Abstract
Severe strongyloidiasis has often been reported to occur in some patients infected with both Strongyloides stercoralis (S. stercoralis) and human T-cell leukaemia virus type 1 (HTLV-1); however, there are few useful predictive markers for the risk of development of strongyloidiasis in these patients. To search for such predictive markers, we examined peripheral blood and stool samples of individuals infected with both S. stercoralis and HTLV-1 in Okinawa, Japan, an area in which both of these are endemic. The HTLV-1 proviral load and antibody titre were examined in relation to the S. stercoralis load as measured by the direct faecal smear method in patients infected with both S. stercoralis and HTLV-1. The Epstein-Barr virus (EBV)-associated nuclear antigen (EBNA) antibody titre was also measured in these patients in order to examine the relationship between host immunity and HTLV-1 proviral load or antibody titre. The direct faecal smear-positive group showed both a higher HTLV-1 proviral load and HTLV-1 antibody titre than the -negative group (P < 0·05). In contrast, inverse correlations of these parameters with the EBNA antibody titre were observed, especially for proviral load (ρ = −0·387, P < 0·05). These results suggest that HTLV-1 proviral load and antibody titre influence the S. stercoralis load via disturbance of the host immunity, and that proviral load would be an especially useful predictive marker of the risk of development of strongyloidiasis in patients infected with both S. stercoralis and HTLV-1.
Keywords: HTLV-1, strongyloidiasis, S. stercoralis load, HTLV-1 proviral load
INTRODUCTION
Human T-cell leukaemia virus type 1 (HTLV-1) is a human retrovirus aetiologically associated with adult T-cell leukaemia (ATL) [1,2] and with chronic inflammatory disorders such as tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) [3,4] and HTLV-1 uveitis (HU) [5,6]. HTLV-1 is a persistent virus, currently infecting 10–20 million people worldwide, most of whom remain healthy.
Strongyloides stercoralis is a common intestinal parasitic nematode that can undergo its life cycle and proliferate within its host. Strongyloidiasis is a chronic, usually asymptomatic, gastrointestinal infection that is mostly found in healthy individuals. However, strongyloidiasis is apparently an opportunistic infection, and in immunocompromised hosts or patients on immunosuppressive therapy, systemic migration of larvae provokes dissemination of the infection and a serious illness due to the unique autoinfective life cycle of this nematode [7,8].
Strongyloidiasis is relatively common in tropical and subtropical areas, while HTLV-1 carriers have a unique geographical distribution in the world [9]. In areas where S. stercoralis and HTLV-1 are both endemic, such as the West Indies and Okinawa, Japan [10,11], patients infected with both S. stercoralis and HTLV-1 are found. Severe strongyloidiasis, with symptoms including meningitis and pneumonia, has been reported to occur in some of these patients [12–14]. Impairment of host immunity has been reported in HTLV-1 carriers [15–17] and suspected of being a cause of severe strongyloidiasis in these patients [13,18]. However, there are also many asymptomatic patients infected with both S. stercoralis and HTLV-1. These findings suggest that immunological differences among individual patients infected with both S. stercoralis and HTLV-1 may account for the differing severity of strongyloidiasis. To identify such differences, these patients should be monitored and evaluated for their risk of development of strongyloidiasis. However, stool examinations are not suitable for quantitative analysis of the S. stercoralis load [19], and neither IgE antibody titre nor eosinophil count in peripheral blood shows a significant correlation with S. stercoralis load in patients infected with both S. stercoralis and HTLV-1 [unpublished observations]. There was a trend toward severe strongyloidiasis in the group with monoclonal integration of HTLV-1 proviral DNA analysed by the Southern blotting procedure, however, this was not statistically significant [20]. Therefore, more useful predictive markers of strongyloidiasis are needed in patients infected with both S. stercoralis and HTLV-1.
