Abstract
Mannan-binding lectin (MBL) triggers complement activation upon binding to microbial surfaces. MBL deficiency has been associated with increased susceptibility to severe bacterial infections. We hypothesized that MBL deficiency may predispose children to Shiga toxin-producing Escherichia coli (STEC) O157:H7 infections and the associated haemolytic uraemic syndrome (HUS). We compared circulating levels of MBL among children with uncomplicated O157:H7 haemorrhagic colitis (HC), patients with O157:H7 HUS, normal and diseases control groups. Circulating MBL concentrations on admission were as follows: 3·22 ± 2·43 µg/ml among normal controls (n = 23); 2·90 ± 2·44 µg/ml in patients with rotavirus enteritis (n = 10); 2·78 ± 1·65 µg/ml in children with HC due to non-STEC bacterial pathogen (n = 15); 2·67 ± 2·44 µg/ml in patients with uncomplicated O157:H7 HC (n = 27); 2·80 ± 2·97 µg/ml in children with O157:H7 HUS (n = 15); 6·70 ± 4·49 µg/ml in patients with chronic renal failure unrelated to O157:H7 infection (n = 6). Higher MBL levels were found in patients with chronic renal failure compared to O157:H7 HC (P < 0·047). However, MBL concentrations <0·5 µg/ml, which have been associated with MBL deficiency in relation to increased susceptibility to infections, were noted at comparable rates between the different groups (P = NS). Our data does not support that MBL deficiency may predispose to O157:H7 infections nor than the development of diarrhoea associated HUS.
Keywords: haemolytic uraemic syndrome, E. coli O157, mannan-binding lectin, complement, enzymed-linked immunosorbent assay, child
INTRODUCTION
Haemolytic uraemic syndrome (HUS) is defined by a triad of microangiopathic haemolytic anaemia, thrombocytopenia and acute renal failure [1]. Familial HUS has been associated with congenital deficiency of factor H, a regulatory protein of the alternative pathway of complement activation [2,3]. Alternatively, HUS most frequently occurs after a diarrhoeal prodrome (D+ HUS) due to Shiga toxin-producing Escherichia coli (STEC) [1]. The pathogenesis of D+ HUS is unclear but involves pathogen virulence factors other than Shigatoxin [1].
Mannan-binding lectin (MBL) is a C-type lectin which binds to terminal sugars, as expressed on the surface of microorganisms [4]. Upon binding, MBL mediates elimination of a wide array of bacteria, yeasts, viruses and protozoa through activation of the complement system or direct interaction with phagocyte receptors [5–7]. MBL deficiency arises from single point mutations in exon 1 of the coding region and/or in the promoter [6,8]. Only 3 mutations affect the coding region at codons 52, 54 and 57 and are referred to as the D, B and C variants, respectively. Markedly decreased MBL serum concentrations are found in heterozygous individuals whereas the protein is absent in homozygotes and compound heterozygotes [8]. MBL deficiency may be the most common immunodeficiency. For instance, the B variant is present in 26% of Caucasians [6]. These individuals have been shown to be at an increased risk of developing serious infectious diseases [9,10].
There is no data on the role of MBL in children with E. coli O157:H7 infections and D+ HUS. We hypothesized that MBL deficiency may predispose to E. coli O157:H7 infections and the development of HUS. We compared circulating MBL concentrations among: normal controls; disease controls with enteritis due to rotavirus gastroenteritis; disease controls with haemorrhagic colitis (HC) due to non STEC pathogens; children with uncomplicated HC due to E. coli O157:H7; children with O157:H7 associated HUS; and children with chronic renal failure unrelated to O157:H7 infection.
MATERIALS AND METHODS
Clinical data
Children, aged less than 18 years, who presented with HUS at Sainte-Justine Hospital, between 1 April 1996, and 1 March 2002, were eligible for the study. Enteritis was defined as the acute onset of diarrhoea (>1 day) with or without abdominal pain. HC was defined as enteritis with grossly bloody stools noted prior to medical consultation. HUS was defined [11] as a prodrome of enteritis or HC with the following ensuing criteria: thrombocytopenia (<150 000 × 109/l); microangiopathic haemolytic anaemia (haemoglobin below the third percentile for age and sex) with fragmented red cells on blood smear; acute renal failure (serum creatinine: >62 µmol/l if aged <5 years; >88 µmol/l if aged 6–12 years; >88 µmol/l (females) or > 102µmol/l (males) if aged >12 years) [12].
