Abstract
Human T lymphotrophic virus type-I (HTLV-I), a human retrovirus, infects CD4+ lymphocytes and is thought to modify their function; a possible association with pulmonary diseases has also been suggested. However, little is known about the influence of HTLV-I on cryptogenic fibrosing alveolitis (CFA), a chronic inflammatory interstitial lung disease of unknown aetiology. In order to clarify the influence of HTLV-I infection on CFA, 72 CFA patients with and without HTLV-I infection were examined. HTLV-I positive CFA patients were likely to have larger affected areas and to show traction bronchiectasis with honeycombing change. An imbalance of matrix metalloproteinases and tissue inhibitor of metalloproteinases were also observed in the BALF of HTLV-I positive CFA patients. CD3+/CD25+ lymphocyte percentage was significantly higher in the BALF of HTLV-I positive patients compared to negative patients. MIP-1α, IP-10 and sICAM levels in BALF were also significantly higher in HTLV-I positive patients than in negative patients. The levels of MCP-1 and IL-8 were not significantly different. In HTLV-I positive patients, the MIP-1α and IP-10 levels showed a significant positive correlation with percentage of CD3+/CD25 lymphocytes. HTLV-I positive CFA patients showed a larger lesion than negative patients and exhibited increased levels of certain cytokines that correlated with activated T cells in the BALF. We suggest that HTLV-I infection may contribute to the development of CFA via activation of T cells. We also propose that these features should be taken into consideration in the treatment of CFA in HTLV-I infected individuals.
Keywords: HTLV-I associated bronchopneumonopathy, matrix metalloproteinases, IP-10, MIP-1α, sICAM
INTRODUCTION
Human T lymphotrophic virus type-I (HTLV-I), a human retrovirus, is known to cause adult T cell leukaemia (ATL) [1] and preferentially infects CD4+ cells in vivo, modifying the immune system as well as T cell functions [2]. HTLV-I is also associated with several non-malignant disorders such as HTLV-I associated myelopathy (HAM) [3], HTLV-I associated uveitis (HAU) [4] and arthropathy [5]. In addition, several studies from HTLV-I pandemic areas have reported that both ATL patients [6,7] and non-ATL HTLV-I positive people develop frequent pulmonary complications [8–12]. Unlike patients with ATL, pulmonary involvement in non-ATL patients does not correlate with leukaemic cell infiltration and pathogens associated with opportunistic infection are not found in the lungs of these HTLV-I positive individuals [8,13,14]. Bronchoalveolar lavage fluid (BALF) findings in these patients are characterized by increased interleukin-2 receptor (IL-2R/CD25) positive T cells and marked elevation of soluble IL-2R [11]. Other studies showed up-regulated HTLV-I tax gene expression in lung tissue and a close correlation between HTLV-I mRNA expression and lymphocytosis in the lung of HTLV-I positive individuals [15,16]. The association of intrapulmonary chemokine production with the HTLV-I product protein has also been reported in transgenic mice [17]. These results suggest that HTLV-I infection could induce chronic inflammation in the lung through the activation of leucocytes; however, there has been no clinical study correlating HTLV-I infection with pulmonary disorders.
Cryptogenic fibrosing alveolitis (CFA) is a chronic interstitial lung disease of unknown cause, characterized pathologically by inflammation and fibrosis of the lung parenchyma and is included in diffuse parenchymal lung diseases (DPLDs) that account for 15% of the patient population seen by respiratory specialists [18]. The incidence of CFA has been estimated at 10·7 cases per 100 000 per year for males and 27 of 100 000 for females and is considered to be one of the most common chronic interstitial lung diseases [18,19]. There have been many reports focusing on the inflammatory components of this disease and there is no doubt that various inflammatory cells and mediators contribute to the pathogenesis of this disease. Taken together, we hypothesized that HTLV-I infection might influence CFA. In this study, we tried to clarify the influence of HTLV-I on CFA by investigating 72 CFA patients (18 patients were positive for HTLV-I and 54 patients were negative) and have compared the clinical features and cytokine levels in the BALF.
