Abstract
Peripheral blood CD8+ T lymphocytes generally express the CD8 coreceptor as an αβ heterodimer. On these cells, the CD8β chain is present either at high (CD8βhigh) or low density (CD8βlow). CD8βhigh cells are CD28+, whereas CD8βlow cells are CD28+ or CD28–. Therefore, three subpopulations of CD8+ T cells can be described: (i) CD8βhighCD28+ (ii) CD8βlowCD28+, and (iii) CD8βlowCD28– cells. Phenotypic and functional characterization of these CD8+ T cell subsets revealed significant differences. CD8βhighCD28+ cells predominantly express CD45RA. In contrast, CD8βlowCD28+ cells frequently express CD45R0 and the activating NK receptor CD161. CD8βlowCD28– cells frequently revert to the CD45RA phenotype. In addition, these cells express CD16, CD56, CD94, and the killer-inhibitory receptors NKB1 and CD158a. Intracellular IL-2 was frequently detected in CD8βhighCD28+ cells and CD8βlowCD28+ cells, but not CD8βlowCD28– cells. CD8βlowCD28+ cells and CD8βlowCD28– cells frequently stained positive for IFN-γ. In addition, these cells contain intracellular perforin and granzyme A. Expression of Fas (CD95) as well as susceptibility to apoptosis is markedly increased in CD8βlowCD28+ and CD8βlowCD28– cells as compared to CD8βhighCD28+ cells. In vitro activation of peripheral blood lymphocytes triggered expansion of CD8βhighCD28+ cells as well as a development into CD8βlowCD28+ and CD8βlowCD28– cells. Similarly, activation of CD8βhighCD28+ cord blood cells resulted in the appearance of CD8βlowCD28+ and CD8βlowCD28– cells. These data suggest that CD8βhighCD28+ cells can differentiate into CD8βlowCD28+ and CD8βlowCD28– cells upon TCR stimulation. Therefore, the CD8β/CD28 subsets in peripheral blood may reflect distinct stages of post-thymic CD8+T cell development.
Keywords: T lymphocytes, co-stimulatory molecules, cytokines, cellular differentiation, cellular activation
INTRODUCTION
CD8+ T lymphocytes are cytotoxic cells relevant in defense against tumours and virally infected cells. CD8 represents a coreceptor binding to MHC class I and stabilizing the complex of TCR, MHC, and peptide. The CD8 molecule consists of two polypeptide chains expressed either as a homodimer (CD8αα) or as a heterodimer (CD8αβ) [1–3]. The CD8αβ heterodimer is 100 times more efficient as a coreceptor for the TCR than CD8αα[4–6]. CD8αα is expressed on the surface of NK cells and intraepithelial lymphocytes (IELs). In contrast, the majority of MHC class I-restricted TCRαβ+ T cells in peripheral blood coexpress CD8α and CD8β. Studies in T cell clones have demonstrated that both CD8αβ heterodimers and CD8αα homodimers can be expressed on the same cell [7]. In this study, we describe peripheral blood T cells that express CD8β in a low density (CD8βlow) suggesting that the inefficient CD8αα coreceptor is predominantly expressed. CD8βlow cells are characterized with respect to the coexpression of CD28, a receptor for the B7 costimulatory molecule. Based on CD8β/CD28 coexpression we define three distinct subsets of CD8+ T cells. Phenotypic and functional characteristics of these subsets are discussed on the background of previous data on CD8+CD28+ and CD8+CD28– T cells.
MATERIALS AND METHODS
Cells
PBL were obtained from blood donors aged 23–80 (median 45) years after informed consent. All donors were healthy, i.e. free of clinical signs of infection, malignancy or rheumatic disease. CBL were obtained from umbilical cords of random newborn children at the day of birth. CBL were collected in heparin and penicillin/streptomycin containing media. PBL and CBL were cultured in RPMI 1640 (Biochrom, Berlin, Germany) containing 10% fetal calf serum (PAA Laboratories, Cölbe, Germany), 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 U/ml IL-2. If indicated, 96 well flat bottom culture dishes were coated with unconjugated UCHT1 (CD3) MoAb (1 µg/well) for stimulation.