The purpose of this study was to identify useful markers for predicting an individual's risk of developing strongyloidiasis in patients infected with both S. stercoralis and HTLV-1. Our findings demonstrated that the magnitude of HTLV-1 proviral load and anti-HTLV-1 antibody titre may be related to the development of strongyloidiasis via disturbance of the immunity, and that proviral load may be an especially useful predictive marker.
MATERIALS AND METHODS
Study population
Patients who were infected with S. stercaralis in Okinawa, Japan, and who received health examinations from 1993 to 1998, were included in the present study. They consisted of 31 HTLV-1-positive (18 males and 13 females) and 11 HTLV-1-negative (7 males and 4 females) patients. The ages (mean ± SD) were 53·6 ± 12·3 (HTLV-1-positives) and 57·1 ± 8·34 (HTLV-1-negatives), respectively. Informed consent for the study was obtained from each participant. Protocols involving human subjects were approved by our local Ethical Committee.
Diagnosis of S. stercoralis and the determination of S. stercoralis load
Patients in this study were diagnosed with S. stercoralis by the triplicate agar plate faecal culture method [21], which can detect more than 95% of positive cases [19]. Triplicate examination of direct faecal smears, which is less effective than the agar plate faecal culture method [19], was also performed at the same time. Patients who were negative in all three examinations in the direct faecal smear tests were assumed to have ‘light’S. stercoralis infection; all other cases were assumed to have ‘heavy’ infection.
Determination of antibody to HTLV-1
Individuals seropositive for HTLV-1 were identified by the particle agglutination test (PA test) (Serodia HTLV-1; Fujirebio, Tokyo, Japan) and also by an indirect immunofluorescence assay [22]. Titration of anti-HTLV-1 antibody was performed by the PA test (Serodia HTLV-1; Fujirebio).
HTLV-1 proviral load in the peripheral blood mononuclear cells
Semi-quantitative polymerase chain reaction (PCR) amplification of the gag region sequence for measuring the HTLV-1 proviral load in peripheral blood mononuclear cells (PBMC) has been described elsewhere [23]. Briefly, heparinized blood was collected and the PBMC were separated by density gradient centrifugation using Lymphoprep (Nicomed, Oslo, Norway). High-molecular-weight genomic DNA of the PBMC was extracted by proteinase K digestion and phenol/chloroform extraction. Using 0·5 µg of genomic DNA samples as templates, the number of HTLV-1 proviral copies was determined by semiquantitative PCR amplification of the gag region of the provirus.
Determination of antibody to Epstein-Barr virus (EBV)-associated nuclear antigen
Antibody to Epstein-Barr virus-associated nuclear antigen (EBNA) were detected by the anticomplement immunofluorescence technique [24]. Raji cells were used as a source of EBNA antigen. Fluorescein (FITC)-conjugated rabbit immunoglobulin to human complement (C3) (DAKO, Glostrup, Denmark) was also used. The titre of antibody was expressed as the reciprocal of the maximum dilution of serum (2-fold serial dilutions from the initial 1 : 10 dilution) that gave detectable fluorescence.
Statistical analysis
The Mann–Whitney U-test and the Fisher's exact probability test were used to analyse the statistical significance of differences. Spearman's rank correlation was used to assess the association between different variables.
RESULTS
Comparison of the results of the direct faecal smear method between HTLV-1-positive and -negative group
First we compared the results of direct faecal smears between the HTLV-1-positive and -negative groups to determine the influence of HTLV-1 infection on the S. stercoralis infection. The number of S. stercoralis-positive direct faecal smears was 19 out of 31 in HTLV-1-positive S. stercoralis patients (61·3%), whereas the number of S. stercoralis-positive direct faecal smears was 2 out of 11 in HTLV-1-negative S. stercoralis patients (18·2%). The positive rate of the direct faecal smear method in the HTLV-1-positive group was significantly higher than that in the -negative group (P < 0·05, by Fisher's exact probability test) (Table 1). Some involvement of HTLV-1 infection in increasing the positive rate of the direct faecal smear method was therefore suspected; however, variations among patients were also observed in the HTLV-1-positive group.