MATERIALS AND METHODS
Subjects
This study was reviewed and approved by the Kagoshima University Faculty of Medicine Committee on Human Research. All cases of patients admitted to the Third Department of Internal Medicine (Kagoshima University Faculty of Medicine) and the Department of Respiratory Medicine (National Minami-Kyushu Hospital) between 1996 and 2001 were reviewed retrospectively by specialists of respiratory medicine. A total of 4782 patients were admitted between 1996 and 2001. The mean age of the patients was 62·4 ± 19·9. Of these, 772 patients (16·1%) were infected with HTLV-I.
Study protocol
The following are the steps undertaken during the review process: (1) three specialists of respiratory medicine reviewed carefully the records of all patients who were admitted to our departments; (2) clinical symptoms were investigated carefully and all previous chest radiographs were reviewed; and (3) a diagnosis of CFA was presumed if a patient had either bilateral interstitial chest radiographic shadowing with bilateral basal inspiratory crackles or lung function parameters compatible with CFA (a restrictive and/or gas transfer defect) and confirmed pathological findings. In addition, a diagnosis of CFA required the patient to have no evidence of allergic alveolitis, sarcoidosis or occupational exposures that would cause pneumoconiosis. In order to evaluate the influence of HTLV-I infection in CFA, we excluded the patients with systemic diseases such as collagen disease, HIV infection, vasculitis and malignant neoplasms as well as patients with immunological abnormalities that predispose them to opportunistic infection, such as diabetes mellitus and acute or chronic liver disease. In order to exclude the influence of genetic and environmental factors, we investigated patients from two separate hospitals (Kagoshima University is in Kagoshima City and National Minami-kyushu Hospital is in Aira-gun, wherein a previous study showed a significant difference in HTLV-I prevalence between these two areas [20]). For HTLV-I positive patients, we examined the counts of abnormal lymphocytes and excluded the ATL patients.
Determination of serum HTLV-I antibody
As a policy of our centres, we performed a serum HTLV-I antibody test routinely on all patients admitted to our hospitals. Serum samples were collected from all subjects and tested for anti-HTLV-I antibody as follows. First, anti-HTLV-I antibody was measured with an EitesT ATL kit (Eisai Inc., Tokyo, Japan), and then the sera were re-examined by Western blot method using MT 2 cell lysate antigens [21] to confirm positivity.
Radiographic analysis
We also examined the affected pulmonary segments on high resolution computed tomography (HRCT) to evaluate the distribution of the lesion in patients with CFA. The ratio of the affected area to the total lung field was judged subjectively by the visual scoring method [22]. Each slice was evaluated individually, and the right and left lungs were graded separately. A score of 0 was given if there were no abnormal shadows on the chest HRCT. If < 25% of the pulmonary parenchyma in a slice was considered to be abnormal the score was 1; between 25% and 50% the score was 2; between 50% and 75% the score was 3; and>75% the score was 4. Therefore, the right and left lungs each received a maximum score of 4, and a maximum score of 8 was given per slice. All slices above the level of the diaphragm were assessed in each patient. For each subject, a visual score (VS) in percentage (total of scores for each slice over the total possible maximum score) was calculated. The radiographs of each subject were evaluated independently by two investigators (a pulmonologist and a radiologist) who have been blinded to the clinical data.
Bronchoalveolar lavage fluid (BALF) analysis
The lavage fluid was spun in a cytometer (KN-70, Kubota Ltd, Tokyo, Japan) at 44× g for 5 min and stained with May–Giemsa stain to identify cell populations. Five hundred cells, excluding epithelial cells, were identified per slide to establish differential cell counts, and the counts were expressed in percentages. The subtypes of lymphocytes were analysed by flow cytometry using CD4, CD8 and CD25 monoclonal antibodies (Becton Dickinson Co., Mountain View, CA, USA) and FITC-conjugated anti-CD3 monoclonal antibody. BALF fluids were stored at −20°C for further analysis routinely in both hospitals.
