Abstract
The aim of this study was to quantify the expression of CD38 on CD8+ T lymphocytes of patients with infectious mononucleosis (IM) caused by Epstein–Barr virus (EBV) and cytomegalovirus (CMV). CD38 quantification technique chosen for this study was based on the enumeration of CD38 antibody binding sites in comparison to the quantification standards rather than determining relative fluorescence, which is difficult to standardize. The study enrolled 19 patients with typical clinical and laboratory parameters compatible with EBV-induced IM as well as 10 patients with atypical clinical presentation of this disease. Furthermore, CD38 expression was analysed in a group of 13 patients with IM caused by CMV infection. CD38 quantification was performed within 6 days of the presentation of symptoms. All three groups of IM patients showed a statistically significant increase in the number of anti-CD38 antibody binding sites (which correspond to the number of CD38 molecules) on bright CD8+ T lymphocytes compared to healthy controls. The numbers of CD38 molecules expressed on CD8+ T lymphocytes did not differ significantly between IM patients with typical and atypical clinical presentation of the disease. Patients with CMV-induced IM had significantly lower numbers of CD38 molecules expressed on CD8+ T lymphocytes. Therefore, we conclude that CD38 quantification could be helpful in differential diagnostics of IM cases with atypical clinical presentation.
Keywords: CD38, EBV, quantitative flow cytometry
INTRODUCTION
CD38 is a 45-kDa type II transmembrane glycoprotein expressed by activated T lymphocytes, thymocytes, B lymphocytes and natural killer (NK) cells in the peripheral blood and, to a lesser extent, by platelets and erythrocytes [1].
It is a multi-functional ectoenzyme capable of catalysing multiple reactions (ADP-ribosylcyclase, NAD-glycohydrolase, cADP-ribosyl cyclase) and generating products involved in calcium signalling [2]. Furthermore, CD38 is an adhesion molecule. It is hypothesized that the interaction between CD38 and the corresponding counter-receptor CD31 mediates a weak adhesion between lymphocytes and endothelial cells during the process of lymphocyte homing [3]. Perhaps the most complex biological feature of CD38 is its role as a signalling molecule. It has been shown that CD38 delivers activation signals in T and B lymphocytes as well as NK cells and monocytes, resulting in cytokine synthesis and secretion as well as in activation of several secondary messengers. It has been hypothesized that CD38 interacts directly with T cell receptors (TCR) and B cell receptors (BCR) in delivering activation signals to the corresponding cells [4]. The role of CD38 as a regulator of T cell-mediated cytotoxicity has also been investigated.
Although some aspects of the biological role of this molecule are controversial, it has a firmly established role in the diagnostic of lymphoproliferative diseases (leukaemia phenotyping and classification) [5,6]. It has been shown that increased percentages of CD38+CD8+ T lymphocytes predict faster disease progression and more intensive depletion of CD4+ T lymphocytes in HIV-1 infected patients [7,8]. More recent studies investigated a possible role of CD38 as a marker of residual viral activity in HIV patients with undetectable viral load after highly active antiretroviral therapy (HAART) [9]. Elevated relative fluorescence intensity of CD38 on CD8+ T lymphocytes has also been proposed as a superior negative prognostic marker in HIV-disease [10].
The aim of this study was to quantify CD38 expression on bright CD8+ T lymphocytes in the acute phase of infectious mononucleosis (IM) caused by Epstein–Barr virus (EBV). The subpopulation of CD8+ T lymphocytes plays an important role in the controlling of acute EBV infection as well as the establishment and maintenance of viral latency (in B lymphocytes).
A number of studies investigated the phenotype of CD8+ T lymphocytes in IM more closely [11,12]. Callan et al. [13] have shown that the majority of EBV-specific T cells have an activated/memory phenotype and express HLA-DR, CD38, memory marker CD38, low levels of CD45RA and have decreased expression of homing receptor CD62L. Uda et al. [14] have also reported an unusual CD28 expression pattern on CD8+ T lymphocytes in IM patients due to the continuous spectrum of CD28 intensity.