Table 1.
Comparison of the results of the direct faecal smear method between HTLV-1-positive and -negative patients with S. stercoralis
| Direct faecal smear | ||
|---|---|---|
| Negative | Positive | |
| HTLV-1 | ||
| Negative (n = 11) | 9 | 2 |
| Positive (n = 31) | 12 | 19 |
P < 0·05, by Fisher's exact probability test.
Comparison of HTLV-1 proviral load and antibody titre between direct faecal smear-negative and -positive groups
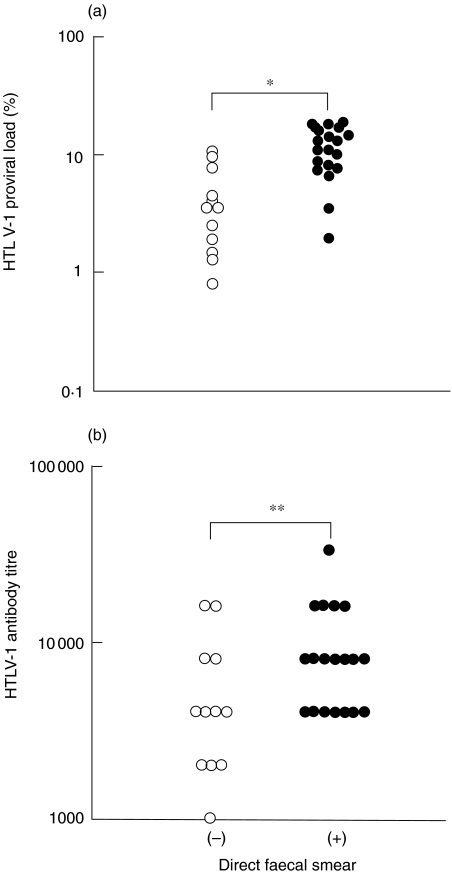
Because varying results of the direct faecal smear method were observed among HTLV-1 carriers, the influence of activity of HTLV-1 infection on the results of the direct faecal smear method was examined. Because the HTLV-1 proviral load has been suggested to reflect the activity of HTLV-1 infection [25–29], the HTLV-1 proviral load was compared between the direct faecal smear-negative and -positive groups. The HTLV-1 proviral load in the direct faecal smear-negative group was 3·5% (median) with a range of 9·7%, whereas the proviral load in the -positive group was 11·0% (median) with a range of 17·0%. The difference in the proviral load between these two groups was significant (P < 0·01, by Mann–Whitney U-test) (Fig. 1a). The results are shown plotted on a semilog scale in Fig. 1a. The HTLV-1 antibody titre, examined by the PA test, was also compared between the direct faecal smear-negative and -positive groups, because HTLV-1 antibody titre has been reported to correlate with HTLV-1 proviral load [30]. The HTLV-1 antibody titre in the direct faecal smear-negative group was 4,096 (median) with a range of 15,360, whereas the antibody titre in the -positive group was 8,192 (median) with a range of 28,672. The difference in the HTLV-1 antibody titre between these two groups was significant (P < 0·05, by Mann–Whitney U-test) (Fig. 1b). These results indicate that the activity of HTLV-1 infection influenced the results of the direct faecal smear method in patients infected with both S. stercoralis and HTLV-1.
Fig. 1.