Cytokines in BALF
To evaluate the immunological effect of HTLV-I on CFA, we measured the macrophage inflammatory protein-1α (MIP-1α), interferron-γ inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8) and soluble form of intercellular adhesion molecule (sICAM) concentrations in BALF using an ELISA kit purchased from R&D Systems, Minneapolis, MN, USA. We also determined the matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinases (TIMPs) titres in the BALF to evaluate the balance of fibrosis and remodelling in the lung lesions. The concentrations of MMP-2, -9 and TIMP-1, -2 in BALF were measured by corresponding sandwich enzyme immunoassay systems using monoclonal antibodies according to the manufacturer's protocols (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The χ2 test was used to evaluate the prevalence of HTLV-I infection in CFA patients. The Bonferroni/Dunn with analysis of variance (anova) test was used to see the difference in laboratory findings, lymphocyte subpopulations. The Mann–Whitney U-test was employed to evaluate the difference in VS and the BALF results. We also employed Spearman's rank correlation test to evaluate the statistical correlation between cytokines or VS and CD3+/CD25+ cell percentage in BALF. A P-value below 0·05 was considered significant. Most values were expressed as mean ± standard deviation (s.d.).
RESULTS
Patients
The prevalence of HTLV-I infection in CFA patients (18/72, 25%) was significantly higher than compared to the prevalence in all patients admitted to the departments between 1996 and 2001 [odds ratio (OR) = 1·75, 95% confidence intervals (95%CI) = 1·02–3·00, P < 0·05]. In HTLV-I positive CFA patients, 16 patients were suffering from HAM, five patients were suffering from HAU and three patients were suffering from HTLV-I associated arthropathy (four patients had HAM and HAU, two patients had HAU and HTLV-I associated arthropathy). Of all the HTLV-I infected patients in our study, 24 patients were suffering from HAM, seven patients were suffering from HAU and four patients were suffering from HTLV-I associated arthropathy. Twelve HTLV-I positive CFA patients were transferred to our centres because of an abnormal shadow found on routine examination for other HTLV-I associated diseases, four HTLV-I positive CFA patients were suffering from slight dyspnoea on effort and two HTLV-I positive CFA patients were suffering from chronic cough.
The affected areas were significantly wider, %FEV1 was significantly lower and the prevalence of upper lobe involvement and traction bronchiectasis with honeycombing were significantly higher in HTLV-I positive than in negative patients (Table 1). The mean age, smoking index, male/female ratio and laboratory findings were not significantly different between two groups.
Table 1.
Comparison of CFA between HTLV-I positive patients and negative patients
| HTLV-I positive patients (n = 18) | HTLV-I negative patients (n = 54) | P-value | |
|---|---|---|---|
| Pulmonary function tests | |||
| %VC (%) | 63·3 ± 13·8 | 71·3 ± 13·2 | P < 0·05 |