Examples of HIV-1 infection and haematology have shown that measurement of the intensity of CD38 expression increases significantly the diagnostic value of this molecule [10,15–17]. Therefore, we have employed quantitative flow cytometry in the model of IM caused by EBV.
CD38 quantification technique chosen for this study is based on the enumeration of CD38 antibody binding sites in comparison to the quantification standards rather than determining relative fluorescence. To our knowledge, this is the first attempt to enumerate CD38 molecules on CD8+ T lymphocytes in EBV-caused IM.
We also tried to investigate the possible diagnostic use of CD38 enumeration on CD8+ T lymphocytes in IM.
The diagnosis of IM is based on clinical presentation and supportive laboratory, findings including an absolute lymphocytosis in which more than 10% of cells are atypical, and appropriate serological tests [18,19]. Differential diagnosis of IM usually includes acute infections with EBV, cytomegalovirus (CMV), toxoplasma, HIV-1/2, human herpesvirus 6 and hepatitis viruses. The combination of the clinical diagnosis, routine laboratory tests and serology data enables a reliable diagnosis in the majority of both adult and paediatric IM patients. However, atypical clinical and laboratory presentations of this disease can be misleading and result in inaccurate diagnosis and unnecessary investigations before the appropriate diagnostic concept has been established [20,21].
Examples of atypical clinical IM presentations include advanced age, very high fever (>40°C), absence of lymphadenopathy, maculopapular skin rash not related to antibiotic use, neurological complications (Guillain–Barré syndrome), autoimmune haemolytic anaemia, etc. Atypical laboratory findings in IM usually include very high lymphocytosis (>30 × 109/l WBC) and only a few ‘reactive’ lymphocytes in peripheral blood smears.
Therefore, we have compared the levels of CD38 expression in patients with typical versus atypical clinical and/or laboratory presentation of EBV-induced IM as well as in patients with CMV-induced IM. Possible diagnostic significance of our finding is discussed.
MATERIALS AND METHODS
Patients and controls
Peripheral blood (PB) samples were obtained from 42 immunocompetent adult patients with clinical symptoms or laboratory findings compatible with IM syndrome.
EBV-infected IM patients (n = 29) were divided into two groups based on the clinical and laboratory presentation of their disease. The median age within the group of EBV-infected IM patients with typical disease presentation (n = 19) was 22 (range 9) with male to female ratio 1 : 1·2. EBV-infected IM patients with atypical clinical and/or laboratory disease presentation (n = 10) had a median age of 31 (range 14) and a male to female ratio 1·5 : 1.
The criteria for atypical (clinical or laboratory) presentation of EBV-induced IM were: advanced age, long incubation period (more than 10 days), very high fever (>40°C), absence of lymphadenopathy, maculopapular skin rash not related to antibiotic use, neurological complications (Guillain–Barré syndrome), autoimmune haemolytic anaemia, very high lymphocytosis (>30 × 109/l WBC), only a few ‘reactive’ lymphocytes in the peripheral blood smears, etc. A group of patients with CMV-induced IM included 13 patients. The median age in this group was 29 (range 15) with male to female ratio 1 : 1·5.
The patients were diagnosed and monitored at the University Hospital for Infectious Diseases ‘Dr Fran Mihaljević’, Zagreb. The hospital's ethics committee approved the study. Informed consent was obtained from all patients and healthy controls.
A group of healthy controls included 35 adults without apparent immunological or haematological disorders. The study excluded children younger than 17 years of age as well as adults above 55 years of age (senescence of the immune system). Immunodeficient patients, autoimmune patients and pregnant women were also excluded from the study.
Serological and other routine laboratory testing
All serum samples were tested for IgM and IgG antibodies to viral capsid antigen (VCA), IgG antibodies to early antigen-diffuse [EA(D)] and EBV nuclear antigen (EBNA), respectively, and for IgM and IgG antibodies to CMV using an enzyme-linked immunosorbent assay (ELISA, DiaSorin, Saluggia, Italy) to determine acute infections. The method used for determination of specific IgM to EBV VCA and CMV was indirect IgM capture assay. Testing and interpretation of the results were performed according to the manufacturer's recommendation.
