal range of T cell responses to Fas-, ceramide- and PDN-induced T cell death was set at the 95th percentile and was 82%, 85%, and 78%, respectively (relative cell survival percentage) (Table 1).
Table 1.
Fas-, ceramide- and PDN-induced cell death in normal controls and different patient groups
| Anti-Fas MoAb | Ceramide | PDN | |||||
|---|---|---|---|---|---|---|---|
| Subject groupa | nb | Relative cell survivalc | Resistant n. (%)d | Relative cell survivalc | Resistant n. (%)d | Relative cell survivalc | Resistant n. (%)d |
| Controls | 65 | 60 (53–68) | 3 (5%) | 61 (48–68) | 3 (5%) | 50 (40–59) | 3 (5%) |
| GD | 28 | 94 (89–100)† | 24 (86%)† | 82 (66–94)† | 12 (42%)† | 54 (48–66) | 1 (4%) |
| HT | 35 | 71 (60–85)*† | 12 (34%)*† | 57 (41–65)* | 4 (11%)* | 59 (45–75) | 2 (6%) |
| HT-u | 15 | 63 (58–73)* | 1 (7%)* | 60 (48–75)* | 2 (13%) | 46 (38–59) | 0 |
| HT-t | 20 | 81 (63–94)*†‡ | 11 (55%)*†‡ | 56 (39–79)* | 2 (10%)* | 60 (52–67) | 2 (10%) |
GD: Graves’ disease; HT: Hashimoto's thyroiditis; HT-u: untreated HT; HT-t: treated HT;
number of subjects in each group;
results are expressed as median and interquartile ranges of the relative cell survival percentage detected after 18 h incubation with the indicated reagent in activated T cells derived from each subject;
results are expressed as number (proportion in the brackets) of subjects resistant to the indicated cell-death stimulus in each group. Resistance was set at the 95th percentile of the control range and was 82% (for anti-Fas), 85% for ceramide and 78% for PDN (relative cell survival percentage).
Significantly different from controls (P < 0·05, relative cell survivals were analysed by the Mann–Whitney test, number of resistant cases with Fisher's exact test);
significantly different from GD;
significantly different from HT-u.
Analysis of the Fas gene
Mutation analysis of the Fas gene (MIM 134637; TNFRSF6) was performed by denaturing high-performance liquid chromatography (DHPLC) and DNA genomic sequencing. DNA was extracted from PBMC with standard methods. All exons and intron–exon boundaries were amplified by polymerase chain reaction (PCR). PCR was performed in 50 µl final volume containing 25 pmol of each primer, 250 ng of genomic DNA, 0·2 mm dNTPs and 1·25 units of AmpliTaq Gold (Applied Biosystems) in the buffer provided by the manufacturer. Amplification was performed in a GeneAmp PCR system 9700 (Applied Biosystems, Perkin-Elmer, Foster City, CA, USA).
DHPLC analysis reveals the presence of a mutation by differential retention of homo- and heteroduplex DNA fragments on the reverse-phase chromatography column under appropriate conditions of partial denaturation. The optimal temperature for DHPLC analysis for each fragment was determined by computation: this was accomplished by electronic submission to a community web site (http://hardy-weinberg.stanford.edu/dhplc/melt.html). DHPLC analysis was performed using the WAVE DNA fragment analysis system (Transgenomic Inc., Santa Clara, CA, USA).
Sequencing of genomic DNA was performed with a dye terminator DNA sequencing kit (Applied Biosystems, Perkin-Elmer) on an ABI Model 3100 automated DNA sequencer (Applied Biosystens, Perkin-Elmer). In particular, the fragment encompassing exon 7 was sequenced in all the subjects without performing DHPLC because of a common polymorphism in this exon, which could alter the interpretation of the results.
Production of hybrid cell lines.
Fusions were performed by centrifuging 5 × 105 activated T cells with an equal number of Fas-sensitive HUT78 cells. Prewarmed 50% polyethylene glycol (PEG) and then serum-free DMEM were added to the mixed-cell pellet drop by drop. Then, cells were washed and cultured in RPMI-1640 + 10% FCS + anti-Fas MoAb (1 µg/ml). Hybrid cells survive if Fas-resistant lymphocytes carry a dominant negative factor inhibiting Fas function, whereas unfused HUT78 cells die following Fas triggering and unfused T cells do not grow in the absence of appropriate stimuli. Control fusions between activated T cells from normal donors and HUT78 cells were performed in each experiment.
