Abstract
We measured serum interferon-gamma-inducible protein 10 (IP-10) and monokine induced by gamma interferon (MIG) levels to investigate the role of these molecules in the pathophysiology of haemophagocytic lymphohistiocytosis (HLH). Serum IP-10 and MIG levels were significantly increased in patients with active HLH compared with those of healthy controls. Serum MIG levels decreased gradually during the course of disease in a patient who recovered without therapy. On the other hand, rapid reduction of MIG and IP-10 levels was observed after chemotherapy in a patient with severe HLH. IP-10 and MIG mRNA expression was enhanced in liver and spleen, and IP-10 mRNA expression was enhanced in bone marrow in the patients, suggesting activated macrophages that infiltrated in these organs as one of the main producers of these cytokines. Serum IP-10 and MIG levels showed a significant correlation with serum IFN-γ levels. In addition, these chemokines had a significant correlation with fever and serum LDH levels, which are clinical indicators of disease activity of HLH. These results suggest that IP-10 and MIG which are produced by activated macrophages by the stimulation of IFN-γ, play an important role in the pathophysiology of HLH, by recruitment of activated Th1 cells into the tissues or organs.
Keywords: Chemokine, IP-10, MIG, Th1 cells, haemophagocytic lymphohistiocytosis
INTRODUCTION
HLH is an unusual syndrome characterized by fever, cytopenias, hepatosplenomegaly, coagulopathy and the pathological finding of haemophagocytosis in bone marrow and other tissues, accompanied by aberrant hyperactivation of macrophages and T lymphocytes with excessive secretion of cytokines [1–5]. HLH is triggered by various infectious agents but familial or nonfamilial immune deficiencies often contribute to the development of the disease [6]. Most of the clinical manifestations are attributable to hypercytokinaemia [5,7,8]. Among them, TNF-α and Th1 cytokines such as IFN-γ play important roles in the pathogenesis of HLH by activation of macrophages [7,9–9]. We previously reported that serum levels of IL-18 were significantly increased in patients with HLH, suggesting the important role of this cytokine in the induction of IFN-γ along with IL-12 [13].
Chemokines are a family of cytokines initially characterized by their capacity to induce chemotaxis or migration of leucocytes, and play important roles in the inflammatory response [14,15]. However, the role of chemokines in the pathophysiology in HLH has not been clarified. Luster et al. [16] reported the isolation of a CXC chemokine gene that encodes a 98-amino acid protein called interferon-gamma-inducible protein 10 (IP-10), which is produced mainly by macrophages stimulated with IFN-γ[17,18]. It is a chemoattractant for human T cells, and monocytes, and promotes T cell adhesion to endothelial cells [16,19]. In addition, it inhibits bone marrow colony formation [20] and angiogenesis, and also has antitumour activity [21]. Monokine induced by gamma interferon (MIG) is also a T cell chemoattractant strongly inducible by IFN-γ through NF-κB dependent pathway, and is very similar to IP-10 in its molecular structure, biological function, and chromosomal location [17,22]. IP-10 and MIG are involved in the selective recruitment of lymphocytes, as the receptor of IP-10 and MIG, CXC receptor 3 (CXCR3), is predominantly expressed on memory/activated T cells, especially on T helper 1 (Th1) cells [23–25]. It is reported that these chemokines play an important role in the pathogenesis of inflammatory and autoimmune diseases by inducing the recruitment of activated Th1 cells [25–27]. To investigate the role of these chemokines in the pathophysiology of HLH, we measured serum levels of these cytokines and analysed the correlation with other cytokines and clinical parameters. We found that serum IP-10 and MIG levels were significantly increased in patients with HLH. These cytokine mRNA expression levels were enhanced in liver and spleen, and IP-10 mRNA expression levels were enhanced in bone marrow of HLH patients. IP-10 and MIG significantly correlated with serum levels of IFN-γ, fever, and serum LDH levels. These results suggest IP-10 and MIG which would be induced by IFN-γ in vivo, play important roles in the pathophysiology of HLH by recruitment of activated Th1 cells into the tissues or organs with macrophage infiltration, leading to further macrophage activation by the interaction of macrophages and T cells.