Antibodies and chemical reagents
For immunofluorescence studies the following mouse MoAbs were used: DJ130C (CD16, FITC-conjugated, obtained from DAKO, Hamburg, Germany), ALB11 (CD45RA, FITC, Immunotech, Hamburg, Germany), UCHL-1 (CD45R0, FITC, Immunotech), DX12 (CD161, FITC, Pharmingen, Hamburg, Germany), DX9 (NKB1, FITC, Pharmingen), NCAM16·2 (CD56, FITC, Becton Dickinson, Heidelberg, Germany), CD28·2 (CD28, APC, Pharmingen), 5F2 (CD8, unconjugated, Immunotech), DK25 (CD8α, FITC, Ortho Biotech, Neckargemünd, Germany), 2ST8·5H7 (CD8β, PE, Immunotech), UCHT1 (CD3, FITC, Pharmingen), 4S.B3 (IFN-γ, FITC, Pharmingen), MQ1–17H12 (IL-2, FITC, Pharmingen), HP-3E4 (CD158a, FITC, Pharmingen), HP-3D9 (CD94, FITC, Pharmingen), CH11 (CD95, FITC, Coulter Immunotech), BU20a (BrdU, FITC, Pharmingen), δG9 (perforin, FITC, Pharmingen), CB9 (granzyme A, FITC, Pharmingen). Matched isotype controls were obtained from Pharmingen. Annexin V-FITC was purchased from Pharmingen. Brefeldin A, PMA, ionomycin, and propidiumiodide (PI) were obtained from Sigma (Deisenhofen, Germany).
Flow cytometry and detection of intracellular antigens
Two, three, and four-colour immunostaining and flow cytometry were performed using standard techniques and equipment (FACSCalibur cytometer, Becton Dickinson) and are presented as histograms (lin/log) or dot plot diagrams (log/log). The mean fluorescent intensity (MFI) was used as an index of fluorochrome staining intensity. For the detection of intracellular cytokines, cells were stimulated with phorbolester (50 ng/ml PMA) and ionomycin (500 ng/ml) at 37°C and 5% CO2 for one hour. For another three hours, cells were incubated in PMA/ionomycin and 0·02 µg/ml Brefeldin A at 37°C and 5% CO2. Washed cells were then stained with CD8β (PE-conjugated) and CD28 (APC) MoAbs. Cells were washed again and fixed in 4% paraformaldehyde (15 min, 20°C). After three washing steps, cells were permeabilized with saponin and stained with IL-2 or IFN-γ MoAbs and subjected to cytometry. For the detection of intracellular perforin and granzyme A, fresh cells were stained for CD8β (PE) and CD28 (APC), fixed in 4% paraformaldehyde, permeabilized with saponin and stained with perforin or granzyme A antibodies or isotype controls (FITC).
BrdU incorporation assay
Cells were incubated with 100 µm BrdU for 2 h (37°C, 5% CO2), washed three times (washing buffer: 0·5% BSA in PBS), and subjected to immunostaining using CD8β (PE-conjugated) and CD28 MoAbs (APC). Cells were suspended in 0·5% ice-cold paraformaldehyde and kept on ice for 30 min. The suspension was protected from light and incubated at 20°C for another 20 min. Residual paraformaldehyde was removed in three washing steps and cells were slowly suspended in 70% ice-cold ethanol. Cells were then incubated at room temperature for 20 min. Washed cells were then incubated in DNase (50 U/ml in washing buffer) at 37°C for 30 min, washed three times, and suspended in 50 µl dilution buffer (0·5% Tween 20 and 0·5% BSA in PBS). 10 µl BrdU MoAb (FITC-conjugated) was added and incubated at room temperature for 20 min. Subsequent to washing, cells were analysed by flow cytometry.
Apoptosis assays
Freshly isolated PBL or cultured cells were washed and stained for CD8β and CD28 (see above). Subsequently, cells were suspended in 100 µl binding buffer (10 mm HEPES/NaOH pH 7·4, 140 mm NaCl, 2·5 mm CaCl2) and annexin V-FITC (5%, v/v, in binding buffer) was added. After 15 min, 100 µl binding buffer and 10 µl propidiumiodide were added and cells were analysed by flow cytometry. Cells stained by annexin, but not by PI were regarded as apoptotic.
Statistics
If not otherwise stated, results are reported as the median among the individuals studied, because not all variables passed Kolmogorov-Smirnoff normality tests. Differences between the described cell populations were tested using two-sided Wilcoxon matched pair signed rank tests. Asterisks indicate significance at P < 0·05 (*), P < 0·01 (**) and P < 0·001 (***). Correlation was assessed by nonparametric correlation and is reported by the Spearman correlation coefficient (r).
RESULTS
Definition of CD8+ T cell subsets in peripheral blood
The expression of the CD8 α and β chains was studied in peripheral blood lymphocytes (PBL) from healthy donors using three antihuman CD8 MoAbs. DK25 recognizes CD8α[8] and binds to human CD8α+ transfectants in the absence of CD8β (Potthast et al. personal communication). 2ST8.H5 recognizes an epitope of the CD8β molecule that depends upon the expression of both CD8α and CD8β[9]. This MoAb immunoprecipitates the CD8αβ complex from cell lines expressing the human α and β cDNA [9]. (3) 5F2 recognizes CD8β irrespective of its pairing partner. This MoAb immunoprecipitates CD8β from cell lines expressing the human β cDNA in the absence of the α cDNA [9,10].