Caspase activity.
Fas-induced activation of caspase-8, and -9 was evaluated on activated T cells obtained by treating PBMC with PHA at days 0 (1 µg/ml) and 8 (0·1 µg/ml) and culturing cells with 10 U/ml IL2. Four days after the second stimulation, T cells were treated or not with the CH11 MoAb on ice for 30 min, then moved to 37°C for 3 h, centrifuged, and caspase activity was evaluated on cell lysates using fluorimetric assays (MBL, Watertown, MA, USA). At least two controls using T cells from normal donors were always run in parallel. Results were expressed as relative caspase activity percentage, calculated as follows: (Fas-induced caspase activity displayed by each subject/mean of the Fas-induced caspase activities displayed by the healthy controls run in the same experiment) × 100.
RESULTS
Fas-induced cell death was assessed in activated T cells derived from 35 HT, 28 GD patients and 65 normal donors. We also evaluated the response to ceramide, whose pathway partially overlaps that of Fas [2], and to PDN, which does not involve the Fas system. An analysis at the single patient level showed that 24/28 GD (86%) and 12/35 (34%) HT were resistant to Fas-induced cell death, whereas 12/28 (42%) GD and 4/35 (11%) HT were resistant to ceramide-induced cell death. All ceramide-resistant GD, but only two ceramide-resistant HT were also resistant to Fas. Resistance to PDN was displayed by one GD and two HT. These three patients were resistant to Fas. Statistical analysis showed that the responses to Fas triggering and ceramide were significantly lower in GD than in HT and normal controls. The response to Fas was also significantly lower in HT than in normal controls, whereas the response to ceramide was similar in both groups. By contrast, the response to PDN was not different in the three groups (Table 1). In all Fas-resistant patients, Fas resistance was confirmed by evaluating Fas-induced apoptosis by annexinV-staining (data not shown).
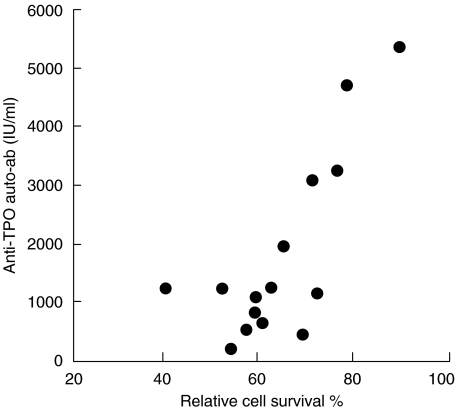
These data show that most GD patients displayed decreased function of Fas. By contrast, Fas function was highly heterogeneous in HT patients. In previous studies on T1DM and MS, we showed that Fas function was mainly decreased in patients displaying signs of aggressive courses [14,15]. Therefore, we performed two analyses to evaluate whether Fas resistance could detect subsets of HT patients with aggressive autoimmunity. First, we compared Fas function in patients that received replacement therapy or did not, assuming that development of hypotyroidism is a sign of aggressive immunological attack of the gland. Disease duration, ages and sex distribution were similar in the two groups (see Materials and Methods). The treated group displayed significantly lower Fas function and higher frequency of Fas resistance than the untreated group (Table 1). Secondly, we evaluated whether Fas function correlated with serum levels of anti-TPO autoantibodies. Anti-TPO autoantibody levels were correlated directly with Fas-resistance in the untreated group: the higher the Fas defect, the higher the autoantibody level (Fig. 1), whereas no correlation was found in the treated group (data not shown). Cell death induced by ceramide or PDN was similar in both groups and no correlation was found with serum levels of autoantibodies. In GD patients, no correlation was found between the autoantibody level and Fas function.
Fig. 1.
Correlation between resistance to Fas-induced T cell death and serum level of anti-TPO autoantibodies in HT patients not requiring replacement therapy. Pearson correlation r = 0·79, p < 0·001.
Fas expression evaluated by direct immunofluorescence on the day when the cell death assay was performed was always in the normal range and was similar in all groups. Search for DN T cells in fresh PBMC did not reveal their expansion in any patient (i.e their count was < 1%) (data not shown).