HTLV-1 proviral load and antibody titre were compared between the direct faecal smear-negative (○) (n = 12) and –positive (•) (n = 19) groups. (a) HTLV-1 proviral load in the direct faecal smear-positive group was higher than that in the -negative group (direct faecal smear-negative group, median 3·5%, range 9·7%; direct faecal smear-positive group, median 11·0%, range 17·0%). (b) HTLV-1 antibody titre in the direct faecal smear-positive group was higher than that in the -negative group (direct faecal smear-negative group, median 4,096, range 15,360; direct faecal smear-positive group, median 8,192, range 28,672). (*P < 0·01, **P < 0·05, by Mann–Whitney U-test)
Correlation between HTLV-1 proviral load and antibody titre
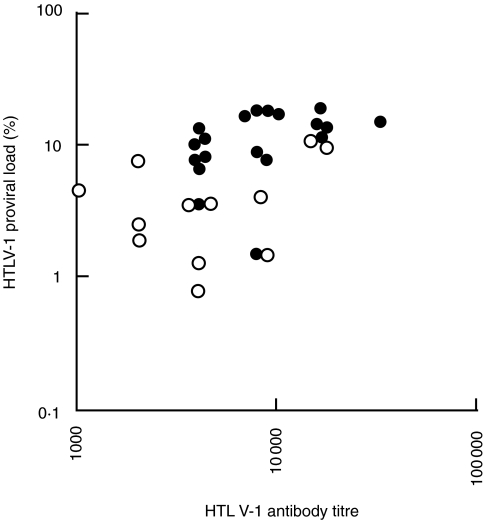
To examine the relationship between the results of the direct faecal smear method and both HTLV-1 proviral load and antibody titre, the correlation between HTLV-1 proviral load and antibody titre was examined in the patients infected with both S. stercoralis and HTLV-1. There was a tendency for the direct faecal smear positive-group to have both higher proviral load and antibody titre. A significant correlation (ρ = + 0·566, P < 0·01, by Spearman's rank correlation) was observed between HTLV-1 proviral load and antibody titre (Fig. 2).
Fig. 2.
Correlation between HTLV-1 proviral load and antibody titre in patients infected with both S. stercoralis and HTLV-1. The direct faecal smear-positive group (•) had both higher HTLV-1 proviral load and antibody titre than the -negative group (○). A correlation between HTLV-1 proviral load and antibody titre was observed in these patients infected with both S. stercoralis and HTLV-1 (ρ = +0·566, P < 0·01, by Spearman's rank correlation).
Comparison between EBNA antibody titre and HTLV-1 proviral load or HTLV-1-antibody titre
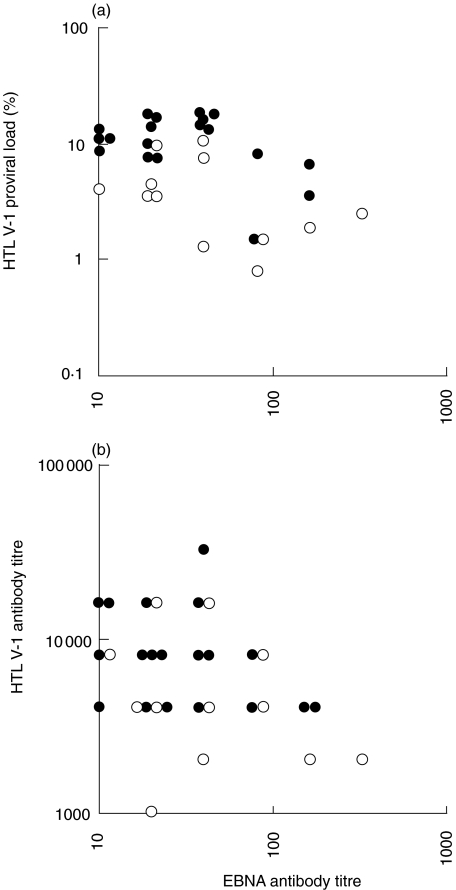
To examine the relationships between immune response level and HTLV-1 proviral load or antibody titre in patients also infected with S. stercoralis, the EBNA antibody titre, which has been reported to reflect the immune status [31–34], was examined. An inverse correlation was observed between the HTLV-1 proviral load and EBNA antibody titre (ρ = −0·387, P < 0·05, by Spearman's rank correlation) (Fig. 3a). Therefore, the increase of HTLV-1 proviral load was suspected to be related to impaired immunity, and almost all direct faecal smear-positive patients were also categorized into both the groups of higher HTLV-1 proviral load and lower EBNA antibody titre. Although HTLV-1 antibody titre and EBNA antibody titre also tended to be inversely correlated, the correlation was not significant (ρ =−0·340, P < 0·1, by Spearman's rank correlation) (Fig. 3b). These results indicate that the increase of the HTLV-1 proviral load was especially related to the lowering of the immune status, perhaps resulting in the increase of the S. stercoralis load.