MATERIALS AND METHODS
Patients
The concentrations of serum IP-10, MIG, IFN-γ, and IL-18 were measured in 12 patients with HLH, including 3 patients with familial haemophagocytic lymphohistiocytosis (FHL), 6 associated with Epstein-Barr virus (EBV) infection, 1 associated with anaplastic large cell lymphoma, and 2 of unknown aetiology ( Table 1). All patients were diagnosed as having HLH by clinical findings (prolonged high fever and hepatosplenomegaly), laboratory data (coagulopathy, pancytopenia, and hyperferritinaemia), and histopathological findings (haemophagocytosis in the bone marrow). Three patients were diagnosed as having FHL by the defect of perforin (case 3), and by the presence of family history (cases 1 and 2). All patients met the criteria for HLH by Henter et al. [28] and Imashuku [29]. Diagnosis of primary EBV infection was made by serological examinations. Controls included 12 patients with infectious mononucleosis (IM) and 21 healthy volunteers. Informed consent was obtained from all patients and healthy donors.
Table 1.
Clinical findings of patients with HLH
| Case no. | Age† | Sex | Type of disease | Fever | Hepato- splenomegaly | Pan- cytopenia | Liver dysfunction | DIC | Hyper- ferritinaemia | Hyper- triglyceridaemia | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1m | F | FHL | + | + | + | + | + | + | + | Dead |
| 2 | 2m | M | FHL | + | + | + | + | + | + | + | Dead |
| 3 | 2m | M | FHL‡ | + | + | + | + | + | + | + | Alive |
| 4 | 6m | M | Unknown | + | + | + | + | + | + | + | Alive |
| 5 | 1y | F | VHAS | + | + | + | + | + | + | + | Alive |
| 6 | 1y | M | VHAS | + | + | + | + | + | + | + | Alive |
| 7 | 2y | F | VHAS | + | + | + | + | + | + | + | Alive |
| 8 | 2y | M | Unknown | + | + | + | + | + | + | + | Alive |
| 9 | 3y | M | VHAS | + | + | + | + | + | + | – | Alive |
| 10 | 8y | M | VHAS | + | + | + | + | + | + | + | Alive |
| 11 | 12y | F | LHAS | + | + | + | + | + | + | + | Alive |
| 12 | 13y | F | VHAS | + | + | + | + | + | + | – | Alive |
m, months; y, years.
Perforin deficient.
Cytokine assay
Serum IP-10 and MIG levels were measured by enzyme-linked immunosorbent assay (ELISA). Murine antihuman IP-10 and MIG monoclonal antibodies (mAb) (R & D Systems, Minneapolis, MN, USA), and biotinylated goat antihuman IP-10 and MIG polyclonal Abs (R & D Systems) were used for IP-10 and MIG ELISA, respectively. ELISAs were performed according to the manufacturers’ instructions. Briefly, each ELISA plate was coated overnight at 37°C with anti-IP-10 or MIG mAb at the concentration of 2 µg/ml. After washing with PBS, each well was filled with blocking solution (PBS containing 1% BSA and 5% sucrose) and incubated for 3 h at room temperature. After washing, IP-10 or MIG standard (31·25–2000 pg/ml) and serum samples were added into each well, and the plates were incubated for 2 h at room temperature. After washing three times, biotinylated antihuman IP-10 or MIG polyclonal antibody was added and incubated at room temperature for two hours. The plates were washed 3 times and 100 µl of a 1 : 1000 dilution of streptavidin (Dako A/S, Denmark) was added to each well. Thirty minutes after incubation, 100 µl of 1 : 1 mixture of H2O2 and tetramethyl benzidine (Genzyme Diagnostics, San Calros, CA, USA) was added to each well. Thirty minutes after incubation at room temperature, the reaction was stopped by the addition of 50 µl of 2 m H2SO4. IP-10 and MIG concentrations were determined from standard curves of human IP-10 and MIG, respectively (R & D system) by absorbance at 450 nm using a micoplate reader (Immuno Reader NJ-2000, Nippon InterMed K.K., Japan). The detection limits of IP-10 and MIG were 62·5 pg/ml. Serum cytokine levels of and IL-18 were determined by ELISA using ELISA kits according to the manufacturers’ instructions (IFN-γ: Biosource International Inc. Camarillo, CA, USA; IL-18: MBL, Nagoya, Japan). The minimum detection limits for IFN-γ and IL-18 were 2 pg/ml and 12·5, respectively.