| %FEV1 (%) | 66·8 ± 10·2 | 74·1 ± 11·3 | P < 0·05 |
| %DLCO (%) | 50·8 ± 16·9 | 55·3 ± 15·2 | n.s. |
| Radiographic appearance | |||
| Visual score | 27·4 ± 12·9 | 20·3 ± 13·1 | P < 0·05 |
| Ratio of upper lobe involvement | 10/18 | 15/54 | P < 0·05 |
| Prevalence of traction brochiectasis and honeycombing change | 12/18 | 20/54 | P < 0·05 |
| BALF analysis | |||
| Total cell count (×105/µl) | 2·88 ± 1·99 | 2·91 ± 1·85 | n.s. |
| Macrophage (×105/µl) | 1·82 ± 1·66 | 1·36 ± 1·41 | n.s. |
| Lymphocyte (×105/µl) | 0·69 ± 0·44 | 0·61 ± 0·38 | n.s. |
| Neutrophile (×105/µl) | 0·13 ± 0·15 | 0·16 ± 0·21 | n.s. |
| Eosinophile (×105/µl) | 0·03 ± 0·06 | 0·03 ± 0·05 | n.s. |
| CD4+ lymphocytes(%) | 35·9 ± 10·1 | 35·8 ± 16·9 | n.s. |
| CD8+ lymphocytes (%) | 34·1 ± 20·1 | 33·5 ± 13·5 | n.s.. |
| CD4+/CD8+ | 1·49 ± 1·01 | 1·33 ± 1·36 | n.s. |
| CD3+ lymphocytes (%) | 92·3 ± 11·3 | 88·9 ± 12·5 | n.s. |
| CD3+/CD25+ (%) | 27·9 ± 16·4 | 18·9 ± 16·6 | P < 0·05 |
| MMP-2 (ng/ml) | 3·89 ± 1·99 | 2·58 ± 2·01 | P < 0·05 |
| MMP-9 (ng/ml) | 7·1 ± 10·9 | 5·29 ± 6·99 | n.s. |
| TIMP-1 (ng/ml) | 10·24 ± 9·97 | 11·43 ± 12·22 | n.s. |
| TIMP-2 (ng/ml) | 11·28 ± 5·22 | 9·67 ± 6·35 | n.s. |
| MIP-1α (pg/ml) | 29·7 ± 9·6 | 20·3 ± 12·2 | P < 0·01 |
| IP-10 (pg/ml) | 27·8 ± 10·2 | 20·2 ± 10·8 | P < 0·05 |
| MCP-1 (pg/ml) | 31·4 ± 12·3 | 34·3 ± 17·7 | n.s. |
| IL-8 (pg/ml) | 29·8 ± 18·6 | 28·7 ± 17·8 | n.s. |
| sICAM (pg/ml) | 104·5 ± 27·7 | 80·4 ± 30·4 | P < 0·01 |
BALF analysis
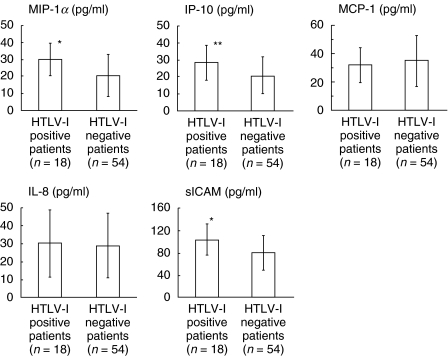
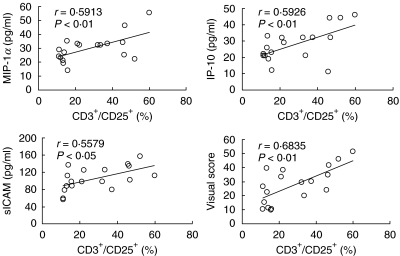
There was no significant difference in lymphocyte numbers and CD3+ lymphocyte percentage, however, CD3+/CD25+ percentage was significantly higher in HTLV-I positive patients than in negative patients. The BALF level of MMP-2 was significantly higher in HTLV-I positive patients than in negative patients while there was no difference in MMP-9, TIMP-1 and TIMP-2 levels between two groups (Table 1). IP-10, MIP-1α and sICAM levels were significantly higher in HTLV-I positive patients than in negative patients. The levels of MCP-1 and IL-8 were not significantly different (Fig. 1). In HTLV-I positive patients, the MIP-1α, IP-10 and sICAM levels showed a significant positive correlation with the CD3+/CD25+ percentage (MIP-1α: r = 0·5913, P < 0·01; IP-10: r = 0·5926, P < 0·01; sICAM: r = 0·5579, P < 0·05, Fig. 2). There was no significant correlation between BALF cytokine levels and CD3+/CD2+ lymphocyte percentages in HTLV-I negative CFA patients. In HTLV-I positive CFA patients, VS showed significant positive correlation with the percentage of CD3+/CD25+ lymphocytes in BALF (r = 0·6835, P < 0·01, Fig. 2) while no significant correlations in HTLV-I negative CFA patients were observed.
Fig. 1.
Comparison of cytokine levels in BALF between HTLV-I positive and negative CFA patients. The levels of MIP-1α, IP-10 and sICAM were significantly higher in HTLV-I positive patients than in negative patients. The levels of MCP-1 and IL-8 were not significantly different between two groups. Bar shows mean value in each group. (*P < 0·01, **P < 0·05, Mann–Whitney test).