The normal control group was composed of 23 children who had an elective surgery for inguinal hernia or strabismus; these patients were matched for age and sex to those with O157:H7 HC. There were 10 children with enteritis due to rotavirus and 15 patients with HC due to non STEC pathogens: Salmonella (n = 4), Yersinia (n = 1), Campylobacter (n = 6), no pathogen identified (n = 4). We enrolled 27 patients with HC due to E. coli O157:H7 and 15 children with O157:H7 associated HUS. Finally, a disease control group for renal insufficiency was composed of 6 patients on chronic peritoneal ambulatory dialysis for renal failure unrelated to O157:H7 infection, including: nephrosis (n = 1), renal dysplasia (n = 3), and reflux nephropathy (n = 2). This group presented no signs of upper respiratory tract infections, had normal temperature, as well as negative urine and dialysate cultures before blood sample collection.
Age, sex, results of stool cultures, and the date of onset of enteritis and HUS were noted. Among patients presenting with HUS, blood samples were obtained on admission, before starting peritoneal dialysis if needed [13].
Laboratory data
Sorbitol-negative colonies grown on MacConkey-sorbitol agar were subcultured onto blood agar and screened for serotype O157 by slide agglutination (Difco, Detroit, MI, USA). Colonies agglutinating with the antiserum were identified as E. coli by standard biochemical reactions. Identification of pathogen was available within 24–48 h of sample collection. Evidence of rotavirus enteritis was obtained by enzyme-linked immunosorbent assay (ELISA) (Pathfinder®, Bio-Rod, Redmond, WA, USA).
Blood samples were collected, allowed to clot and centrifuged at 3000 g for 10 min at 4°C. Specimens were aliquoted and stored at −80°C until assayed. Serum MBL levels were determined using a commercial ELISA kit according to the manufacturer's instructions (AntibodyShop, Copenhagen, Denmark). The detection limit of the assays was 0·005 µg/ml.
Ethics
Written informed consent was obtained from the parents of all children. The study was approved by the Ethics Committee of Sainte-Justine Hospital.
Statistics
Descriptive statistics are presented as mean ± standard deviation for data with a normal distribution; median and range were used otherwise. The Student t-test was used to compare continuous data with a normal distribution and the Mann–Whitney U-test if the distribution was abnormal. The Kruskal–Wallis test (α = 0·05) was used to compare age, leucocytes, Δ time and MBL concentrations between the following independent groups: (i) normal controls; (ii) disease controls due to rotavirus gastroenteritis; (iii) disease controls with HC due to non STEC pathogens; (iv) children with uncomplicated O157:H7 HC; (v) children with O157:H7 associated HUS; and (vi) children with chronic renal failure unrelated to O157:H7 infection. Orthogonal, 2 × 2 comparisons were then performed using the test of Dunn (α = 0·0033). Due to the small sample size in group (vi), results were confirmed with the non parametric Mann–Whitney U-test. All statistical tests were two-sided. In order to control for Δ time, children in group (iv) and (v) were also compared using the Mann–Whitney U-test after matching for the time interval between the onset of enteric symptoms and the day of blood sample collection. The proportions of significantly decreased circulating MBL concentrations (<0·5 µg/ml) were compared between groups using a contingency table. Finally, a linear regression analysis between MBL concentrations and age was also performed.
RESULTS
Evidence of E. coli O157:H7 in stools was present in 93% of children with HUS. Moreover, 30% of patients within the HUS group required peritoneal dialysis. Children with HUS had abnormal serum creatinine level (197 ± 162 µmol/l), haemoglobin level (6.0 ± 1.5 g/dl), and platelet count (51 600 ± 28 873 × 109/l). The mean creatinine level in the disease control group for renal failure was: 506 ± 328 µmol/l. The clinical characteristics of 96 children included among the different groups are presented in Table 1. The sex distribution was comparable between groups. Children with chronic renal failure were older than other patients (P < 0·0003). Moreover, compared to patients with uncomplicated O157:H7 HC, children with HUS were younger (P < 0·003), and presented a trend for a higher white blood cell count on admission (P < 0·06). The time interval between the onset of enteritis and that of blood sample collection was also longer in the HUS group (P < 0·001). The vast majority of patients were Caucasians: 87% (20/23) normal controls; (80%) 8/10 rotavirus; 87% (13/15) non O157 HC; 88% (24/27) O157 HC; 93% (14/15) O157 HUS; 83% (5/6) chronic renal failure. Groups were thus comparable with regard to ethnic origin.