A current acute EBV (typical or atypical clinical presentation) and CMV infections were defined by the detection of specific IgM antibodies in the first serum sample, their subsequent decrease in the second serum sample and further increase in IgG antibodies specific for EB VCA, EA(D) and EBNA. The absence of anti-EBNA IgG antibodies in the first serum sample was also a criterion for the acute EBV infection.
Patients presenting with non-specific viral syndromes have also been assessed for HIV risk behaviours and, following written consent, tested appropriately.
Haematological analysis of PB, biochemical tests for liver enzyme activity as well as cytological analysis of PB smears were performed in all patients. Flow cytometry was performed by using an aliquot of PB drawn for haematological analysis.
Flow cytometry
PB immunophenotyping was performed by using a whole blood non-wash method on Multi-Q-Prep System with the ImmunoPrep Reagent System (Beckman Coulter, USA). The samples were analysed on Epics XL-MLC Flow cytometer (Beckman Coulter, USA) as recommended. Absolute counts of CD4+ T lymphocytes were performed directly on the cytometer by using Flow-Count Fluorospheres (Beckman Coulter, USA).
Three-colour flow cytometry was performed by using a panel of monoclonal antibodies specific for CD3, CD4, CD8, CD14, CD16, CD19, CD45, CD56, HLA-DR (Dako, Denmark) conjugated with FITC, PE or PerC5 with appropriate isotypic controls. Immunophenotyping was performed within 6 days from the onset of symptoms.
Quantitative flow cytometry (enumeration of CD38 molecules on CD8+ T lymphocytes)
CD38 expression on CD8+ T lymphocytes was performed using the CELLQUANT CD38/CD8-PE flow quantitative flow cytometry kit (Biocytex, Marseille, France). The number of CD38 antigenic sites on CD8+ T lymphocytes was determined by converting the CD38 fluorescence intensity into the corresponding number of sites per cell based on a calibrated bead standard curve. The calibrated suspension contains beads which are coated with increasing and accurately known quantities of mouse IgG. The procedure was performed according to the manufacturer's instructions.
Briefly, for each sample of PB mixed with anticoagulant K3EDTA, two tubes were prepared. Aliquots of PB (50 µl) were mixed with 25 µl of negative isotypic control (mouse IgG antibody) and anti-CD38 antibody, respectively. Calibrated bead suspension (50 µl) was also mixed with 25 µl of negative isotypic control (mouse IgG antibody). After incubation (10 min, room temperature), 25 µl of FITC-labelled antimouse IgG polyclonal antibody was added to all three tubes. Following incubation (10 min, room temperature), 25 µl of neutralization reagent was added to all tubes. After another incubation (10 min, room temperature), 10 µl of anti-CD8-PE antibody was added to the two tubes with PB. The red cell lysis and fixation in these two tubes was performed by using automated Multi-Q-Prep System with the ImmunoPrep Reagent System (Beckman Coulter, USA).
All three tubes were centrifuged at 300 g for 5 min, supernatants were discarded and the cells (or beads) were resuspended in 2 ml of washing buffer. The procedure was repeated and cells or beads were resuspended in 1 ml of washing buffer. The tubes were mixed well and incubated at 2–8°C for at least 30 min before analysis on the flow cytometer.
In order to stabilize the mean fluorescence intensity (MFI) values, we collected data for at least 2000 (bright) CD8+ T lymphocytes. The selected statistics for MFI was Mn (x).
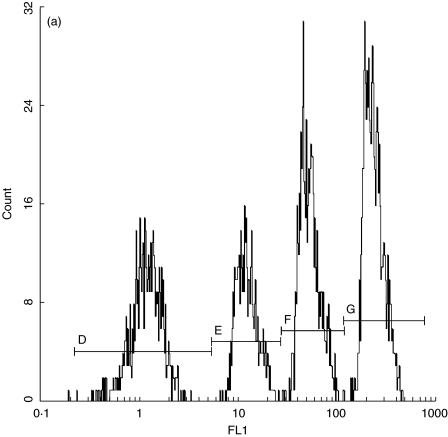
The compensation set-up was performed using calibration beads and negative isotypic control. Figure 1a shows an example of FL1 LOG histogram versus a calibrated bead solution count. The calibrator solution contains four distinct subpopulations of beads with different Mn (x) values. The calibration curve was constructed by plotting Mn (x) values for all four subpopulations of calibrator beads on the abscissa (x-axis) against their corresponding number of monoclonal antibody molecules (provided by the manufacturer) on the ordinate (y-axis).