Fig. 2.
Correlation between percentage of CD3+/CD25+ lymphocytes and cytokine levels in BALF of HTLV-I positive CFA patients. The levels of MIP-1α, IP-10 and sICAM showed significant positive correlations with the percentage of CD3+/CD25+ lymphocytes. VS also showed significant positive correlations with the percentage of CD3+/CD25+ lymphocytes.
DISCUSSION
In this study, we evaluated clinical and immunological HTLV-I infection influence on CFA. There have been several reports describing possible association of HTLV-I infection and pulmonary disorders [13,22,23]. Accordingly, a new clinical entity, namely HTLV-I associated bronchopneumonopathy (HAB), has been suggested [8, 13,23]. However, to our knowledge there has been no report to suggest a high prevalence of HTLV-I infection in patients with CFA. In our study, the percentage of symptomatic HTLV-I infection status is higher than in previous reports [24,25]. We think this is a potential bias of the study in hospitalized patients and there may be more HTLV-I infected asymptomatic carriers living in our area. It is interesting that all HTLV-I positive CFA patients were suffering from HTLV-I associated diseases and most HTLV-I positive CFA patients did not have major respiratory symptoms. Therefore, we propose that it is possible that either more intensive investigation of minor symptoms or routine investigation of lung function testing may have resulted in an increased diagnosis of CFA among HTLV-I infected individuals.
In this report, we showed that HTLV-I positive CFA patients were likely to have wide lesions and bronchiectasis with honeycombing change. Of course, we cannot deny the possibility of coincidence; however, we think that CFA patients with HTLV-I infection are likely to have incurred more damage to the lung parenchyma because the BALF chemokine levels were correlated with activated T cell percentages only in HTLV-I positive CFA patients. In addition, our study showed that HTLV-I positive CFA patients showed significantly increased BALF levels of MMP-2 compared to negative patients; no differences in the TIMP-2 levels were noted. MMPs are a family of zinc- and calcium-dependent endopeptidases capable of proteolytically degrading many of the components of the extracellular matrix [26] and TIMPs are the endogenous inhibitors of MMPs [27]. MMPs are thought to be associated with wound repair of human respiratory epithelium [28], while TIMPs are thought to be associated with irreversible pulmonary structure remodelling via myofibroblast [29]. The imbalance of these factors has been thought to contribute to the development of interstitial lung diseases [30]. Taken together, we propose the possibility that HTLV-I positive patients are likely to have an imbalance of MMPs and TIMPs, which induce pulmonary fibrosis.
Our study also showed increased MIP-1α and IP-10 BALF levels that correlated with activated T cell percentages (CD3+/CD25+ lymphocytes) in HTLV-I positive patients. MIP-1α is known to regulate the trafficking and activation state of select subgroups of inflammatory cells, including lymphocytes [31], modulate leucocyte adhesion to the endothelium and contribute to leucocyte recruitment into the lungs [32]. On the other hand, IP-10 is chemotactic for activated T cells and plays an important role in recruiting activated effector T cells into sites of tissue inflammation [33]. IP-10 is also reported to be involved in the pathogenesis of pulmonary fibrosis [34], bleomycin-induced pulmonary fibrosis [35], radiation-induced pulmonary fibrosis [36] and human immunodeficiency virus (HIV)-associated alveolitis [37]. T cells can be the cellular source of MIP-1α [38] and IP-10 [39]. An in vivo study showed that p40tax protein of HTLV-I could induce chemokine production in the lung including MIP-1α and IP-10 in mice [17]. To our knowledge, this is the first report that showed a possible correlation of HTLV-I infection and BALF IP-10 levels. Taken together, we propose the possibility that HTLV-I infected lymphocytes may contribute to the differential levels of chemokines in BALF between HTLV-I positive and negative CFA patients.