Fig. 3.
Relationship between EBNA antibody titre and HTLV-1 proviral load or antibody titre. ○ direct faecal smear-negative patients; • direct faecal smear-positive patients. (a) An inverse correlation was observed between the HTLV-1 proviral load and EBNA antibody titre (ρ = −0·387, P < 0·05, by Spearman's rank correlation). The direct faecal smear-positive group had a higher HTLV-1 proviral load and a lower EBNA antibody titre than the -negative group. (b) An inverse correlation tended to be present between the HTLV-1 antibody titre and EBNA antibody titre, however, it was not significant (ρ = −0·340, P < 0·1, by Spearman's rank correlation).
DISCUSSION
Severe strongyloidiasis is often seen in patients infected with both S. stercoralis and HTLV-1; however, patients with such dual infections are thought to have various immunological differences. Therefore, markers useful for predicting the development of strongyloidiasis are needed for evaluating these patients. In the present study, we found that the magnitude of the HTLV-1 proviral load was related to the rate of detection of S. stercoralis in the direct faecal smear test via the impairment of the immunity in the patients infected with both S. stercoralis and HTLV-1.
Impairment of host immunity has already been reported in HTLV-1 carriers [15–17]. The influence of impaired immunity due to HTLV-1 infection on the results of stool examinations have also been reported in patients infected with both S. stercoralis and HTLV-1 [35]. Our results support these previous observations and demonstrated that S. stercoralis was more frequently detected in the HTLV-1-positive group than -negative group. However, there were also direct faecal smear-negative patients in the HTLV-1-positive group. Therefore, it was suspected that the immunological level varied among the HTLV-1 positive-patients.
Because strongyloidiasis is thought to be an opportunistic infection [7,8], patients with impaired immunity are suspected of having an increased S. stercoralis load, and the larvae of S. stercoralis should be easily found in their stools by the direct faecal smear method. In fact, increased S. stercoralis load or dissemination of S. stercoralis has been reported in patients with impaired immunity [13,36]. Therefore, direct faecal smear-positive patients were assumed to have heavier infection of S. stercoralis than the smear-negative patients. If the S. stercoralis load increased in such patients, autoinfection of S. stercoralis would be activated and the possibility of severe strongyloidiasis would increase. Based on these assumptions, patients infected with both S. stercoralis and HTLV-1 were divided into two groups: direct faecal smear-negative and –positive groups, as an indicator of S. stercoralis load in this study.
A significant increase of HTLV-1 proviral load, which has been suggested to reflect the activity of HTLV-1 infection [25–29], and of HTLV-1 antibody titre in the direct faecal smear-positive group suggested that increased HTLV-1 proviral load and antibody titre were related to increased S. stercoralis load. In ATL patients, whose HTLV-1 proviral load is increased [2, 23, 25], the S. stercoralis load also increased and disseminated strongyloidiasis was reported [37–39], in accord with our findings. The HTLV-1 antibody titre was suspected to have a similar relationship with S. stercoralis load as the HTLV-1 proviral load, because the direct faecal smear-positive group had both higher HTLV-1 proviral load and antibody titre. HTLV-1 antibody titre was reported to correlate with HTLV-1 proviral load [30], and our results support this notion also in patients infected with both S. stercoralis and HTLV-1.