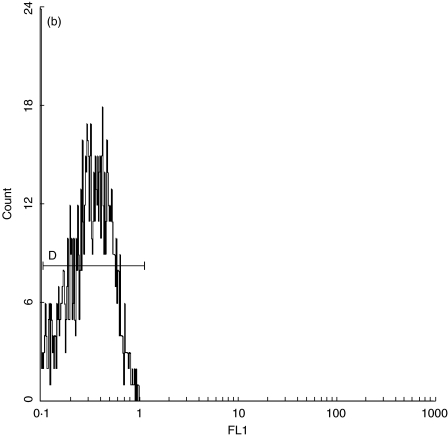
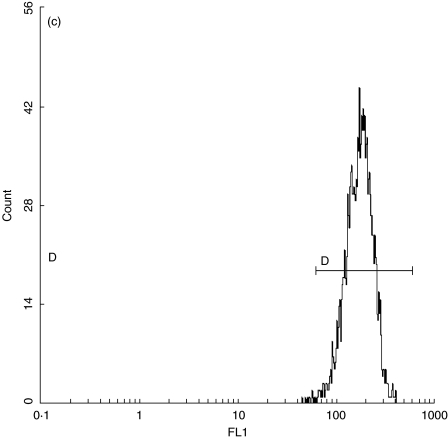
Fig. 1.
(a) Quantification standard contains four subpopulations of beads with known numbers of anti-CD38 antibody binding sites (CELLQUANT, Biocytex). (b) CD38 quantification: negative control. (c) Expression of CD38 on bright CD8+ T lymphocytes in a patient with EBV-induced IM.
By analysing FL1 log in the subopulation of bright CD8+ T lymphocytes in the negative control and unknown sample, we determined their corresponding Mn (x) values (Fig. 1b,c). By interpolating Mn (x) values of negative control and unknown sample on the calibration curve, the corresponding numbers of monoclonal antibodies were read directly from the curve. The number of specific anti-CD38 antibody binding sites was obtained by subtracting the negative control value from the value for unknown sample.
RESULTS
Clinical and laboratory findings in IM
The majority of IM patients in the group 1 (typical clinical presentation) exhibited usual signs and symptoms associated with this disease, including fever, lymphadenopathy, laryngitis, malaise, myalgias, arthalgias, diarrhoea, unproductive cough and splenomegaly. The complications of the clinical course included thrombocytopenic purpura (one patient) and myocarditis (one patient). The majority of patients had leucocytosis, lymphocytosis and increased percentages of atypical lymphocytes (mean 18%, range 16%) as well as abnormal liver function tests (almost all patients had increased lactate dehydrogenase (LDH) values). Most patients showed lymphadenopathy. Cytological examination of lymph nodes was performed in 15 patients and showed reactive hyperplasia consistent with IM. Patients with uncomplicated clinical courses were treated conservatively with supporting measures.
Atypical clinical and laboratory presentations of EBV-induced IM included: absence of lymphadenopathy (n = 1), maculopapular skin rash not related to antibiotic use (n = 1), autoimmune haemolytic anaemia (n = 1), very high lymphocytosis (>30 × 109/l WBC, n = 4) and only a few ‘reactive’ lymphocytes in the peripheral blood smears (n = 3).
The most pronounced clinical signs and symptoms in the CMV group were similar to those described for the previously mentioned EBV-infected patients. Biochemical and haematological findings were also similar to those in the EBV-infected patients. Complications described in the CMV group included pneumonitis (one patient) and thrombocytopenic purpura (one patient).