ICAM-1 belongs to the immunoglobulin supergene family and is expressed by many airway cells including bronchial epithelium cells, endothelial cells, T cells, mast cells, eosinophils and alveolar macrophages. It facilitates cell-to-cell interaction, which could potentiate chronic inflammation and is particularly important in neutrophil adhesion to epithelial cells. ICAM-1 plays an important role in microvascular leucocyte recruitment in the bleomycin-induced pulmonary fibrosis [40] and its expression was increased in CFA patients [40]. In vitro, HTLV-I infection of T cells resulted in constitutive expression of ICAM-1 [41]. Besides, HTLV-I infected individuals showed elevated levels of sICAM in BALF and the concentration of sICAM-1 correlated with the percentage of activated T cells [13]. Our study also showed significantly increased sICAM levels and a significantly positive correlation of sICAM levels with the percentage of CD3+/CD25+ lymphocytes (activated T cells) was observed in HTLV-I positive patients who exhibited wider lesions than HTLV-I negative CFA patients. Although we cannot deny the possibility that increased sICAM-1 levels reflected the increased movement of cells into the lungs, we suggest that ICAM may be involved in the pathogenesis of CFA in HTLV-I positive patients.
In our study, there was no significant difference of MCP-1 and IL-8 levels between HTLV-I positive and negative patients. MCP-1 is a major chemoattractant for monocytes in inflammation and immune responses [42]. MCP-1 was detected in the BALF of CFA [43] and suggested the possible association with the pathogenesis of CFA [44]. However, macrophages and epithelial cells, not lymphocytes that were the targets of HTLV-I infection, were the main cellular source of MCP-1 production in CFA [45]. On the other hand, IL-8 is a potent chemoattractant for neutrophils and plays a pivotal role in acute inflammation by recruiting and activating neutrophils [42]. Because of this function, IL-8 is considered to play a prominent role in the attraction of neutrophils to the lung in CFA [46] and HTLV-I tax protein can trans-activate the human IL-8 gene through acting concurrently on the AP-1 and nuclear factor-kappaB-like sites [47]. However, in CFA the cellular sources of IL-8 were considered to be alveolar macrophages [48]. We cannot deny the possibility that lymphocytes from HTLV-I positive patients can potentially secrete more IL-8 than from those of negative patients; we consider that the lymphocytes are not the major cellular source of IL-8 production in HTLV-I positive CFA patients.
In summary, CFA in HTLV-I positive patients have the following features: (1) patients with CFA were likely to have large lesions, bronchiectasis with honeycombing change and imbalance of MMPs and TIMPs; and (2) increased BALF levels of MIP-1α, IP-10 and sICAM-1 that correlated with the percentage of CD3+/CD25+ T cells. Also, VS, which reflect the width of lesion, showed a significant positive correlation with the percentage of activated T cells, which are the target of HTLV-I infection. Therefore, we suggest that the HTLV-I status should be checked when treating patients with CFA to evaluate the role of HTLV-I in the pathogenesis of fibrosing alveolitis. We propose a possible new clinical entity, namely HTLV-I-associated fibrosing alveolitis (HAFA), because we consider that HTLV-I infection may cause the differences between patients with HTLV-I infection and fibrosing alveolits compared with patients with CFA alone. However, we also propose that this study must be repeated with a second cohort to confirm these findings, because of the potential problems encountered when undertaking multiple comparisons. Further studies addressing these points are necessary to clarify the influence of HTLV-I in CFA.
Acknowledgments
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research (OPSR) Japan. We wish to thank in particular Dr Koichirou Usuku (Department of Medical Information, Kagoshima University Faculty of Medicine) and Ikkou Higashimoto (Third Department of Internal Medicine, Kagoshima University Faculty of Medicine) for their critical reviews and statistical analysis and appreciation to Mrs Rumi Matsuyama (Third Department of Internal Medicine, Kagoshima University Faculty of Medicine) for her technical assistance. We also appreciate Dr Carole L. Galligan (Laboratory of Molecular Immunoregulation, National Cancer Institute-Frederick, Frederick, MD, USA) for her excellent language editing.