Analysis of IP-10 and MIG mRNA expression
Total RNA was extracted from peripheral blood mononuclear cells, bone marrow mononuclear cells, liver, and spleen using ISOGEN (NIPPON GENE, Osaka, Japan), according to the manufacturer's instructions. Random hexamer-primed reverse transcription (RT) of RNA was performed on total RNA by using First-Strand cDNA synthesis kit (Amersham Pharmacia Biotech, Uppsala, Sweden). IP-10 and MIG mRNA levels were compared by semiquantitative RT-PCR with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels as an internal control. The primer pair for IP-10 was 5′-GGA ACC TCC AGT CTC AGC ACC-3′, and 5′-GCG TAC GGT TCT AGA GAG AGA GGT AC-3′. Primer pair for MIG was 5′-TTC CTC TTG GGC ATC ATC TTG CTG-3′, and 5′-GAA GAT GGT GAT GGG ATT TC-3′. Primer pair for GAPDH was 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′.
Statistical analysis
The differences in serum cytokine levels among patients with HLH, IM, and healthy controls were analysed by Kruskal–Wallis test and multiple comparisons (Scheffe's test). The difference of serum cytokine levels between active phase and remission phase of the disease were analysed by Wilcoxon test. Spearman's rank/product moment correlation was used to determine an association between two variables.
Ethics
Informed consent was obtained from all donors or their parents in this study. This study was approved by Regional Committee of Ethics for Human research at Faculty of Medicine of Kyushu University.
RESULTS
Serum IP-10 and MIG levels in patients with HLH
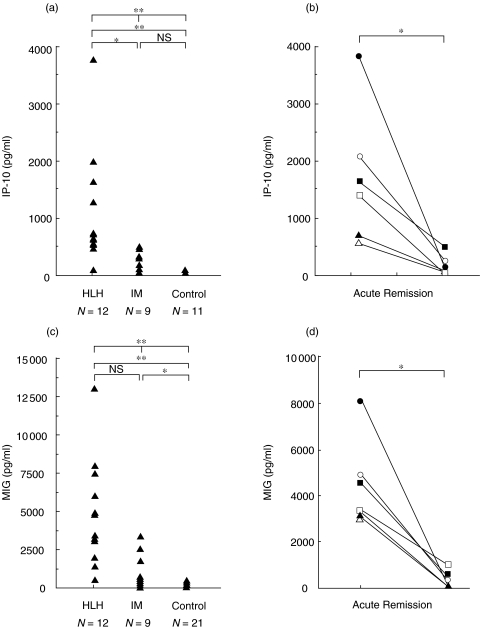
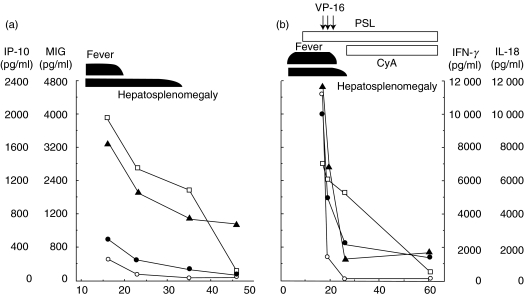
To investigate the role of these chemokines in the pathophysiology of HLH, we measured serum IP-10 and MIG levels in patients with HLH. As shown in Fig. 1, serum IP-10 (Fig. 1a,b) and MIG (Fig. 1c,d) levels were significantly increased compared with those of healthy controls (P < 0·01). In addition, serum IP-10 levels of HLH patients were significantly elevated compared with patients with IM (Fig. 1a,b P < 0·01). The IP-10 and MIG levels decreased in remission phase of the disease (Fig. 1). The serum IP-10 and MIG levels were serially examined in two patients in the course of the disease: in one, spontaneous remission was achieved (case 10 in Table 1), and in the other, chemotherapy with VP-16 was performed (case 5 in Table 1). In case 10, high serum MIG levels sustained for longer period, similar to IL-18, compared with IP-10 or IFN-γ (Fig. 2). In contrast, IP-10 and MIG levels dropped shortly after the effective VP-16 treatment along with IFN-γ in case 5, although IL-18 levels seemed to decrease more gradually (Fig. 2).