Lymphocyte subpopulations in IM
Table 1 shows percentages of PB lymphocyte subpopulations in patients with atypical and typical presentations of EBV- and CMV-induced IM compared to healthy controls (median, minimum and maximum values). The comparison of lymphocyte subpopulations in all three groups of IM patients with healthy controls revealed a similar pattern.
Table 1.
Peripheral blood lymphocyte subpopulations in patients with infectious mononucleosis (IM) caused by Epstein–Barr virus (EBV) or cytomegalovirus (CMV)
| Cellular subpopulation (%) median (minimum–maximum) | EBV-induced IM typical disease presentation | EBV-induced IM atypical disease presentation | CMV-induced infectious mononucleosis | Healthy controls |
|---|---|---|---|---|
| T lymphocytes | 88·2* | 86·8* | 80·3 | 75·2 |
| (84·5–92·5) | (79·4–91·6) | (66·2–90·1) | (68·9–88·2) | |
| B lymphocytes | 1·6* | 1·3* | 2·0 | 8·9 |
| (0·2–4·5) | (0·6–3·2) | (0·5–5·1) | (5·6–26·8) | |
| CD4+ | 13·3* | 11·5* | 23·1 | 46·5 |
| T lymphocytes | (6·9–28·9) | (7·5–26·8) | (10·2–30·5) | (35·5–63·4) |
| CD8+ T lymphocytes | 68·1* | 69·8* | 52·2 | 23·4 |
| (43·3–78·3) | (39·4–76·2) | (35·1–70·5) | (19·2–31·7) | |
| NK cells | 5·7 | 6·3 | 4·6 | 6·0 |
| (0·5–14·8) | (1·3–11·9) | (1·5–13·5) | (0·5–25·9) | |
| HLA-DR+ T lymphocytes | 53·9* | 30·9* | 36·8 | 5·6 |
| (13·7–81·4) | (15·6–75·1) | (12·1–54·6) | (1·0–9·2) |
The differences in all lymphocytes subpopulations (except NK cells) of both patient groups were significant compared to healthy controls (Mann–Whitney test, P < 0·05).
Statistically significant differences compared to patients with CMV-induced IM (Mann–Whitney test, P < 0·05).
All three groups of IM patients (atypical and typical EBV-infected and CMV-infected people) showed significantly increased percentages of T lymphocytes (P = 0·002 for both EBV groups, P = 0·01 for CMV group), CD8+ T lymphocytes (P = 0·001 for typical EBV and CMV groups, P= 0·0001 for atypical EBV group) and activated HLA-DR+ T lymphocytes compared to healthy controls (P < 0·0001 for EBV groups, P < 0·01 for CMV group).
Percentages of CD4+ T lymphocytes and B lymphocytes in both groups of IM patients were decreased significantly compared to healthy controls (P < 0·0001 for atypical and typical EBV groups, P = 0001 for CMV group).
Despite the reduced percentages, absolute counts of CD4+ T lymphocytes of all three IM groups were within referral values (median 744, range 1156 cells/ml). In the EBV group, typical patients had a median of 873 CD4+ T lymphocytes per µl (range 856 cells/ml) while atypical patients had a median of 743 CD4+ T lymphocytes per µl (range 756 cells/ml). CMV-infected patients had a median of 963 CD4+ T lymphocytes per µl (range 856 cells/ml).
NK cells were the only PB lymphocyte subpopulation within referral values in all three groups of IM patients.
Both groups of patients with EBV-induced IM showed significantly higher percentages of T lymphocytes (P < 0·001), CD8+ T lymphocytes (P = 0·02 for typical, P = 0·01 for atypical EBV-infected patients) and activated HLA-DR+ T lymphocytes (P = 0·02) compared to CMV-infected patients.
Both groups of EBV-infected IM patients had significantly lower percentages of B lymphocytes (P = 0·02 for typical, P= 0·001 for typical EBV group) and CD4+ T lymphocytes (P = 0·002 for both groups) than CMV-infected IM patients. The differences between absolute counts of CD4+ T lymphocytes and percentages of NK cells in three patient groups were not statistically significant.