The CD8α surface density was homogeneous on all CD3+CD8+ PBL (Fig. 1a). In contrast, CD8β was expressed either in high density (CD8βhigh) or in low density (CD8βlow; Fig. 1b). Double-staining for CD8α (DK25) and CD8β (5F2) demonstrated that the CD8β staining intensity was three to four fold higher on CD8βhigh cells (CD8β MFI 2·1·102) compared to CD8βlow cells (CD8β MFI 0·6·102). In contrast, the CD8α staining intensity was only slightly higher on CD8βhigh cells (CD8α MFI 3·9·103) as compared to CD8βlow cells (CD8α MFI 3·2·103). This indicates that excess CD8α is present in the form of αα homodimers on CD8βlow cells (Fig. 1c). CD8βlow cells were equally stained by MoAbs 5F2 and 2ST8.H5 indicating that CD8β is entirely present in αβ heterodimers on these cells (Fig. 1d). All CD8βlow cells were CD3 positive confirming their identity as T cells (Fig. 1e). CD3 negative PBL including NK cells did not express CD8β.
Fig. 1.
CD8 expression on peripheral blood lymphocytes from a representative healthy young donor. (a) CD8α on CD3+ gated PBL. (b) D8β on CD3+ gated PBL. CD8β is expressed either on a high level (CD8βhigh, marker 1) or on a low level (CD8βlow, marker 2). (c) –(f) PBL gated based on FSC/SSC. Percentages in respective quadrants and regions are indicated. (c) CD8βhigh cells (R1) and CD8βlow cells (R2) express comparable amounts of CD8α. (d) CD8β staining intensity is equal for MoAbs 2ST8·5H7 and 5F2 indicating that all CD8β is present in αβ heterodimers. (e) CD8βhigh cells (R1) and CD8βlow cells (R2) are CD3 positive. (f) Coexpression of CD8β and CD28 defines three populations: CD8βhighCD28+ (R1), CD8βlowCD28+ (R2), CD8βlowCD28– (R3). (g) Frequency of the three CD8β/CD28 populations in 49 healthy subjects aged 23–80 years (given as per cent of CD8β+ cells; horizontal lines indicate the median).
Double-staining for CD8β (2ST8.H5) and CD28 demonstrated that CD8βlow cells can be subdivided into CD28+ and CD28– cells (Fig. 1f). In contrast, CD8βhigh cells are uniformly positive for CD28. Therefore, three distinct subsets were defined: CD8βhighCD28+ CD8βlowCD28+ CD8βlowCD28–. CD8βlowCD28– cells showed an intermediate CD8β staining intensity (MFI 1·6·102) compared to CD8βhighCD28+ cells (MFI 6·6·102) and CD8βlowCD28– cells (MFI 1·0·102). The CD28 staining intensity was higher on CD8βlowCD28+ cells (MFI 3·1·102) compared to CD8βhighCD28+ cells (MFI 1·8·102). Cell size as indicated by FSC distribution was not different among the three populations. The frequency of cells falling into the three subsets was highly variable among blood donors (Fig. 1g). With increasing age there was a trend towards a decreased relative number of CD8βhigh CD28+ cells (r = −0·3) and, conversely, an increased number of CD8βlowCD28– cells (r = + 0·4). The relative number of CD8βlowCD28+ cells did not change with age (r = 0·0).
Characterization of CD8+ T cell subsets in peripheral blood
We asked whether differences in CD8β/CD28 expression would be associated with other characteristic markers of T cell function. Surface phenotyping of peripheral blood T lymphocytes from healthy donors revealed that the expression of CD8β/CD28 correlates with the expression of CD45 splice variants (Fig. 2a): CD8βhighCD28+ cells were almost completely CD45R0–CD45RA+. In contrast, CD8βlowCD28+ cells were CD45R0+CD45RA–, whereas the majority of CD8βlowCD28– cells developed into CD45R0–CD45RA+. A significant number of CD8βlowCD28– cells was found to coexpress the NK cell receptors CD56, CD94, CD16, CD158a, and NKB1 (Fig. 2d). CD8βlowCD28+ cells expressed less CD56 and CD94, but not CD16, NKB1, or CD158a. CD8βhighCD28+ cells were negative for these markers. The activating NK receptor CD161 was present in high density on CD8βlowCD28+ cells, and in low density on an subset of CD8βlowCD28– cells (Fig. 2i). Expression of NK receptors on CD8βlowCD28+ and CD8βlowCD28– cells was not confined to a distinct subset of cells that were positive for all of these markers. For example, staining for CD8β, CD16, and CD158a revealed that approximately 10% of CD8βlow T cells were positive for CD16 or CD158a. Of these, approximately 20% expressed CD158a, 40% CD16, and 40% both markers (data not shown). As the density of NK receptors on CD8+ T cells was often low compared to CD3-negative NK cells, PI counter-staining was used to exclude unspecific staining of dead cells (data not shown).