Table 1.
Clinical characteristics of patients
| NC (23) | Rotavirus GE (10) | Non-O157 HC (15) | O157:H7 HC (27) | O157:H7 HUS (15) | CRF (6) | |
|---|---|---|---|---|---|---|
| Sex (M/F) | 14/9 | 3/7 | 5/10 | 12/15 | 6/9 | 3/3 |
| Age* (months) | 57.0 ± 39.0 | 21.0 ± 14.0¶ | 59.0 ± 44.0 | 68.0 ± 47.0 | 30.0 ± 15.0§ | 125.0 ± 51.0† |
| WBC** (×109/l) | ND | 10·6 ± 4·0 | 8·9 ± 2·7 | 15·2 ± 8·5 | 19·6 ± 8·4‡§§†† | ND |
| Δ Time** (days) | – | 2·9 ± 1·3 | 3·6 ± 2·7 | 5·3 ± 2·8 | 8·0 ± 9·4‡‡ | – |
GE, gastroenteritis; HC, haemorrhagic colitis; HUS, haemolytic uraemic syndrome; CRF, chronic renal failure; M/F, male/female; WBC, white blood cell count on admission; Δ Time, refers to the time interval between the day of blood sample collection and the onset of enteric symptoms; ND, not done.
P < 0·0001
P < 0·0007, *** P < 0·001; comparison of the six groups together.
P < 0·002, viral gastroenteritis versus O157:H7 HC;
P < 0·003, HUS versus O157:H7 HC;
P < 0·0003, CRF compared to any other group;
P < 0·003, HUS versus viral gastroenteritis;
P < 0·0002, HUS versus non-O157 HC;
P < 0·06, HUS versus O157:H7 HC;
P < 0·001, HUS versus any other group.
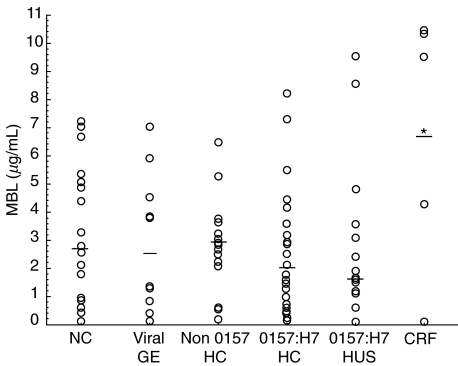
Circulating MBL concentrations are presented in Fig. 1. It shows that circulating MBL levels were comparable between groups, when all groups are considered together (P = 0·34). However, orthogonal comparisons (α = 0·0033) showed increased MBL levels among children with CRF (group vi) compared to HUS (P < 0·003), O157:H7 HC (P < 0·001), and non O157:H7 HC (P < 0·002). As there were few patients in group (vi), non parametric test analysis confirmed higher MBL levels during chronic renal failure compared to O157:H7 HC (P < 0·047) only. MBL concentrations were not correlated with age (P = NS). In order to control for time after the onset of enteric symptoms, patients with HUS (n = 11) were matched to children with uncomplicated O157:H7 HC (n = 11) at 5·6 ± 3·5 days versus 5·7 ± 3·6 days, respectively. MBL levels were also comparable between these subgroups (mean ± standard deviation, median (range)): 2·33 ± 2·5, 2·65 (0·12–8·10) µg/ml in HUS versus 3·05 ± 2·60, 1·64 (0·01–8·60) µg/ml in uncomplicated O157:H7 HC; (P = 0·4).
Fig. 1.