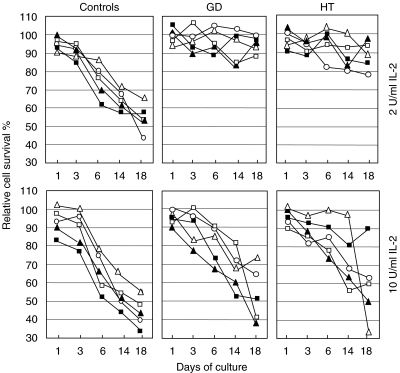
To compare Fas function in Fas-sensitive and Fas-resistant individuals at different times of T cell culture, PBMC from 20 Fas-resistant patients (10 GD and 10 HT) and 15 Fas-sensitive normal donors were cultured with PHA ± IL2 and Fas-induced cell death was assessed at different times. Cultures were performed in the presence of either 2 U/ml (i.e. the standard culture condition) or 10 U/ml of IL-2. Figure 2 shows that, with the low dose of IL-2, T cells from the normal donors were initially resistant to Fas-induced cell death, but became gradually sensitive during the culture. By contrast, T cell cultures from the patients were always Fas-resistant. With the high dose of IL-2, sensitivity was increased greatly and the differences between patients and normal controls were lost in most cases. These results are in line with the notion that Fas connection to the death signalling pathway is a late event in T cell activation [1] and IL-2 sensitizes cells to Fas-induced cell death [23,24].
Fig. 2.
T cell sensitivity to Fas-induced cell death at different culture times and conditions in HT and GD patients and normal controls. Only Fas-resistant patients were recruited according to the results shown in Table 1. Peripheral blood T cells were activated with PHA and cultured with either 2 U/ml IL2 (upper panels) or 10 U/ml IL2 (lower panels); in each group, each subject is marked with the same symbol in the upper and lower panels. Fas-induced cell death was assessed at days 1, 3, 6, 14 and 18 of culture (cells were restimulated with PHA at day 12). Results are expressed as the relative percentage of cell survival. The experiment was performed on 15 normal controls, 10 GD and 10 HT patients; the figure shows representative data obtained from five subjects of each group.
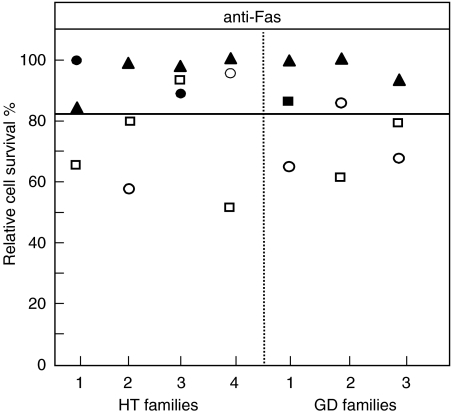
To evaluate the familial component of the decreased function of Fas, we assessed susceptibility to cell death in the father and mother of seven Fas-resistant patients (four treated HT and three GD) (Fig. 3). Most of these parents were healthy; only the mothers of patient HT-1 and HT-3 and the father of patient GD-1 displayed HT. We found that four mothers (three HT and one GD) and two fathers (one HT and one GD) were resistant to Fas-induced cell death. Moreover, two further fathers (one HT and one GD) displayed a response that was near the upper limit of the normal range. All parents with HT were Fas-resistant, but the defect was displayed also by several healthy parents.
Fig. 3.
Fas-induced T-cell death in the mother (circles) and father (squares) of four HT and three GD patients (triangles). Black symbols mark subjects with autoimmune thyroid diseases, white symbols mark healthy subjects. Activated T cells were treated with anti-Fas MoAb and survival was assessed after 18 h. Results are expressed as percentage of specific cell survival. The horizontal lines indicate the upper limit of the normal range calculated as the 95th percentile from 65 normal donors.
In ALPS-Ia, decreased function of Fas is due to mutations of the Fas gene. Therefore, we searched for mutations of the Fas gene in all Fas-resistant patients. DNA changes were found in fragments encompassing exons 2, 3 and 7. All of them corresponded to silent DNA variations or common polymorphisms. No causal mutation was identified.