Fig. 1.
Serum IP-10 and MIG levels in HLH. Serum IP-10 (a, b) and MIG (c, d) levels were determined in active phase of HLH, IM and healthy controls (a, c), and acute and remission phases of HLH (b, d). The differences of serum cytokine levels among 3 groups were analysed by Kruskal–Wallis test, and multiple comparison (Scheffe's test). The difference of the cytokine levels between active and remission phase of the disease was analysed by Wilcoxon test. *P < 0·05, **P < 0·01, NS not significant.
Fig. 2.
Time course of serum IP-10 and MIG levels in patients with HLH. Serum IP-10 (•) and MIG (▴) levels were determined during the course of the disease in (a) patient 10 and (b) patient 5 (Table 1), together with IFN-γ (○) and IL-18 (□). VP-16, etoposide; PSL, prednisolone; CyA, cyclosporin A.
Correlation between IP-10, MIG and clinical data
We analysed the relationships between the levels of the chemokines and those with IFN-γ, IL-18 and clinical data. Three serial samples (in active, partial remission at 5–10 afebrile days after active phase, and complete remission phase of the disease) from each patient (2 VAHS: patients 5 and 10, and 1 FHL: patient 3) were available for the analysis. As shown in Table 2, Spearman's rank sum test revealed the significant positive correlation between serum IP-10 or MIG levels with IFN-γ (Correlation coefficient (CC) 0·863 and 0·929, respectively, P < 0·05, Table 2). In addition, serum IP-10 levels significantly correlated with serum IL-18 levels (CC 0·721, P < 0·05), body temperature (CC 0·904, P < 0·05), and the levels of LDH (CC 0·879, P < 0·05) and ferritin (CC 0·908, p < 0·05) (Table 2). Also, serum MIG levels showed significant correlation with serum LDH levels (CC 0·779, P < 0·05), known as one of the important clinical parameters.
Table 2.
Correlation between IP-10 and MIG levels and clinical data in HLH
| IP-10 | MIG | IFN-γ | IL-18 | Body temperature | LDH | Ferritin | |
|---|---|---|---|---|---|---|---|
| IP-10 | – | NS | 0·863 | 0·721 | 0·904 | 0·879 | 0·908 |
| MIG | – | – | 0·929 | NS | NS | 0·779 | NS |
Each value represents Spearman's correlation coefficient with significant correlation between the two factors. (P < 0·05). NS, no significant correlation.
IP-10 and MIG mRNA expression
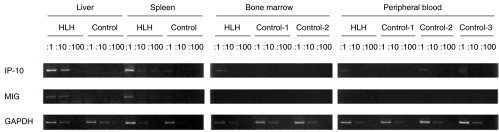
To investigate the organs where IP-10 and MIG are mainly produced in patients with HLH, we analysed the mRNA expression levels in liver, spleen, bone marrow, and peripheral blood mononuclear cells of the patients. As shown in Fig. 3, significant IP-10 and MIG mRNA expression was observed in liver and spleen in a patient with FHL (case 2 in Table 1). A slight increase of IP-10 mRNA level in bone marrow was observed in the FHL patient. In addition, significant mRNA expression of CXCR3, the receptor for IP-10 and MIG, was observed in liver of patient with HLH (data not shown). These results suggested that liver and spleen with the infiltration of activated macrophages would be the main organs where these chemokines are produced, which leads to the further infiltration of CXCR3 expressing T cells there.