A significant inverse correlation between HTLV-1 proviral load and EBNA antibody titre showed that increased HTLV-1 proviral load reflected impairment of host immunity, because a decrease of EBNA antibody titre has been demonstrated to reflect decreased immunity [31–34]. The finding that the direct faecal smear-positive group had a higher HTLV-1 proviral load and a lower EBNA antibody titre suggested that the increase of HTLV-1 proviral load was related to the increase of S. stercoralis load via the impairment of immunity. This impairment may have caused failure of the usual immune mechanisms against S. stercoralis, resulting in the increase of S. stercoralis load. The HTLV-1 antibody titre tended to be related to the EBNA antibody titre as was the proviral load; however, this relationship was not significant. The reason for the difference of the results between the HTLV-1 proviral load and antibody titre remains unclear. The fact that one is a continuous variable (HTLV-1 proviral load) and the other is a discrete variate (HTLV-1 antibody titre) might be one of the reasons.
Cases of severe strongyloidiasis were not included in this study. However, a severe case of strongyloidiasis in an HTLV-1 carrier whom we have experienced [40] has been shown to have increased HTLV-1 proviral load, and the faecal smears were positive. This case further support our findings in the present study. In previous studies, increased HTLV-1 proviral load has been suggested to be related to the activity of HTLV-1 infection [25–29] and disease activity [41], suggesting the possibility of a relationship of the proviral load with the host's immunity. Therefore, we propose that HTLV-1 proviral load is one of the useful predictive markers for the risk of the development of strongyloidiasis. HTLV-1 antibody titre could also be used as such a marker; however, as an indicator of S. stercoralis load or the host's immunity, the proviral load is more reliable and useful for quantitative analysis.
In conclusion, the magnitude of HTLV-1 proviral load and antibody titre reflected the increase of rate of detection of S. stercoralis in the direct faecal smear test via the impairment of the host's immunity. Especially, HTLV-1 proviral load would be a useful predictive marker for the risk of development of strongyloidiasis in patients infected with both S. stercoralis and HTLV-1.
Acknowledgments
The authors thank all patients for participating in the study and all of our colleagues of the Department of Parasitology, School of Medicine, University of the Ryukyus and Izumizaki Hospital for their co-operation.
REFERENCES
- 1.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retro-virus from cell lines of human adult T cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–5. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–7. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. Antibodies to human T lymphotropic virus type-I in patients with tropic spastic paraparesis. Lancet. 1985;2:407–10. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 4.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I-associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki M, Yamaguchi K, Takatsuki K, Watanabe T, Mon S, Tajima K. HTLV-I and uveitis. Lancet. 1992;339:1110. doi: 10.1016/0140-6736(92)90699-4. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki M, Watanabe T, Yamaguchi K, et al. An uveitis associated with human T-lymphotropic virus type-I (HTLV-I). seroepidemiological, clinical and virological studies. J Infect Dis. 1992;166:943–4. doi: 10.1093/infdis/166.4.943. [DOI] [PubMed] [Google Scholar]
- 7.Grove DI. Human Strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 8.Heyworth MF. Parasitic diseases in immunocompromised hosts. Criptosporidiosis, isosporiasis and strongyloidiasis. Gastroenterol Clin North Am. 1996;25:691–707. doi: 10.1016/s0889-8553(05)70269-7. [DOI] [PubMed] [Google Scholar]