REFERENCES
- 1.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–5. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 4.Nakao K, Ohba N, Matsumoto M. Noninfectious anterior uveitis in patients infected with human T lymphotropic virus type I. Jpn J Ophthalmol. 1989;33:472–81. [PubMed] [Google Scholar]
- 5.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;1:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Yokota T, Mashita R, Tamura S. Pulmonary manifestations in adult T cell leukemia at the time of diagnosis. Respiration. 1993;60:115–9. doi: 10.1159/000196184. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka R, Yamaguchi K, Yoshinaga T, Takatsuki K. Pulmonary complications in patients with adult T cell leukemia. Cancer. 1985;55:2491–4. doi: 10.1002/1097-0142(19850515)55:10<2491::aid-cncr2820551030>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama I, Thihara J, Sakashita R, et al. HTLV-I associated Bronchopneumonopathy − a new clinical entity? Am Rev Respir Dis. 1988;137:46. [Google Scholar]
- 9.Sugimoto M, Nakashima H, Watanabe S, et al. T lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet. 1987;2:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto M, Nakashima H, Kawano O, Ando M, Araki S. Bronchoalveolar T lymphocytosis in HTLV-1-associated myelopathy. Chest. 1989;95:708. doi: 10.1378/chest.95.3.708a. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto M, Nakashima H, Matsumoto M, Uyama E, Ando M, Araki S. Pulmonary involvement in patients with HTLV-I-associated myelopathy: increased soluble IL-2 receptors in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1989;139:1329–35. doi: 10.1164/ajrccm/139.6.1329. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto M, Mita S, Tokunaga M, et al. Pulmonary involvement in human T cell lymphotropic virus type-I uveitis: T lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur Respir J. 1993;6:938–43. [PubMed] [Google Scholar]
- 13.Seki M, Higashiyama Y, Kadota J, et al. Elevated levels of soluble adhesion molecules in sera and BAL fluid of individuals infected with human T cell lymphotropic virus type 1. Chest. 2000;118:1754–61. doi: 10.1378/chest.118.6.1754. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi U, Nishihara H, Miyasaka K. HTLV-1-associated bronchopneumonopathy (HAB): CT pathological correlation. Clin Radiol. 2001;56:664–6. doi: 10.1053/crad.2001.0677. [DOI] [PubMed] [Google Scholar]
- 15.Seki M, Higashiyama Y, Mizokami A, et al. Up-regulation of human T lymphotropic virus type 1 (HTLV-1) tax/rex mRNA in infected lung tissues. Clin Exp Immunol. 2000;120:488–98. doi: 10.1046/j.1365-2249.2000.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashiyama Y, Katamine S, Kohno S, et al. Expression of human T lymphotropic virus type 1 (HTLV-1) tax/rex gene in fresh bronchoalveolar lavage cells of HTLV-1-infected individuals. Clin Exp Immunol. 1994;96:193–201. doi: 10.1111/j.1365-2249.1994.tb06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazato A, Kawakami K, Iwakura Y, Saito A. Chemokine synthesis and cellular inflammatory changes in lungs of mice bearing p40tax of human T lymphotropic virus type 1. Clin Exp Immunol. 2000;120:113–24. doi: 10.1046/j.1365-2249.2000.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cushley MJ, Davison AG, Lewis RA, du Bois RM, et al. The diagnosis, assessment and treatment of diffuse parenchymal lung disease in adults. Thorax. 1999;54:S1–S30. doi: 10.1136/thx.54.suppl_1.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki T, Nakagawa M, Osame M. Human T lymphotropic virus type-I (HTLV-I) seroprevalence in Kagoshima Prefecture. Med J Kagoshima Univ. 1993;45:135–41. [Google Scholar]
- 21.Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–1. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 22.Matsuyama W, Kubota R, Hamasaki T, et al. Enhanced inhibition of lymphocyte activation by Mycobacterium avium complex in human T lymphotrophic virus type I carriers. Thorax. 2001;56:394–7. doi: 10.1136/thorax.56.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki M, Kadota JI, Higashiyama Y, et al. Elevated levels of beta-chemokines in bronchoalveolar lavage fluid (BALF) of individuals infected with human T lymphotropic virus type-1 (HTLV-1) Clin Exp Immunol. 1999;118:417–22. doi: 10.1046/j.1365-2249.1999.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington WJ, Jr, Ucar A, Gill P, et al. Clinical spectrum of HTLV-I in south Florida. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:466–73. doi: 10.1097/00042560-199504120-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990;3:1096–101. [PubMed] [Google Scholar]