Fig. 3.
IP-10 and MIG mRNA expression in various tissues. mRNA expression levels of IP-10 and MIG were determined by semiquantitative RT-PCR, as described in materials and methods. GAPDH mRNA expression was used as an internal control. PCR was performed on 10-folds serially diluted samples of cDNA for the comparison of IP-10 or MIG mRNA expression levels.
DISCUSSION
Recent studies have indicated important roles of IP-10, MIG, and CXCR3+ lymphocytes in the pathophysiology of inflammatory and autoimmune diseases [27], such as rheumatoid arthritis, ulcerative colitis [30], hepatitis C virus-infected liver [31], autoimmune thyroid disorders [32,33], and multiple sclerosis [34,35]. This study first demonstrated that serum IP-10 and MIG levels were significantly elevated in patients with active phase of HLH. In the remission phase of the disease, serum IP-10 or MIG levels were still slightly higher than healthy controls in a part of HLH patients (Figs 1 and 2), suggesting the persistent existence of IP-10/MIG-producing cells even in this stage. It is possible that serum levels of these chemokines are useful for the evaluation of the smoldering disease activity similar to IL-18 in HLH [13].
In addition to the function as a chemoattractant for T cells, IP-10 promotes the adhesion of activated T cells to both naive and primed human umbilical vein endothelial cells (HUVECs) [36]. In HLH, IP-10 and MIG would be mainly produced from activated macrophages by the stimulation of IFN-γ, which is overproduced in HLH patients [7,37]. As the receptor for IP-10 and MIG, CXCR3, is predominantly expressed on Th1 cells [23–25], IP-10 and MIG may play important roles in the pathophysiology of HLH, by inducing the accumulation and infiltration of activated Th1 cells into tissues or organs with macrophage infiltration. Actually we observed enhanced expression of CXCR3 in liver of the patient of HLH (data not shown). Furthermore, CXCR3 ligands (IP-10, MIG, and I-TAC) are natural antagonists for CCR3 [38]. It is possible that overproduction of CXCR3 suppresses the migration of Th2 cells even in the presence of CCR3 ligands, enhancing the polarization of T cell recruitment. Recruited T cells would be further activated to produce IFN-γ by the effect of IL-12 [10] and IL-18 [13] from macrophages, and contribute to the maintenance or further enhancement of macrophage activation. The significant correlation of IFN-γ and the two chemokines (Table 2) might be the result of the interaction of macrophages and T cells.
IP-10 and MIG were produced mainly from liver and spleen (Fig. 3). It is likely that macrophages which infiltrated in these organs would be the main producer of these chemokines. However, IP-10 expression in hepatocytes has been reported in patients with type 1 autoimmune hepatitis [39]. IP-10 and MIG were selectively up regulated on sinusoidal epithelium in hepatitis C-virus infected liver [31]. It is possible that hepatocytes and sinusoidal epithelium in liver, which were stimulated with inflammatory cytokines, are partially responsible for the overproduction of IP-10 in patients with HLH. Similarly, enhanced expression of IP-10 or MIG is reported in cerebrospinal fluid in meningitis [40] and demyelinating neuropathies [41], microglial cells/macrophages in multiple sclerosis [42] and herpes simplex virus infection [43], and astrocytes in brain in multiple sclerosis [34]. In experimental autoimmune encephalomyelitis, IP-10 blocking caused reduction of the brain manifestation [44]. Thus, possible expression of IP-10 and MIG in hepatocytes, sinusoidal epithelium in liver, and brain lesions suggest that these chemokines also play important roles by enhancing recruitment of activated T cells there in HLH.
CXCR3 or IP-10 deficient mice showed impaired T cell proliferation and IFN-γ production in response to antigenic stimulation, reduced contact hypersensitivity response, and impaired ability to control the virus replication [45,46]. These data suggested that IP-10 influences the generation of activated effector T cells. One of the possible mechanisms of this effect would be that constitutive IP-10 expression in lymphoid tissues might contribute to antigen-loaded dendritic cell trafficking to lymphoid tissues [47]. Recently, it was found that IFN-α/β stimulation of mouse spleen cells induced IP-10 mRNA expression in a IRF-9 dependent mechanism, and CXCR3 signalling pathway is critical for CD8+ T cell activation, suggesting that IP-10 may costimulate T cell activation in an autocrine loop [48]. In HLH, overproduction of IP-10 would be partially responsible for the dysregulation of activated T cells.