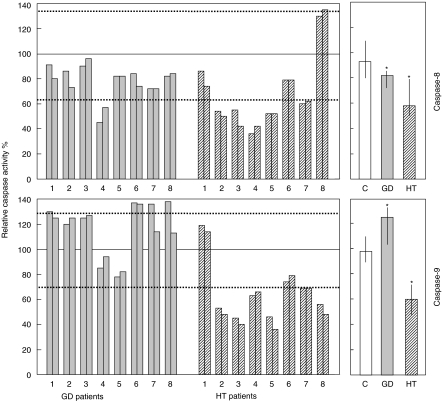
In ALPS-II, decreased Fas function is due probably to mutations hitting the Fas pathway. Therefore, we analysed the Fas pathway by evaluating Fas-induced activation of caspase-8 and -9 in 16 Fas-resistant patients (eight GD and eight HT) and 16 Fas-sensitive normal controls (Fig. 4). In HT patients, activation of both caspase-8 and -9 was significantly lower than in normal controls (P ≤ 0·001). By contrast in GD patients, activation of caspase-9 was significantly higher (P ≤ 0·01) and activation of caspase-8 was slightly, but significantly, lower (P ≤ 0·05) than in normal controls.
Fig. 4.
Fas-induced activation of caspase-8 (upper panels) and -9 (lower panels) in activated T cells from patients with GD, HT and normal controls. Grey bars: GD patients (n = 8); striped bars: HT patients (n = 8); white bar: normal controls (n = 16). Activated T cells were treated with anti-Fas MoAb and caspase activation was assessed after 3 h. Left panels: single patient analysis of the caspase activity evaluated in GD and HT patients (results from two independent experiments are shown for each patient); results are expressed as relative caspase activity percentage (see Materials and methods); the continuous horizontal lines indicate 100% of activity, which was the mean of the activities displayed by the two to four healthy donors run in parallel with the patient samples in each experiment; the dotted horizontal lines indicate the 95th and 5th percentile of the activity displayed by all normal controls (n = 16). Right panels: bulk caspase activity displayed by GD and HT patients and normal controls; results are expressed as median values and interquartile ranges; asterisks mark data that are significantly different from normal controls (P < 0·05, Mann–Whitney test).
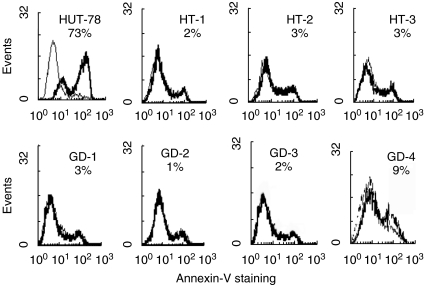
In previous studies, we showed that Fas-resistant patients with T1DM or MS produce molecules exerting a dominant negative effect on Fas function [14,15]. To evaluate whether these molecules are also detectable in GD and HT, we fused activated CD4+ T cells derived from eight Fas-resistant (five GD and three HT) and 18 Fas-sensitive subjects (four HT and 14 normal controls) with the Fas-sensitive HUT78 T cell line, and cultured hybrid cells under the selective pressure of anti-Fas MoAb. Most fusions from Fas-resistant patients (4/5 GD and 3/3 HT) gave rise to Fas-resistant hybrid cell lines, whereas no fusion from Fas-sensitive subjects did so (Fig. 5).
Fig. 5.
Cell death response to Fas triggering of control HUT78 cells and hybrid cell lines obtained by fusing HUT78 cells with Fas-resistant T cells from four Fas-resistant GD and three Fas-resistant HT patients. Hybrid cells are resistant to Fas-induced cell death, whereas control HUT78 cells are not. Cells were treated with the anti-Fas MoAb and cell death was evaluated after 12 h by staining cells with annexin V and propidium iodide. The FACS profiles show staining with annexin V of propidium iodide-negative cells from the untreated (grey lines) and anti-Fas MoAb treated (black lines) cells. The numbers indicate the proportion of annexin V+ cells in the anti-Fas MoAb-treated sample less the proportion of annexin V+ cells in the untreated sample. Similar results were obtained when propidium iodide-positive cells were also included to detect both early and late apoptotic cells. Fas-sensitive hybridomas could not be raised, as they were killed by the selection media (containing anti-Fas MoAb) during the early phases of hybridoma production.