Fig. 2.
Expression of characteristic cell surface molecules on CD8βhighCD28+ (red), CD8βlowCD28+ (green), and CD8βlowCD28– cells (blue). Scatter plots are representative examples from the same healthy donor. Percentages in respective quadrants and regions are indicated. (a)Gated for PBL based on FSC/SSC. (b)– (i)Gated for CD8β+ cells. Bar charts under the plots show the median percentage ± SD of cells expressing the indicated marker in a number of individuals (n). Significant differences are indicated by asterisks.
The PMA/ionomycin-induced cytokine production was markedly different among the three CD8+ T cell subsets (Fig. 3a). Positive staining for IL-2 was primarily found in CD8βlowCD28+ cells. CD8βhighCD28+ cells stained less frequently positive for IL-2, whereas CD8βlowCD28– cells were almost exclusively IL-2 negative. In contrast, CD8βlowCD28– cells most frequently stained positive for IFN-γ. Only few CD8βhighCD28+ cells contained IFN-γ. Expression of cytotoxic effector molecules was studied using intracellular immunostaining of freshly isolated PBL (Fig. 3c). CD8βlowCD28+ cells and CD8βlowCD28– cells frequently expressed intracellular perforin and granzyme A. Intensity of perforin expression as indicated by the MFI was markedly higher in CD8βlowCD28– cells as compared to perforin-positive CD8βlowCD28+ cells (data not shown). The apoptosis receptor Fas (CD95) was expressed more often on CD8βlowCD28+ and CD8βlowCD28– cells than on CD8βhighCD28+ cells (Fig. 3e). However, the Fas surface density as indicated by the MFI was lower on Fas positive CD8βlowCD28– cells compared to Fas positive CD8βhighCD28+ and CD8βlowCD28+ cells (Fig. 3f). Apoptotic cells as detected by annexin V staining of freshly isolated PBL were rarely detected in the CD8βhighCD28+ subset but were more frequent in the CD8βlowCD28+ and CD8βlowCD28– subsets (Fig. 3g). With increasing age the relative number of annexin V-positive cells increased within the CD8βhighCD28+ subset (r = + 0·1), but decreased within the CD8βlowCD28+ (r = −0·2) and CD8βlowCD28– (r = −0·2) subsets.
Fig. 3.
Intracellular cytokines, effector molecules and susceptibility to apoptosis of peripheral blood CD8+ T cell subsets. Bars show the median percentage ± SD of cells staining positive for the indicated molecule in a number of individuals, with the exception of panel (f). Significant differences are indicated by asterisks. (a,b) Intracellular IL-2 and IFN-γ after PMA/ionomycin activation of PBL. (c,d) Intracellular perforin and granzyme A in freshly isolated PBL. (e)Fas expression by anti-CD95 staining of freshly isolated PBL. (f)Mean fluorescence intensity (MFI) of cells staining CD95 positive. (g)Spontaneous apoptosis by annexin V-FITC and PI staining of freshly isolated PBL.
CD8+ T cell subsets after stimulation of peripheral blood lymphocytes in vitro
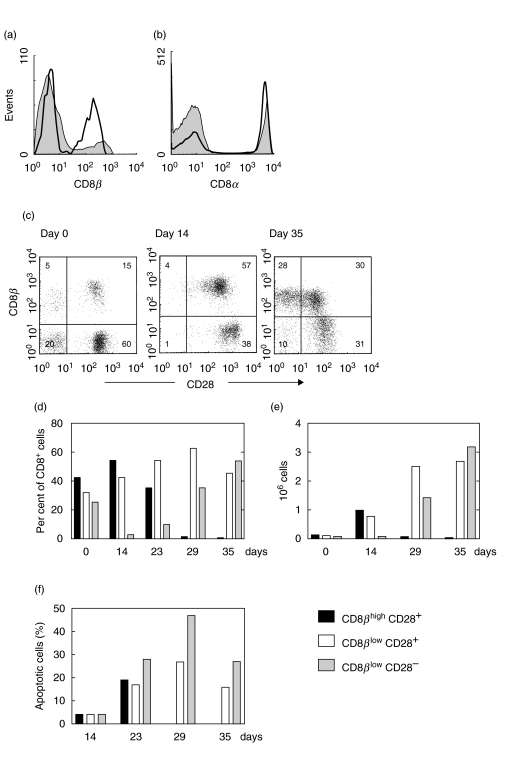
PBL obtained from healthy blood donors were stimulated with immobilized CD3 MoAb and IL-2 for one week. CD8+ T cell subsets were then analysed weekly during further culture in the presence of IL-2. A typical experiment is shown in Fig. 4. CD3 stimulation was observed to decrease the expression of CD8β, corresponding to a change from CD8βhigh to CD8βlow (Fig. 4a). The MFI of CD8β expression on CD28+ cells decreased from 3·7·102 (day 0) to 1·5·102 (day 35), whereas the MFI of CD8α expression remained constant at 5·2·103 (Fig. 4b). CD8β/CD28 double-staining revealed that the decrease of CD8β is followed by down-regulation of CD28 (Fig. 4c). Interestingly, CD28-negative cells present on day 0 almost completely disappeared on day 14. They were replaced by CD8βlow cells that diminished CD28 expression during further culture. Figure 