Circulating levels of Mannan-binding lectin (MBL) among children with O157:H7 infections and control groups. The figure shows comparable MBL concentrations between groups (P = NS), including: (i) (n = 23) normal controls (NC), 3·22 ± 2·43, median 2·74 µg/ml; (ii) (n = 10) patients with viral gastroenteritis (GE) due to rotavirus, 2·90 ± 2·44, median 2·55 µg/ml; (iii) (n = 15) patients with haemorrhagic colitis (HC) due to non O157:H7 pathogen, 2·78 ± 1·65, median 2·84 µg/ml; (iv) (n = 27) children with uncomplicated HC due to E. coli O157:H7 (O157:H7 HC), 2·67 ± 2·44, median 1·97 µg/ml; (v) (n = 15) patients with E. coli O157:H7 associated haemolytic uraemic syndrome (O157:H7 HUS), 2·80 ± 2·97, median 1·64 µg/ml; and (vi) (n = 6) children with chronic renal failure (CRF) unrelated to O157:H7 infection, 6·70 ± 4·49, median 7·10 µg/ml. *increased MBL levels were noted among children with CRF compared to HUS (P < 0·003), O157:H7 HC (P < 0·001), and non O157:H7 HC (P < 0·002).
Among the whole study population, 15% (14/96) of patients presented circulating MBL levels < 0·5 µg/ml. The proportions of such decreased concentrations were comparable between the different groups (P = NS): (i) normal controls 14% (3/22); (ii) patients with rotavirus gastroenteritis 20% (2/10); (iii) children with HC due to non-STEC bacterial pathogen 7% (1/15); (iv) patients with uncomplicated O157:H7 HC 18% (5/27); (v) children with O157:H7 HUS 13% (2/15); and (vi) patients with chronic renal failure unrelated to O157:H7 infection 16% (1/6).
DISCUSSION
We showed that children with uncomplicated O157:H7 HC and those with O157:H7 HUS presented similar circulating MBL concentrations. These were both comparable to levels found in the viral and the bacterial disease control groups and were also almost identical to those of normal controls. Age-dependent variation in serum MBL concentrations has been demonstrated, with gradually decreasing levels starting from infancy through adulthood [14]. As expected [1], children with O157:H7 associated HUS were younger than those with O157:H7 HC, but age in the latter group was comparable to that found in the bacterial and the normal control groups. Although patients with chronic renal failure were significantly older, they presented slightly higher median circulating MBL concentrations than children with O157:H7 HC. Moreover, they also presented two-fold higher serum creatinine levels than those with HUS. Similarly, Satomura et al. [15] recently reported abnormally increased serum MBL levels among adults with chronic renal failure, which further increased after haemodialysis. As MBL is an acute-phase protein [16], we cannot exclude that this may have modified our measurements.
However, we noted that MBL concentration <0·5 µg/ml, which has been associated with an increased susceptibility to infection [17], was found at the same frequency in children with uncomplicated O157:H7 HC and patients with HUS, and those from any control group. The cut-off value used to define MBL deficiency was based on a study conducted in the adult population [17]. However, a median circulating MBL concentration of 0·3 µg/ml was also noted in children with MBL deficiency, whereas validation of diagnosis was performed by genotype analysis [18]. Furthermore, circulating MBL levels did not increase in these patients during an acute phase reaction [18]. Therefore, one can assume MBL deficiency when levels are < 0·5 µg/ml, irrespective of the clinical condition. Accordingly, our data do not suggest that MBL deficiency may predispose to O157:H7 enteritis nor to the development of D+ HUS.
Familial HUS has been associated with congenital deficiency in factor H, a regulatory protein of the alternative pathway of complement activation which prevents formation of the C3 convertase [2,3]. Patients with factor H deficiency who show decreased serum C3 levels have a 16-fold increased risk of developing this form of HUS [2]. According to Warwicker, there are evidences for a role of the complement system in all forms of HUS [3]. Decreased serum C3 concentrations have been reported in children with D+ HUS and persistently low levels are associated with a poor prognosis [19–23]. Deposits of IgM, C1q, properdin and C3 have also been found within kidney sections of children with D+ HUS [24]. One weakness of our study comes from the fact that we were unable to simultaneously characterize the classical, the alternative and the mannan-binding lectin pathway of complement activation. Accordingly, our data on circulating MBL do not clarify what is the biological significance of complement abnormalities in D+ HUS. The lack of any association between circulating MBL levels and severity of illness in children with E. coli O157:H7 infections do not suggest that MBL deficiency may predispose to either O157:H7 enteritis or the development of D+ HUS. Nevertheless, based on our findings, we cannot rule out any interaction of MBL with the causative bacterium within the gastrointestinal tract.