DISCUSSION
This work shows that a substantial proportion of paediatric patients with HT or GD display decreased function of Fas and this defect is significantly more frequent in GD than in HT. Fas function was not abolished in Fas-resistant patients as the defect was overcome by sensitizing Fas function by culturing cells in high levels of IL2, which decreases expression of inhibitory molecules such as FLIP and bcl-2 [23,24]. The observation that fusion of Fas-resistant T cells from these patients with a Fas-sensitive T cell line generates Fas-resistant hybrid cells suggests that Fas-resistance is due to molecules with a dominant negative effect on Fas function.
One possibility is that the defect is due to inherited genetic alterations similar to those causing ALPS, which often display a dominant negative effect [8–13]. The defect was different from that displayed by ALPS-Ia or -Ib patients, as the Fas gene was not mutated, Fas was normally expressed and FasL was not involved because the test used (i.e. triggering of Fas by MoAb) is independent of FasL. Therefore, the defect might be similar to that displayed by ALPS-II patients and involve the Fas signalling pathway.
This cross-sectional study cannot rule out the possibility that Fas-resistance may have been acquired during the course of disease by selection of constitutively Fas-resistant T cell subsets, driven by the chronic immune activation, the hormone defect, or the therapy. However, the genetic component is supported by the observation that Fas function was decreased in several healthy parents of Fas-resistant patients. It is noteworthy that our patients were mainly children and their disease might have a stronger genetic component than that developed by adults.
In contrast to ALPS patients, our Fas-resistant patients did not display any sign of lymphoproliferation, i.e. no lymphoadenopathy, splenomegaly or expansion of DN T cells. However, this feature was not surprising, because we did not detect signs of lymphoproliferation in Fas-resistant patients with other autoimmune diseases or in the Fas-resistant parents of ALPS patients [12,14,15]. The finding that Fas resistance was detected only in cells cultured with low doses of IL-2 suggests that in these subjects the defect may be subtle and not sufficient to induce massive lymphocyte accumulation after strong immune responses with high IL-2 production. In contrast, the defect may inhibit the shutting-off of weak chronic immune responses such as those involved in autoimmune diseases. It is noteworthy that thyroid autoimmunities are known to favour development of Fas-resistant thyroid-associated lymphomas [25], which calls to mind the observation that ALPS patients display high risk of lymphoma development [26].
The defect seemed to be partly different in GD and HT, as 12/24 Fas-resistant GD, but only 2/12 Fas-resistant HT, were also resistant to ceramide. Moreover, the caspase activation pattern was different in the two groups. In most HT, activity of both caspase-8 and -9 were decreased significantly, which suggests a defect hitting both the extrinsic and the mitochondrial pathway of Fas signalling. By contrast, in most GD caspase-9 activity was increased significantly, whereas caspase-8 activity was decreased slightly, which suggests a defect hitting the extrinsic pathway and inducing a compensatory hyperactivation of the mitochondrial pathway. It is noteworthy that this difference was not absolute, as two GD patients (GD-4 and GD-5 in Fig. 4) displayed an ‘HT-like’ pattern of caspase activation, whereas one HT patient (HT-1) displayed a ‘GD-like’ pattern. Moreover, one HT patient (HT-8) displayed a further pattern with increased activity of caspase-8 and decreased activity of caspase-9. These data suggest that the defect can be heterogeneous in different patients.
HT and GD are driven by different pathogenetic mechanisms. In HT, thyroid-specific autoantibodies and TDTH induce heavy inflammatory infiltration of the gland and tissue damage. Fas and FasL are up-regulated in HT thyrocytes, due probably to cytokines produced by inflammatory cells, which suggests that tissue damage may be due in part to Fas/FasL interaction triggering thyrocyte fratricide or suicide [3,26–28]; further damage has been ascribed to FasL + inflammatory cells [3, 27–31]. This assumption is apparently contradicted by our suggestion that genetically based deficiencies of Fas may favour HT development. However, our data do not imply that the Fas defect is a general cause of HT, because we detected it only in about 30% of patients. Moreover, tissue damage may be ascribed to residual Fas function in a cytokine-rich microenvironment and to other proapoptotic systems, such as tumour necrosis factor (TNF), TNF-related-apoptosis-inducing ligand (TRAIL) and granzymes [1,32]. It is noteworthy that Fas-resistance seems to identify HT patients with aggressive forms of autoimmunity (i.e. those requiring replacement therapy and those not requiring therapy but displaying high levels of auto-antibodies), which is in line with our previous reports on MS and T1DM [14,15]. The lack of correlation between the defect and the autoantibody level in the treated patients might be due to the effect that extensive damage of the thyroid tissue may exert on the autoimmune response.