- 9.Tajima K, Cartier L. Epidemiological features of HTLV-1 and adult T cell leukemia. Intervirol. 1995;38:238–46. doi: 10.1159/000150438. [DOI] [PubMed] [Google Scholar]
- 10.Plumelle Y, Pascaline N, Nguyen D, Panelatti G, Jouannelle A, Jouault H, Imbert M. Adult T-cell leukemia lymphoma. A clinico-pathologic study of twenty-six patients from Martinique. Hematol Pathol. 1993;7:251–62. [PubMed] [Google Scholar]
- 11.Satoh M, Tsukidate S, Fujita K, Yamamoto K. Strongyloidiasis influences the elevation of adult T-cell leukemia-associated antigen antibody titer. Int Arch Allergy Appl Immunol. 1991;96:95–6. doi: 10.1159/000235541. [DOI] [PubMed] [Google Scholar]
- 12.Furuya N, Shimoji K, Nakamura H, et al. A case report of meningitis and sepsis due to Enterococcus faecium complicated with strongyloidiasis. Kansenshogaku Zasshi. 1989;63:1344–9. doi: 10.11150/kansenshogakuzasshi1970.63.1344. [DOI] [PubMed] [Google Scholar]
- 13.Newton RC, Limpuangthip P, Greenberg S, Gam A, Neva FA. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. Am J Med. 1992;92:202–8. doi: 10.1016/0002-9343(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 14.Adedayo AO, Grell GAC, Bellot P. Case study: Fatal strongyloidiasis associated with human T-cell lymphotropic virus type 1infection. Am J Trop Med Hyg. 2001;65:650–1. doi: 10.4269/ajtmh.2001.65.650. [DOI] [PubMed] [Google Scholar]
- 15.Hinuma Y, Imai J. Epstein-Barr virus-specific antibodies in patients with adult T-cell leukemia (ATL) and healthy ATL virus-carriers. Int J Cancer. 1983;31:197–200. doi: 10.1002/ijc.2910310210. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi J, Kishihara Y, Yoshimura E, Furusho N, Yamaji K, Kawakami Y, Murakami H, Kashiwagi S. Correlation between human T cell lymphotropic virus type-1 and Strongyloides stercoralis infections and serum immunoglobulin E responses in residents of Okinawa. Japan Am J Trop Med Hyg. 1997;56:71–5. doi: 10.4269/ajtmh.1997.56.71. [DOI] [PubMed] [Google Scholar]
- 17.Porto AF, Filho JO, Neva FA, Orge G, Alcantara L, Gam A, Carvalho EM. Influence of human T cell lymphotropic virus type-1 infection on serologic and skin tests for strongyloidiasis. Am J Trop Med Hyg. 2001;65:610–3. doi: 10.4269/ajtmh.2001.65.610. [DOI] [PubMed] [Google Scholar]
- 18.Marsh BJ. Infectious complications of T cell leukemia/lymphoma virus type 1 infection. Clin Infect Dis. 1996;23:138–45. doi: 10.1093/clinids/23.1.138. [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–50. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 20.Nakada K, Yamaguchi K, Furugen S, et al. Monoclonal integration of HTLV-1 proviral DNA in patients with strongyloidiasis. Int J Cancer. 1987;40:145–8. doi: 10.1002/ijc.2910400203. [DOI] [PubMed] [Google Scholar]
- 21.Arakaki T, Hasegawa H, Asato R, Ikeshiro T, Kinjo F, Saito A, Iwanaga M. A new method to detect Strongyloides stercoralis from human stool. Jpn J Trop Med Hyg. 1988;16:11–7. [Google Scholar]
- 22.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I. Adult T-cell leukemia. Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–80. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono A, Mochizuki M, Yamaguchi K, Miyata N, Watanabe T. Increased number of circulatiing HTLV-1 infected cells in peripheral blood mononuclear cells of HTLV-1 uveitis patients: a quantitative polymerase chain reaction study. Br J Ophthalmol. 1995;79:270–6. doi: 10.1136/bjo.79.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reedman B, Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated compliment-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973;11:499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Kiyokawa T, Nakada K, et al. Polyclonal integration of HTLV-1 proviral DNA in lymphocytes from HTLV-1 seropositive individuals: an intermediate state between the healthy carrier state and smouldering ATL. Br J Haematol. 1988;68:169–74. doi: 10.1111/j.1365-2141.1988.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 26.Takatsuki K, Yamaguchi K, Watanabe T, Mochizuki M, Kiyokawa T, Mori S, Miyata N. Adult T-cell leukemia and HTLV-1 related diseases. In: Takatsuki K, Hinuma Y, Yoshida M, editors. Gann Monograph on Cancer Research. Tokyo: Kitagawa T; 1992. pp. 1–15. [Google Scholar]