- 26.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–60. [PubMed] [Google Scholar]
- 27.Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992;7:120–5. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- 28.Buisson AC, Zahm JM, Polette M, et al. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol. 1996;166:413–26. doi: 10.1002/(SICI)1097-4652(199602)166:2<413::AID-JCP20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-2 stimulates fibroblast proliferation via a cAMP-dependent mechanism. J Biol Chem. 1995;270:13453–9. doi: 10.1074/jbc.270.22.13453. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1-2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest. 1998;78:687–98. [PubMed] [Google Scholar]
- 31.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 32.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 33.Loetscher M, Gerber B, Loetscher P, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keane MP, Arenberg DA, Lynch JP, III, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–43. [PubMed] [Google Scholar]
- 35.Tager A, Luster A, Kradin R. T cell chemokines interferon-inducible protein-10 and monokine induced by interferon-gamma are upregulated in bleomycin-induced lung injury. Chest. 1999;116:90S. [PubMed] [Google Scholar]
- 36.Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation-induced pulmonary fibrosis: examination of chemokine and chemokine receptor families. Radiat Res. 2002;157:256–65. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Agostini C, Facco M, Siviero M, et al. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T cell alveolitis. Am J Respir Crit Care Med. 2000;162:1466–73. doi: 10.1164/ajrccm.162.4.2003130. [DOI] [PubMed] [Google Scholar]
- 38.Ward SG, Westwick J. Chemokines: understanding their role in T lymphocyte biology. Biochem J. 1998;333(3):457–70. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattass CR, King LB, Luster AD, Ashwell JD. Constitutive expression of interferon gamma-inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179:1373–8. doi: 10.1084/jem.179.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N, Suzuki Y, Nishio K, et al. Roles of ICAM-1 for abnormal leukocyte recruitment in the microcirculation of bleomycin-induced fibrotic lung injury. Am J Respir Crit Care Med. 2000;161:1681–8. doi: 10.1164/ajrccm.161.5.9907104. [DOI] [PubMed] [Google Scholar]
- 41.Owen SM, Rudolph DL, Dezzutti CS, et al. Transcriptional activation of the intercellular adhesion molecule 1 (CD54) gene by human T lymphotropic virus types I and II Tax is mediated through a palindromic response element. AIDS Res Hum Retroviruses. 1997;13:1429–37. doi: 10.1089/aid.1997.13.1429. [DOI] [PubMed] [Google Scholar]
- 42.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 43.Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J. 1999;14:376–82. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- 44.Iyonaga K, Takeya M, Saita N, et al. Monocyte chemoattractant protein-1 in idiopathic pulmonary fibrosis and other interstitial lung diseases. Hum Pathol. 1994;25:455–63. doi: 10.1016/0046-8177(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 45.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 1992;89:5371–5. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glynn PC, Henney EM, Hall IP. Peripheral blood neutrophils are hyperresponsive to IL-8 and Gro-alpha in cryptogenic fibrosing alveolitis. Eur Respir J. 2001;18:522–9. doi: 10.1183/09031936.01.00057901. [DOI] [PubMed] [Google Scholar]
- 47.Mori N, Mukaida N, Ballard DW, Matsushima K, Yamamoto N. Human T cell leukemia virus type I Tax transactivates human interleukin 8 gene through acting concurrently on AP-1 and nuclear factor-kappaB-like sites. Cancer Res. 1998;58:3993–4000. [PubMed] [Google Scholar]
- 48.Carre PC, Mortenson RL, King TE, Jr, Noble PW, Sable CL, Riches DW. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest. 1991;88:1802–10. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]