Enumeration of CD38 molecules in IM
All three groups of IM patients (atypical and typical presentation of acute EBV infection and CMV-infected persons) showed significantly increased numbers of CD38 antigenic sites on bright CD8+ T lymphocytes (Table 2, Fig. 1c, P < 0·0001 for both EBV groups, P = 0·01 for CMV group).
Table 2.
Increased numbers of CD38 molecules on bright CD8+ T lymphocytes in infectious mononucleosis (IM) caused by Epstein–Barr virus and cytomegalovirus
| The number of anti-CD38 antibody binding sites (per CD8+ T lymphocyte) | |||
|---|---|---|---|
| Group | Median | Minimum | Maximum |
| EBV-induced IM typicaldisease presentation | 52 095* | 5403 | 87 109 |
| EBV-induced IM atypicaldisease presentation | 49 672* | 4902 | 86 395 |
| CMV-induced IM | 25 420 | 3252 | 37 262 |
| Healthy controls | 556 | 350 | 988 |
The difference in CD38 numbers in all patient groups was significant compared to healthy controls (Mann–Whitney test, P < 0·001).
Statistically significant differences compared to patients with CMV-induced IM (Mann–Whitney test, P < 0·001).
IM patients with CMV-induced IM had significantly smaller numbers of CD38 antigenic sites on CD8+ T lymphocytes compared to both groups of patients with EBV-induced IM (P < 0·001 for both groups).
The difference in the numbers of CD38 antigenic sites CD8+ T lymphocytes in patients with typical and atypical clinical and laboratory presentation of EBV-induced IM was not statistically significant.
Statistical methods
Statistical analysis was performed by using the statistica software package. Healthy controls and experimental groups were compared by the non-parametric Mann–Whitney U-test with P < 0·05 considered significant.
DISCUSSION
Acute EBV-induced IM is characterized by an expansion of CD8+ T lymphocytes, which are responsible for the control of virus replication and subsequent establishment of latent infection. A previous study has described increased numbers of CD38+CD8+ T lymphocytes in acute EBV infection [22].
However, diagnostic application of CD38 determination in other models (HIV-1/2 infection, haematological diseases) suggests strongly that quantification of CD38 expression increases the diagnostic value of this marker [15–17,23]. To our knowledge, this is the first attempt to determine the numbers of CD38 molecules expressed on bright CD8+ T lymphocytes in EBV- and CMV-induced IM and to compare it to healthy people.
Although no definite guidelines for the technology of choice in CD38 quantification are available, we have chosen to determine both MFI and numbers of anti-CD38 antibody binding sites (corresponding to the number of CD38 molecules) in comparison with standard calibration. The majority of CD38 quantification efforts (mainly in HIV-1 model) have been limited to MFI determination either without calibrators or with biological calibrators (cord blood, CD4+ T lymphocytes), which are difficult to standardize [10,24–26]. Experts have suggested that the conversion of fluorescence intensity to numbers of antibody binding sites can increase the diagnostic, prognostic or therapeutic value of quantitative flow cytometry [23,26].
The results of this study show that acute EBV and CMV infections increase the numbers of CD38 molecules expressed on CD8+ T lymphocytes of IM patients compared to healthy controls. We have also observed that acute EBV infection induced significantly higher levels of CD38 expression compared to CMV infection.
In the context of infectious diseases, CD38 is viewed as an activation marker. The expression of CD38 in HIV-disease is considered to be a result of the immune reaction to viral antigens. This view is supported by the parallel decrease in plasma viraemia and CD38 expression on CD8+ T lymphocytes. This molecule has also been investigated as a possible surrogate marker of low-level HIV-1 replication in patients with undetectable viral load in plasma as a warning signal for possible viral rebound. Therefore, we hypothesize that CD38 expression is partially a result of the immune reaction to EBV antigens.
However, some authors have proposed a model for CD38 as an ‘active player’ and not only as an activation marker in HIV-1 infection [17]. It is believed that, because of its enzymatic activity, CD38 functions as a rescue signal for nucleotide-starved virus-infected cells, inhibits binding of HIV-1 gp120 to CD4, etc. The possible roles of CD38 in EBV and CMV life cycles and the protection of infected cells from nucleotide deficiency and apoptosis remain to be seen.