Thus, IP-10 and MIG seems to have important roles in the pathophysiology of HLH by their various functions on lymphocytes.
Acknowledgments
We thank Dr Naoko Kinukawa for helping the statistical analysis.
REFERENCES
- 1.Risdall RJ, Mckenna RW, Nesbit ME, et al. Virus–associated hemophagocytic syndrome. A benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979;44:993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Cline MJ. Histiocytes and histiocytosis. Blood. 1994;84:2840–53. [PubMed] [Google Scholar]
- 3.Arico M, Janka G, Fischer A, et al. Hemophagocytic lymphohistiocytosis. Report of children from the International Registry. FEL Study Group of the Histiocyte Society. Leukemia. 1996;10:197–203. [PubMed] [Google Scholar]
- 4.Sullivan JL, Woda BA. Lymphohistiocytic disorders. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. 5th edn. Philadelphia: WB, Saunders; 1997. pp. 1359–80. [Google Scholar]
- 5.Tsuda H. Hemophagocytic syndrome in children and adults. Int J Hematol. 1997;65:215–26. doi: 10.1016/s0925-5710(96)00560-9. [DOI] [PubMed] [Google Scholar]
- 6.Difpircq-Lagelouse R, Pastural E, Barrat F, et al. Genetic basis of hemophagocytic lymphohistiocytosis syndrome. Int J Mol Med. 1999;4:127–33. doi: 10.3892/ijmm.4.2.127. [DOI] [PubMed] [Google Scholar]
- 7.Henter JI, Elinder G, Soder O, et al. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–22. [PubMed] [Google Scholar]
- 8.Fujiwara E, Hibi S, Imashuku S. Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15:92–8. doi: 10.1097/00043426-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Akashi K, Hayashi S, Gondo H, et al. Involvement of interferon-γ and macrophage colony-stimulation factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol. 1994;87:243–50. doi: 10.1111/j.1365-2141.1994.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 10.Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;1:4100–3. [PubMed] [Google Scholar]
- 11.Lay JD, Tsao CJ, Chen JY, Kadin ME, Su IJ. Upregulation of tumor necrosis factor-α in Epstein-Barr virus-infected T cells in the pathogenesis of hemophagocytic syndrome. J Clin Invest. 1997;100:1969–79. doi: 10.1172/JCI119728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohga S, Matsuzaki A, Nishizaki M, et al. Inflammatory cytokines in virus–associated hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15:291–8. [PubMed] [Google Scholar]
- 13.Takada H, Ohga S, Mizuno Y, et al. Oversecretion of IL-18 in haemophagocytic lymphohistiocytosis: a novel marker of disease activity. Br J Haematol. 1999;106:182–9. doi: 10.1046/j.1365-2141.1999.01504.x. [DOI] [PubMed] [Google Scholar]
- 14.Adams DH, Shaw S. Leukocyte–endothelial interactions and regulation of leukocyte migration. Lancet. 1998;343:831–6. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 16.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 17.Liao F, Rabin RL, Yannelli JR, et al. Human Mig Chemokine. Biochemical and functional charatereization. J Exp Med. 1995;182:1301–14. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3. regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon inducible cytokine (IP-10) J Exp Med. 1987;166:1084–97. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarris AH, Broxmeyer HE, Wirthmueller U, et al. Human interferon-inducible protein 10. expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J Exp Med. 1993;178:1127–32. doi: 10.1084/jem.178.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HH, Farber JM. Localization of the gene for the human MIG cytokine on chromosome 4q21 adjacent to INP 10 reveals a chemokine ‘mini-cluster’. Cytogenet Cell Genet. 1996;74:255–8. doi: 10.1159/000134428. [DOI] [PubMed] [Google Scholar]
- 23.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;5:129–32. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;16:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–43. [PubMed] [Google Scholar]