4d summarizes these changes in the expression of CD8β and CD28 by showing the relative frequency of CD8βhighCD28+, CD8βlowCD28+ and CD8βlowCD28– cells. Three distinct events were reproducibly observed (i) an initial decrease of the CD8βlowCD28– subset (days 0–14) (ii) a shift from the CD8βhighCD28+ to the CD8βlowCD28+ subset (day 0–29), and (iii) a shift from the CD8βlowCD28+ to CD8βlowCD28– subset (day 29–35). These changes were accompanied by a marked proliferation of CD8+ lymphocytes as shown in Fig. 4e. In fact, the absolute number of CD8βhighCD28+ cells increased significantly (∼ 8fold from day 0–14) before these cells shifted to CD8βlowCD28+. Likewise, CD8βlowCD28+ cells continued to expand (∼3fold from day 14–29) before the shift to CD8βlowCD28– occurred. Annexin V staining demonstrated that apoptotic cells are most frequent in the CD8βlowCD28– subset (27–50% between days 23 and 35) compared to the CD8βhighCD28+ and CD8βlowCD28+ subsets (17–27%; Fig. 4f).
Fig. 4.
Anti-CD3 stimulation of PBL. (a) CD8β and (b) CD8α expression before (▪) and four weeks after stimulation (□). (c) Coexpression of CD8β and CD28 before (day 0) and after stimulation (days 14 and 35). (d,e) Changes in the number of CD8βhighCD28+, CD8βlowCD28+, and CD8βlowCD28– cells given in per cent and absolute numbers. Representative for five independent experiments. (f) Frequency of apoptotic cells determined by annexin V-FITC and PI staining.
CD8+ T cell subsets after stimulation of cord blood lymphocytes (CBL)
In contrast to CD8+ T cells from adult peripheral blood, CD8+ T cells from cord blood are almost exclusively CD8βhighCD28+. As shown in a representative example in Fig. 5a, in vitro stimulation of CBL using CD3 MoAb and IL-2 resulted in the occurrence of CD8βlowCD28+ and CD8βlowCD28– cells. As CD8βlowCD28+ and CD8βlowCD28– cells were virtually absent in the starting sample (day 0), it seemed likely that these cells differentiated from CD8βhighCD28+ cells by decreasing CD8β and CD28 expression. We also applied BrdU incorporation assays to study the proliferative activity of each subset at various time points during culture (Fig. 5b). These experiments showed that proliferation occurred in all subsets, but was most frequent in CD8βhighCD28+ cells. However, these cells gradually decreased during culture, which may reflect their differentiation into CD8βlowCD28+ and CD8βlowCD28– cells.
Fig. 5.
Anti-CD3 stimulation of cord blood lymphocytes (CBL). (a) Changes in the number of CD8βhighCD28+, CD8βlowCD28+, and CD8βlowCD28– cells in per cent. Representative for nine independent experiments. (b) BrdU incorporation assays performed at the indicated time points. Bars show the percentage of BrdU incorporating cells. Representative for three independent experiments.
DISCUSSION
Previous studies have been set out to characterize a subset of CD8+ T cells that lack surface expression of CD28. CD8+CD28– T cells are expanded in the elderly [11–15], as well as in chronic viral infection [16–18], haemato-oncological malignancy [19] and autoimmunity [20]. In contrast, CD8+CD28– cells are exceptionally rare in cord blood [21]. The TCR repertoire of these cells is oligoclonal suggesting they had proliferated after exposure to antigen [11,13, 22]. Findings of a poor proliferative capacity, the lack of activation markers such as HLA-DR, CD69, and CD25, expression of killer-inhibitory receptors [14, 23,24], as well as significantly shortened telomeres indicate that these cells have reached a state of ‘replicative senescence’[21,25,26]. A major functional property of these cells is the exertion of cytolytic toxicity [27–29]. Others have suggested a role as noncytolytic suppressor cells [30,31].