Acknowledgments
We are indebted to Roxane Trahan and Diane Delorme for collecting patient data and blood samples. Dr Toledano is supported by the Fonds de Recherche en Santé du Québec and the Hospital for Sick Children Foundation. Dr Wagner is supported by the Foundation Charles Bruneau, Sainte-Justine Hospital.
REFERENCES
- 1.Proulx F, Seidman E, Karpman D. The pathogenesis of Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome. Pediatr Res. 2001;50:1–9. doi: 10.1203/00006450-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Ruggenenti P, Perna A, et al. Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: Role of factor H abnormalities. J Am Soc Nephrol. 1999;10:281–93. doi: 10.1681/ASN.V102281. [DOI] [PubMed] [Google Scholar]
- 3.Warwicker P, Goodship J, Goodship THJ. Factor H-US? Nephrol Dial Transplant. 1998;13:1921–3. doi: 10.1093/ndt/13.8.1921. [DOI] [PubMed] [Google Scholar]
- 4.Turner MW. Mannose-binding lectin. The pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 5.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz RAB. The collectins in innate immunity. Curr Opinion Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 6.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin. Targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–9. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 7.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin gene by polymerase chain reaction with sequence-specific primers. J Immunol Meth. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 9.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiol. 1998;199:327–39. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 10.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein with childhood infection in consecutive hospital series. Br Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe PC, Milner R, Orrbine E, Klassen TP, Mackenzie AM, Wells GA. A phase II randomized controlled trial of SYNSORB Pk for the prevention of hemolytic uremic syndrome in children with verotoxin producing E. coli gastroenteritis [abstract] Pediatr Res. 1997;41:283A. [Google Scholar]
- 12.Vaughan VC, III, Litt IF. Assessment of growth and development. In: Behrman RE, editor. Nelson Textbook of Pediatrics. Philadelphia: W.B. Saunders; 1992. pp. 32–43. [Google Scholar]
- 13.Brady HR, Brenner BM, Clarkson MR, Lieberthal W. Acute renal failure. In: Brenner BM, editor. The Kidney. Philadelphia: W.B. Saunders; 2000. pp. 1201–62. [Google Scholar]
- 14.Aittoniemi J, Miettinen A, Laippala P, et al. Age-dependent variation in the serum concentration of mannan-binding protein. Acta Paediatr. 1996;85:906–9. doi: 10.1111/j.1651-2227.1996.tb14182.x. [DOI] [PubMed] [Google Scholar]
- 15.Satomura A, Endo M, Ohi H, Sudo S, Ohsawa I, Fujita T. Significant elevations in serum mannose-binding lectin levels in patients with chronic renal failure. Nephron. 2002;92:702–4. doi: 10.1159/000064089. [DOI] [PubMed] [Google Scholar]
- 16.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 18.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–8. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 19.Cameron JS, Vick R. Plasma-C3 in haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Lancet. 1973;2:975. doi: 10.1016/s0140-6736(73)92645-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan BS, Thomson PD, MacNab GM. Serum-complement levels in haemolytic uraemic syndrome. Lancet. 1973;2:1505–6. doi: 10.1016/s0140-6736(73)92782-7. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Miller K, Michael AF. Breakdown of C3 and factor B in hemolytic–uremic syndrome. J Laboratory Clin Med. 1977;89:845–50. [PubMed] [Google Scholar]
- 22.Monnens L, Molenaar PH, Lambert PH, Prosmanns W, van Munster P. The complement system in hemolytic–uremic syndrome. Clin Nephrol. 1980;4:168–71. [PubMed] [Google Scholar]
- 23.Robson WL, Leung AK, Fick GH, McKenna AI. Hypocomplementemia and leukocytosis in diarrhea-associated hemolytic uremic syndrome. Nephron. 1992;3:296–9. doi: 10.1159/000187063. [DOI] [PubMed] [Google Scholar]
- 24.Nolin L, O'Regan S, Pelletier M, Rivard GE, Mongeau JG, Robitaille P. Possible C1q bypass loop activation in the haemolytic uraemic syndrome. Clin Exp Immunol. 1979;35:107–11. [PMC free article] [PubMed] [Google Scholar]