GD is due mainly to anti-TSH receptor autoantibodies causing thyroid hyperplasia. Therefore, it is not surprising that Fas resistance may favour GD development, because ALPS patients also develop mainly antibody-mediated autoimmune diseases such as haematological cytopenias and glomerulonephritis. A different point is that the defect may also result in thyroid hyperplasia by altering interactions between Fas+ and FasL+ thyrocytes, which may play a role in thyroid homeostasis [28,29,33–36]. It is noteworthy that GD patients have been reported to display high levels of soluble Fas in serum, which may further inhibit the function of membrane Fas [8,9,37,38].
In conclusion, this work shows that Fas function is decreased in a substantial proportion of patients developing GD or HT at the paediatric stage. In HT, the defect correlates with aggressive forms of autoimmunity. We suggest that it may be due to genetic alterations affecting the immune response shutting-off system similar to those causing ALPS. Accumulation of several of them in the same subject may cause the rare ALPS, whereas less severe defects may favour development of common autoimmune diseases such as thyroid disease, T1DM [15] or MS [14].
Acknowledgments
This work was supported by Telethon grant no. E1170 (Rome), Fondazione Italiana Sclerosi Multipla (FISM, Genoa), MURST cofin-projects (Rome), AIDS Project (Istituto Superiore di Sanità, Roma).
REFERENCES
- 1.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 2.De Maria R, Lenti L, Malisan F, et al. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 1997;277:1652–5. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- 3.De Maria R, Testi R. Fas–FasL interactions: a common pathogenetic mechanism in organ-specific autoimmunity. Immunol Today. 1998;19:121–5. doi: 10.1016/s0167-5699(97)01202-4. [DOI] [PubMed] [Google Scholar]
- 4.Itoh N, Imagawa A, Hanafusa T, et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1997;186:613–8. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chervonsky AV, Wang Y, Wong FS, et al. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 6.Sabelko KA, Kelly KA, Nahm MH, Cross AH, Russel JH. Fas and Fas ligand enhance the pathogenesis of experimental allergic encephalomyelitis, but are not essential for immune privilege in the central nervous system. J Immunol. 1997;159:3096–9. [PubMed] [Google Scholar]
- 7.Waldner H, Sobel RA, Howard E, Kuchroo VK. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol. 1997;159:3100–3. [PubMed] [Google Scholar]
- 8.Fisher GN, Rosemberg FJ, Strans SF, et al. Dominant interfering Fas gene mutations impair apoptosis in a human lymphoproliferative syndrome. Cell. 1995;81:935–46. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 9.Rieux-Loucat R, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 10.Straus SE, Sneller M, Lenardo MJ, Puck JM, Strober W. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- 11.Dianzani U, Bragardo M, DiFranco D, et al. Deficiency of the Fas apoptosis patway without Fas gene mutations in pediatric patients with autoimmunity/lymphoproliferation. Blood. 1997;89:2871–9. [PubMed] [Google Scholar]
- 12.Ramenghi U, Bonissoni S, Migliaretti G, et al. Deficiency of the fas apoptosis pathway without fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood. 2000;95:3176–82. [PubMed] [Google Scholar]
- 13.Wang J, Zheng L, Lobito A, et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 14.Comi C, Leone M, Bonissoni S, et al. Defective T cell Fas function in patients with multiple sclerosis. Neurology. 2000;55:921–7. doi: 10.1212/wnl.55.7.921. [DOI] [PubMed] [Google Scholar]
- 15.DeFranco S, Bonissoni S, Cerutti F, et al. Defective function of Fas in patients with type I (insulin-dependent) diabetes mellitus associated with other autoimmune diseases. Diabetes. 2001;50:483–8. doi: 10.2337/diabetes.50.3.483. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz HM, Herrmann M, Winkler T, Gaipi U, Kalden JR. Role of apoptosis in autoimmunity. Apoptosis. 2000;5:443–9. doi: 10.1023/a:1009692902805. [DOI] [PubMed] [Google Scholar]