- 26.Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:765–74. doi: 10.1002/1529-0131(200004)43:4<765::AID-ANR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 28.Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FEL Study Group of the Histiocyte Society. Semin Oncol. 1991;18:29–33. [PubMed] [Google Scholar]
- 29.Imashuku S. Differential diagnosis of hemophagocytic syndrome: underlying disorders and selection of the most effective treatment. Int J Hematol. 1997;66:135–51. doi: 10.1016/s0925-5710(97)00584-7. [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;15:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields PL, Morland CM, Salmon M, et al. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–43. [PubMed] [Google Scholar]
- 32.Garcia-Lopez MA, Sancho D, Sanchez-Madrid F, Marazuela M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 an MIG and attract CXCR3+ lymphocytes. J Endocrinol Metab. 2001;86:5008–16. doi: 10.1210/jcem.86.10.7953. [DOI] [PubMed] [Google Scholar]
- 33.Romagnani P, Rotondi M, Lazzeri R, et al. Expression of IP-10/CXCL1 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-oncet Graves’ disease. Am J Pathol. 2002;161:195–206. doi: 10.1016/S0002-9440(10)64171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balashov KE, Rottman JB, Weiner HL, Hancock KK. CCR5 (+) and CXCR3 (+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are increased in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorensen TL, Sellebjerg F, Jensen CV, Strieter RM, Ransohoff RM. Chemokines CXCL10 and CCL2: differential involvement in intrathecal inflammation in multiple sclerosis. Eur J Neurol. 2001;8:665–72. doi: 10.1046/j.1468-1331.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 36.Taub DD, Lloyd AR, Conlon K, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imashuku S, Ikushima S, Esumi N, Todo S, Saito M. Serum levels of interferon-gamma, cytotoxic factor and soluble interleukin-2 receptor in childhood hemophagocytic syndromes. Leuk Lymph. 1991;3:287–92. doi: 10.3109/10428199109107916. [DOI] [PubMed] [Google Scholar]
- 38.Loetscher P, Pellefrino A, Gong JH, et al. The ligands of CXC chemokine receptor 3, I–TAC, Mig, and IP−10 are natural antagonists for CCR3. J Immunol. 2001:2986–91. doi: 10.1074/jbc.M005652200. 276: [DOI] [PubMed] [Google Scholar]
- 39.Nagayama K, Enomoto N, Miyasaka Y, et al. Overexpression of interferon γ-inducible protein 10 in the liver of patients with type I autoimmune hepatitis identified by suppression subtractive hybridization. Am J Gastroenterol. 2001;96:2211–7. doi: 10.1111/j.1572-0241.2001.03959.x. [DOI] [PubMed] [Google Scholar]
- 40.Lahrtz F, Pali L, Nadal D, et al. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-gamma inducible protein-10 and monocyte chemotactic protein-1. Eur J Immunol. 1997;27:2484–9. doi: 10.1002/eji.1830271004. [DOI] [PubMed] [Google Scholar]
- 41.Kieseier BC, Tani M, Mahad D, et al. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002;125:823–34. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 42.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26:133–42. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 43.Lokensgard JR, Hu S, Sheng W, et al. Robust expression of TNF-alpha, IL-1 beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplexvirus. J Neurovirol. 2001;7:208–19. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- 44.Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;15:7617–24. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 45.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dufour JH, Dziejman M, Liu MT, et al. IFN-γ-inducible protein 10 (IP-10; CXCL10) -deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 47.Guttass CR, King LB, Luster AD, Ashwell JD. Constitutive expression of IP-10 in lympohid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179:1373–8. doi: 10.1084/jem.179.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogasawara K, Hida S, Weng Y, et al. Requirement of the IFN-α/β-induced CXCR3 chemokine signalling for CD8+ T cell activation. Genes Cells. 2002;7:309–20. doi: 10.1046/j.1365-2443.2002.00515.x. [DOI] [PubMed] [Google Scholar]