- 27.Etoh K, Tamiya S, Yamaguchi K, et al. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 1997;57:4862–7. [PubMed] [Google Scholar]
- 28.Gabet AS, Mortreux F, Talarmin A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–60. doi: 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 29.Yamano Y, Nagai M, Brennan M, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara S, Okayama A, Stuver S, et al. Association of HTLV-1 antibody profile of asymptomatic carriers with proviral DNA levels of peripheral blood mononuclear cells. J Acquir Immune Defic Syndr. 1994;7:199–203. [PubMed] [Google Scholar]
- 31.Henle W, Henle G. Epstein-Barr virus–specific serology in immunologically compromised individuals. Cancer Res. 1981;41:4222–5. [PubMed] [Google Scholar]
- 32.Nagafuchi S, Fujisaki T, Ohshima K, et al. Immunodeficiency and carcinogenesis in patients with chronic active Epstein-Barr virus infection. Kansenshogaku Zasshi. 1997;71:56–64. doi: 10.11150/kansenshogakuzasshi1970.71.56. [DOI] [PubMed] [Google Scholar]
- 33.Bruu AL, Hjetland R, Holter E, et al. Evaluation of 12 commercial tests for detection of Epstein-Barr virus-specific and heterophile antibodies. Clin Diagn Lab Immunol. 2000;7:451–6. doi: 10.1128/cdli.7.3.451-456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osullivan CE, Peng R, Cole KS, Montelaro RC, Sturgeon T, Jenson HB, Ling PD. Epstein-Barr virus and human immunodeficiency virus serological responses and viral burdens in HIV-infected patients treated with HAART. J Med Virol. 2002;67:320–6. doi: 10.1002/jmv.10080. [DOI] [PubMed] [Google Scholar]
- 35.Arakaki T, Asato R, Ikeshiro T, Sakiyama K, Iwanaga M. Is. the prevalence of HTLV-1 infection higher in Strongyloides carriers than in non-carriers? Trop Med Parasitol. 1992;43:199–200. [PubMed] [Google Scholar]
- 36.Lessnau KD, Can S, Talavera W. Disseminated Strongyloides stercoralis in human immunodeficiency virus-infected patients. Treatment failure and a review of the literature. Chest. 1993;104:119–22. doi: 10.1378/chest.104.1.119. [DOI] [PubMed] [Google Scholar]
- 37.Patey O, Gessain A, Breuil J, et al. Seven years of recurrent severe strongyloidiasis in an HTLV-I infected man who developed adult T-cell leukaemia. AIDS. 1992;6:575–9. doi: 10.1097/00002030-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Maekawa I, Kawamura T, Miyake T. Chronic adult T-cell leukemia (ATL) complicating disseminated strongyloidiasis. Rinsho Ketsueki. 1988;29:64–7. [PubMed] [Google Scholar]
- 39.Udaka M, Maehara N, Tamaki K, et al. A case of Pneumocystis carinii pneumonia with hyperinfection of Strongyloides stercoralis complicated with smoldering adult T-cell leukemia. Kansenshogaku Zasshi. 1990;64:630–5. doi: 10.11150/kansenshogakuzasshi1970.64.630. [DOI] [PubMed] [Google Scholar]
- 40.Ishida H, Inoue T, Nishioka Y, Maruno K, Satoh M, Mishima Y, Furuse H. A case of biliary strongyloidiasis. J Jpn Soc Clin Surg. 1991;52:2151–5. [Google Scholar]
- 41.Ono A, Ikeda E, Mochizuki M, et al. Provirus load in patients with human T-cell leukemia virus type 1 uveitis correlates with precedent Graves’ disease and disease activities. Jpn J Cancer Res. 1998;89:608–14. doi: 10.1111/j.1349-7006.1998.tb03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]