In our study we now demonstrate that, based on the coexpression of CD8β and CD28, three distinct subsets of CD8+ T cells can be described in peripheral blood, namely CD8βhighCD28+, CD8βlowCD28+, and CD8βlowCD28– cells. Based on this subset formation we re-evaluated previously obtained results regarding the different phenotypes of CD8+CD28+ and CD8+CD28– T cells. We found that the CD8+CD28+ population is very heterogeneous in the expression of other characteristic cell surface molecules. CD8βhighCD28+ cells express the CD45RA variant, whereas CD8βlowCD28+ cells preferentially convert to the CD45R0 variant, which is a hallmark of memory T cells. A significant portion of CD8βlowCD28– cells revert to the CD45RA variant again, which may explain the accumulation of CD45RA+ cells within the CD8+ T cells from older individuals [14]. Expression of CD45RA has been described previously in armed effector T cells [32] and memory-like T cells after prolonged exposure to antigen [33]. A significant subset of CD8βlowCD28–, but also CD8βlowCD28+ cells appear to function as mature cytotoxic effector cells, since these cells express high amounts of intracellular perforin and granzyme A. They also express IFN-γ more frequently than CD8βhighCD28+ cells. In contrast, CD8βlowCD28– cells rarely stain positive for IL-2 after stimulation with PMA and ionomycin, which was observed in CD8βhighCD28+ cells and, even more frequently in CD8βlowCD28+ cells.
We found that CD8βhighCD28+ cells were less susceptible to spontaneous apoptosis induction as compared to CD8βlowCD28+ and CD8βlowCD28– cells. Likewise, expression of the Fas receptor was found more frequently on CD8βlow cells compared to CD8βhighCD28+ cells. In contrast, the surface density of Fas expression on CD8βlowCD28– cells was lower than on the other populations, which may partially explain the highly variable frequency of apoptotic cells within the CD8βlowCD28– subset. Others have also found decreased Fas surface density on CD28-negative T cells [21,28]. However, CD28-negative CD8+ T cells are highly susceptible to activation-induced cell death [34], which we also have observed during CD3 stimulation in vitro. Conflicting data exist on the frequency of CD28-negative cells spontaneously undergoing apoptosis. Posnett et al. [28] have found CD8+CD28– cells relatively resistant to apoptosis induction using annexin V staining. In contrast, Lewis et al. [35] described the accumulation of CD8+CD28– cells in HIV-infected individuals, which were highly susceptible to spontaneous apoptosis as detected by a quantitative DNA fragmentation assay. Differences in the study population may partially account for these discrepancies. Among the individuals studied here, we found a relative expansion of CD8βlowCD28– cells and a decreasing frequency of apoptosis with increasing age. Thus, the accumulated CD8βlowCD28– cells observed in older individuals might be partially resistant to apoptosis.
The expression of NK cell receptors, which are important regulatory molecules on so-called NKT cells [36,37], closely correlates with expression of CD8β and CD28. CD56 and CD94 are present on a substantial portion of CD8βlowCD28+ and CD8βlowCD28– cells, but rarely on CD8βhighCD28+ cells. In contrast, CD161 is expressed at high density only on CD8βlowCD28+ cells. Both CD161 and CD94 are members of the C-type lectin superfamily of type-II transmembraneous receptors. The CD94 molecule is able to form a disulphide-linked heterodimer with members of the NKG2 family resulting in ambivalent effects on cellular functions. On T cell clones, crosslinking of the CD94/NKG2H receptor is able to trigger redirected cytotoxicity in a TCR-independent manner [38]. In contrast, CD94/NKG2A may inhibit the lysis of autologous tumour cells by cytotoxic lymphocytes [39]. The CD56 adhesion molecule might also play a role in target cell lysis, because its expression closely correlates with the amount of intracellular perforin and granzyme B in circulating CD8+ T cells [29] as well as in NK cells [40,41].
CD8βlow NKT cells share phenotypic features with a population of IELs that have been studied most extensively in mice (for review, see [42]). These thymus-independent IELs express the CD8αα coreceptor together with either TCR αβ or γδ. In addition, they display NK cell receptors, are capable of cellular toxicity and may recirculate from the gut epithelium into peripheral blood via the thoracic duct [43]. Another population of thymus-dependent IELs expresses both CD8α and CD8β. It will be interesting to further study the relationship between peripheral blood CD8βlow populations and these IELs.