- 17.Rovere P, Sabbadini G, Fazzini F, et al. Remnants of suicidal cells fostering systemic autoaggression. Arthritis Rheum. 2000;43:1663–72. doi: 10.1002/1529-0131(200008)43:8<1663::AID-ANR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophage that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE-2 and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravirajan CT, Pittoni V, Isemberg DA. Apoptosis in human autoimmune diseases. Int Rev Immunol. 1999;18:563–89. doi: 10.3109/08830189909088499. [DOI] [PubMed] [Google Scholar]
- 20.Rosen A, Casciola-Rosen L. Autoantigen as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Diff. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 21.Trudeau JD, Ductz JP, Arany E, Hill DJ, Fieldus WE, Finegood T. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Sostre S, Reyes MM. Sonographic diagnosis and grading of Hashimoto's thyroiditis. J Endocrinol Invest. 1991;14:115–21. doi: 10.1007/BF03350281. [DOI] [PubMed] [Google Scholar]
- 23.Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–8. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 24.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–23. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 25.Takakuwa T, Dong Z, Takayama H, Matsuzuka F, Nagata S, Aozasa K. Frequent mutations of Fas gene in thyroid lymphoma. Cancer Res. 2001;61:1382–5. [PubMed] [Google Scholar]
- 26.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 27.Giordano C, Stassi G, De Maria R, et al. Potential involvment of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science. 1997;255:960–3. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 28.Kotani T, Aratake Y, Hirai Y, Fukazawa Y, Sato H, Ohtaki S. Apoptosis in thyroid tissue from patients with Hashimoto's thyroiditis. Autoimmunity. 1995;20:231–6. doi: 10.3109/08916939508995700. [DOI] [PubMed] [Google Scholar]
- 29.Borgerson KL, Bretz JD, Baker JR., Jr The role of Fas-mediated apoptosis in thyroid autoimmune disease. Autoimmunity. 1999;30:251–64. doi: 10.3109/08916939908993806. [DOI] [PubMed] [Google Scholar]
- 30.Mitsiades N, Poulaki V, Kotoula V, et al. Fas/Fas ligand up-regulation and Bcl-2 down-regulation may be significant in the pathogenesis of Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1998;83:2199–203. doi: 10.1210/jcem.83.6.4853. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Hocker J. Expression of bcl-2, Bax and Fas in oxyphil cells of Hashimoto thyroiditis. Virchows Arch. 2000;436:602–7. doi: 10.1007/s004280000188. [DOI] [PubMed] [Google Scholar]
- 32.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:750–3. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 33.Hiromatsu Y, Hoshino T, Yagita H, et al. Functional Fas ligand expression in thyrocytes from patients with Graves’ disease. J Clin Endocrinol Metab. 1999;84:2896–902. doi: 10.1210/jcem.84.8.5682. [DOI] [PubMed] [Google Scholar]
- 34.Feldkamp J, Pascher E, Perniok A, Scherbaum WA. Fas-mediated apoptosis is inhibited by TSH and iodine in moderate concentrations in primary human thyrocytes in vitro. Horm Metab Res. 1999;31:355–8. doi: 10.1055/s-2007-978753. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami A, Matsuoka N, Tsuboi M, et al. CD4+ T cell-mediated cytotoxicity toward thyrocytes: the importance of Fas/Fas ligand interaction inducing apoptosis of thyrocytes and the inhibitory effect of thyroid-stimulating hormone. Lab Invest. 2000;80:471–84. doi: 10.1038/labinvest.3780053. [DOI] [PubMed] [Google Scholar]
- 36.Tamura M, Kimura H, Koji T, et al. Role of apoptosis of thyrocytes in a rat model of goiter. A possible involvement of Fas system. Endocrinology. 1998;139:3646–53. doi: 10.1210/endo.139.8.6140. [DOI] [PubMed] [Google Scholar]
- 37.Hiromatsu Y, Bednarczuk T, Soyejima E, et al. Increased serum soluble Fas in patients with Graves’ disease. Thyroid. 1999;9:341–5. doi: 10.1089/thy.1999.9.341. [DOI] [PubMed] [Google Scholar]
- 38.Shimaoka Y, Hidaka Y, Okumura M, Takeoka K, Tada H, Amino N. Serum concentration of soluble Fas in patients with autoimmune thyroid diseases. Thyroid. 1998;8:43–7. doi: 10.1089/thy.1998.8.43. [DOI] [PubMed] [Google Scholar]