In an attempt to investigate the possible diagnostic application of this technique, we have compared the numbers of CD38 molecules on CD8+ T lymphocytes in patients with typical and atypical clinical and laboratory presentation of EBV-induced IM. Our study showed identical levels of CD38 expression on CD8+ T lymphocytes in serologically confirmed cases of EBV-induced IM, irrespective of their clinical and laboratory presentation. The results of our study suggest that CD38 quantification could be helpful in diagnosing patients with atypical clinical and/or laboratory disease presentation.
The present study confirms our previous observation that acute EBV-induced IM changes the distribution and activation state of PB lymphocytes [27].
The pattern of these changes (immunophenotyping profile) includes decreased percentages of B and CD4+ T lymphocytes accompanied by an increase in total T and CD8+ T lymphocytes and activated HLA-DR+ T lymphocytes. Despite decreased percentages of CD4+ T lymphocytes, absolute counts of these cells remained within normal range. The comparison of lymphocyte subpopulations in the PB of patients with CMV-induced infectious mononucleosis with healthy controls showed a completely identical pattern of changes.
Our conclusion is that CD38 quantification as well as flow cytometric analysis of PB lymphocytes can be useful for the identification of patients with atypical clinical or laboratory presentation of EBV-induced infectious mononucleosis.
REFERENCES
- 1.Reinherz EL, Kung PC, Goldstein C, Levey RH, Schlowwman SF. Discrete stages of human intrathymic differentiation. analysis of normal thymocytes and leukemic lymphoblasts of T cell lineage. Proc Natl Acad Sci USA. 1980;77:1588–92. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthelier V, Tixier JM, Muller-Steffner H, Schuber F, Deterre P. Human CD38 is an authentic NAD (P) + glycohydrolase. Biochem J. 1998;330:1383–90. doi: 10.1042/bj3301383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deaglio S, Mallone R, Baj G, et al. CD38/CD31, a receptor/ligand system ruling adhesion and signalling in human leukocytes. Chem Immunol. 2000;75:99–102. [PubMed] [Google Scholar]
- 4.Morra M, Zubiaur M, Terhorst C, Sancho J, Malavasi F. CD38 is functionally dependent on the TCR/CD3 complex in human T cells. FASEB J. 1998;12:581–92. doi: 10.1096/fasebj.12.7.581. [DOI] [PubMed] [Google Scholar]
- 5.Morabito F, Mangiola M, Oliva B, et al. Peripheral blood CD38 expression predicts survival in B cell chronic lymphocytic leukemia. Leuk Res. 2001;25:927–32. doi: 10.1016/s0145-2126(01)00049-2. [DOI] [PubMed] [Google Scholar]
- 6.Mizoguchi C, Ushara S, Takatsu K. Interleukin-5 induces IgG1 isotype switch recombination in mouse CD38-activated sIgD-positive B lymphocytes. J Immunol. 1999;162:2812–9. [PubMed] [Google Scholar]
- 7.Mocroft A, Bofil M, Lipman M, et al. CD8+CD38+ lymphocyte percent: a useful immunologic marker for monitoring of HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:158–62. doi: 10.1097/00042560-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Burgisser P, Hammann C, Kaufman D, Battegay M, Rutschmann OT. Expression of CD28 and CD38 by CD8+ T lymphocytes in HIV-1 infection correlates with markers of disease severity and changes towards normalisation under treatment. The Swiss HIV Cohort Study. Clin Exp Immunol. 1999;115:458–63. doi: 10.1046/j.1365-2249.1999.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilling R, Kinloch S, Goh L-E, et al. Parallel decline of CD8+/CD38++ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS. 2002;16:589–96. doi: 10.1097/00002030-200203080-00010. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Hultin LE, Cumberland EG, et al. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino Y, Morishima T, Kimura H, Noshikawa K, Tsurumi T, Kuzushima K. Antigen-driven expansion and contraction of CD8+- activated T cells in primary EBV infection. J Immunol. 1999;163:5735–40. [PubMed] [Google Scholar]