It has been suggested that CD28-negative CD8+ T cells may arise from CD28-positive precursors. Accordingly, our data indicate that CD8βhighCD28+ cells may differentiate into CD8βlowCD28+ and CD8βlowCD28– cells, because CD8βhighCD28+ cells decrease after in vitro stimulation although they proliferate more actively and are less susceptible to apoptosis then CD8βlowCD28+ and CD8βlowCD28– cells; in vitro stimulation of CBL, which contain almost exclusively CD8βhighCD28+ cells, results in the appearance of both CD8βlowCD28+ and CD8βlowCD28– cells. Interestingly, the cell surface phenotype of the CD8+ T cell populations developing upon in vitro stimulation of CBL resembles the phenotype of their counterparts in vivo (data not shown). However, proliferation and apoptosis as observed in the populations in vitro may not necessarily reflect the situation in vivo, because our cell culture conditions may preferentially support certain populations while eliminating others.
Based on their phenotypic appearance in peripheral blood, the CD8β/CD28 subsets may be viewed as a simplified but useful model of major developmental stages: CD8βhighCD28+ cells are primarily naïve cells without effector function, but capable of rapid proliferation upon TCR stimulation. CD8βlowCD28+ cells display an activated and effector/memory phenotype by expressing activation receptors, the CD45R0 variant as well as intracellular effector molecules. CD8βlowCD28– cells contain the highest amount of effector molecules, frequently produce the T cell inhibitory cytokine IFN-γ, but proliferate poorly. They are more susceptible to apoptosis, and rapidly decease upon TCR stimulation.
Expression of CD8β at a low density results in a relative excess of CD8α. Therefore, CD8βlow cells primarily express the less efficient CD8αα homodimeric receptor. The phenotype of CD8βlowCD28+ as well as CD8βlowCD28– cells suggests that these cells have already encountered their antigen. Replacement of the efficient CD8αβ by the less efficient CD8αα receptor may therefore reflect that these cells require only weak signals for activation. In addition, expression of a less sensitive coreceptor may also protect against unspecific TCR stimulation. Together with down-regulation of the costimulatory CD28 molecule and induction of killer inhibitory receptors, the decrease in CD8β expression might help to control the proliferative capacity and cytotoxic activity of primed CD8+ T cells. It will be interesting to track the development of the described T cell subsets under pathological conditions such as infection, immunodeficiency, and autoimmunity. Insight into specific changes in the CD8+ T cell compartment may provide a better understanding of disease pathogenesis and help to define markers for progression and outcome.
Acknowledgments
E.L. Reinherz, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA, is gratefully acknowledged for providing MoAb 2ST8·5H7. This work has been supported by grant DFG Wi 1031/4–1 and Hannover Medical School (HiLF to T. Witte).
REFERENCES
- 1.Littman DR, Thomas Y, Maddon PJ, Chess L, Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985;40:237–46. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- 2.Liaw CW, Zamoyska R, Parnes JR. Structure, sequence, and polymorphism of the Lyt-2 T cell differentiation antigen gene. J Immunol. 1986;137:1037–43. [PubMed] [Google Scholar]
- 3.Norment AM, Littman DR. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988;7:3433–9. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renard V, Romero P, Vivier E, Malissen B, Luescher IF. CD8 beta increases CD8 coreceptor function and participation in TCR-ligand binding. J Exp Med. 1996;184:2439–44. doi: 10.1084/jem.184.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renard V, Delon J, Luescher IF, Malissen B, Vivier E, Trautmann A. The CD8 beta polypeptide is required for the recognition of an altered peptide ligand as an agonist. Eur J Immunol. 1996;26:2999–3007. doi: 10.1002/eji.1830261227. [DOI] [PubMed] [Google Scholar]
- 6.Witte T, Spoerl R, Chang HC. The CD8beta ectodomain contributes to the augmented coreceptor function of CD8alphabeta heterodimers relative to CD8alphaalpha homodimers. Cell Immunol. 1999;191:90–6. doi: 10.1006/cimm.1998.1412. [DOI] [PubMed] [Google Scholar]
- 7.Moebius U, Kober G, Griscelli AL, Hercend T, Meuer SC. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 8.Ledbetter JA, Evans RL, Lipinski M, Cunningham-Rundles C, Godd RA, Herzenberg LA. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981;153:310–23. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiSanto JP, Terry LA, Flomenberg N. Generation of anti-human CD8 beta-specific antibodies using transfectants expressing mixed-species CD8 heterodimers. J Immunol Meth. 1991;141:123–31. doi: 10.1016/0022-1759(91)90218-5. [DOI] [PubMed] [Google Scholar]
- 10.Devine L, Kieffer LJ, Aitken V, Kavathas PB. Human CD8 beta, but not mouse CD8 beta, can be expressed in the absence of CD8 alpha as a beta beta homodimer. J Immunol. 2000;164:833–8. doi: 10.4049/jimmunol.164.2.833. [DOI] [PubMed] [Google Scholar]
- 11.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to ‘benign monoclonal gammapathy’. J Exp Med. 1994;179:609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–7. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, Kourilsky P, Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–9. [PubMed] [Google Scholar]
- 14.Nociari MM, Telford W, Russo C. Postthymic development of CD28-CD8+ T cell subset: age-associated expansion and shift from memory to naive phenotype. J Immunol. 1999;162:3327–35. [PubMed] [Google Scholar]
- 15.Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8 (+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–8. [PubMed] [Google Scholar]
- 16.Saukkonen JJ, Kornfeld H, Berman JS. Expansion of a CD8+CD28- cell population in the blood and lung of HIV-positive patients. J Acquir Immune Defic Syndr. 1993;6:1194–204. [PubMed] [Google Scholar]
- 17.Lewis DE, Tang DS, Adu-Oppong A, Schober W, Rodgers JR. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–20. [PubMed] [Google Scholar]
- 18.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Van den Hove LE, Van Gool SW, Vandenberghe P, Boogaerts MA, Ceuppens JL. CD57+/CD28- T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia. 1998;12:1573–82. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 20.Sfikakis PP, Zografou A, Viglis V, Iniotaki-Theodoraki A, Piskontaki I, Tsokos GC, Sfikakis P, Choremi-Papadopoulou H. CD28 expression on T cell subsets in vivo and CD28-mediated T cell response in vitro in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:649–54. doi: 10.1002/art.1780380512. [DOI] [PubMed] [Google Scholar]
- 21.Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 22.Mingari MC, Schiavetti F, Ponte M, et al. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc Natl Acad Sci USA. 1996;93:12433–8. doi: 10.1073/pnas.93.22.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speiser DE, Valmori D, Rimoldi D, et al. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990–9. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Slifka MK, Pagarigan RR, Whitton JL. NK Markers are expressed on a high precentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–15. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–90. [PubMed] [Google Scholar]
- 26.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Fiorentino S, Dalod M, Olive D, Guillet JG, Gomard E. Predominant involvement of CD8+CD28- lymphocytes in human immunodeficiency virus-specific cytotoxic activity. J Virol. 1996;70:2022–6. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28- cytotoxic effector clones. Int Immunol. 1999;11:229–41. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 29.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 30.Freedman MS, Ruijs TC, Blain M, Antel JP. Phenotypic and functional characteristics of activated CD8+ cells: a CD11b-CD28- subset mediates noncytolytic functional suppression. Clin Immunol Immunopathol. 1991;60:254–67. doi: 10.1016/0090-1229(91)90068-l. [DOI] [PubMed] [Google Scholar]
- 31.Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28- T cells. Transplantation. 2000;69:1304–10. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- 32.Höflich C, Docke WD, Busch A, Kern F, Volk HD. CD45RA (bright)/CD11a (bright) CD8+ T cells: effector T cells. Int Immunol. 1998;10:1837–45. doi: 10.1093/intimm/10.12.1837. [DOI] [PubMed] [Google Scholar]
- 33.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JG. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo. CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162:7080–7. [PubMed] [Google Scholar]
- 34.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8 (+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–13. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DE, Yang L, Luo W, Wang X, Rodgers JR. HIV-specific cytotoxic T lymphocyte precursors exist in a CD28-CD8+ T cell subset and increase with loss of CD4 T cells. AIDS. 1999;13:1029–33. doi: 10.1097/00002030-199906180-00005. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RE, Bartley GT, Lee SS, et al. Expression of the NKTa clonotype in a series of human natural killer clones with identical cytotoxic specificity. J Exp Med. 1986;163:812. doi: 10.1084/jem.163.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt RE, Murray C, Daley JF, Schlossman SF, Ritz J. A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J Exp Med. 1986;164:351. doi: 10.1084/jem.164.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellón T, Heredia AB, Llano M, Minguela A, Rodriguez A, López-Botet M, Aparicio P. Triggering of effector functions on a CD8+ T cell clone upon the aggregation of an activatory CD94/kp39 heterodimer. J Immunol. 1999;162:3996–4002. [PubMed] [Google Scholar]
- 39.Speiser DE, Pittet MJ, Valmori D, et al. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med. 1999;190:775–82. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs R, Stoll M, Stratmann G, Leo R, Link H, Schmidt RE. CD16- CD56+ natural killer cells after bone marrow transplantation. Blood. 1992;79:3239–44. [PubMed] [Google Scholar]
- 41.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Rocha B, Guy-Grand D, Vassalli P. Extrathymic T cell differentiation. Curr Opin Immunol. 1995;7:235–42. doi: 10.1016/0952-7915(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 43.Arstila T, Arstila TP, Calbo S, Selz F, Malassis-Seris M, Vassalli P, Kourilsky P, Guy-Grand D. Identical T cell clones are located within the mouse gut epithelium and lamina propria and circulate in the thoracic duct lymph. J Exp Med. 2000;191:823–34. doi: 10.1084/jem.191.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]