- 12.Roos MT, van Lier RA, Hamann D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein–Barr and human immunodeficiency virus infections in humans. J Infect Dis. 2000;182:451–8. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 13.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during primary immune response to Epstein–Barr virus in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uda H, Mima T, Yamaguchi N, et al. Expansion of CD28-intermediate subset among CD8 T cells in patients with infectious mononucleosis. J Virol. 2002;76:6602–8. doi: 10.1128/JVI.76.13.6602-6608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniou-Fowler T, Dignum H, Taylor PR, et al. Quantification improves the prognostic value of CD38 expression in B cell chronic lymphocytic leukaemia. Br J Haematol. 2002;118:755–61. doi: 10.1046/j.1365-2141.2002.03673.x. [DOI] [PubMed] [Google Scholar]
- 16.Lenkei R, Bratt G, Holmberg V, Murhead K, Sandstrom R. Indicators of T cell activation: correlation between quantitative CD38 expression and soluble CD8 levels in asymptomatic HIV+ individuals and healthy controls. Cytometry. 1998;33:115–22. doi: 10.1002/(sici)1097-0320(19981001)33:2<115::aid-cyto5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T cell activation or an active player in virus/host interactions? AIDS. 2000;14:1979–89. doi: 10.1097/00002030-200006160-00004. [DOI] [PubMed] [Google Scholar]
- 18.Andersson J. An overview of Epstein–Barr virus: from discovery to future directions for treatment and prevention. Herpes. 2002;7:76–82. [PubMed] [Google Scholar]
- 19.Weller TH. Cytomegaloviruses: a historical perspective. Herpes. 2000;7:66–9. [PubMed] [Google Scholar]
- 20.Rea TD, Russo JE, Katon W, Ashley RL, Buchwald DS. Prospective study of the natural history of infectious mononucleosis caused by Epstein–Barr virus. J Am Board Fam Pract. 2001;14:234–42. [PubMed] [Google Scholar]
- 21.Bonnet F, Neau D, Viallard JF, et al. Clinical and laboratory findings of cytomegalovirus infection in 115 hospitalised non-immunocompromised adults. Ann Med Interne. 2001;152:227–35. [PubMed] [Google Scholar]
- 22.Lynne JE, Schmid I, Matud JL, et al. Major expansion of select CD8+ subsets in acute Epstein–Barr virus infection: comparison with chronic human immunodeficiency virus disease. J Infect Dis. 1998;177:1083–7. doi: 10.1086/517400. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz A, Fernandez-Repollet E. Quantitative flow cytometry. Clin Lab Med. 2001;21:743–61. [PubMed] [Google Scholar]
- 24.Hultin LE, Matud JL, Giorgi JC. Quantitation of CD38 activation antigen expression on CD8+ T cells in HIV-1 infection using CD4 expression on CD4+ T lymphocytes as a biological calibrator. Cytometry. 1998;33:123–32. doi: 10.1002/(sici)1097-0320(19981001)33:2<123::aid-cyto6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Gratama JW, D’Hautcourt JL, Mandy DF, et al. Flow cytometric quantitation of immunofluorescence intensity: problems and perspectives. European Working Group on Clinical Cell Analysis. Cytometry. 1998;33:166–78. doi: 10.1002/(sici)1097-0320(19981001)33:2<166::aid-cyto11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Janossy G, Bikoue A, Tilling RE, Reilly JT, Granger V, Bernett D. Stabilized cellular immunofluorescent analysis (SCIFA): a new concept for quantitative flow cytometry in routine immunohaematology. Cytometry. 1998;101(Suppl. 1):330. [Google Scholar]
- 27.Židovec S, Ćulig Z, Begovac J, Jeren T. Comparison of lymphocyte subpopulations in the peripheral blood of patients with infectious mononucleosis and human immunodeficiency virus infection: a preliminary report. J Clin Lab Immunol. 1998;50:63–9. [PubMed] [Google Scholar]