Abstract
Ulcerative colitis (UC), a chronic inflammatory bowel disease, exhibits pronounced increase of T lymphocytes in the inflamed mucosa. To understand the role of intestinal T lymphocytes in the pathogenesis of UC their cytokine production in the mucosa was analysed. Intestinal T lymphocytes of UC, Crohn's disease and control patients were analysed for cytokine mRNA levels by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) directly after isolation without in vitro stimulation. Frequencies of cytokine positive cells were determined in UC and control colon by immunomorphometry. T lymphocytes in normal colon expressed interleukin (IL)-2, interferon (IFN)-γ, tumour necrosis factor (TNF)-α and transforming growth factor (TGF)-β1, but not IL-4, IL-5 or IL-10. In UC, a highly significant increase in IL-10 mRNA levels in T lymphocytes and an increased frequency of IL-10 positive cells was seen in colon. IL-10 mRNA levels were also elevated in T lymphocytes of the non-inflamed ileum and correlated with disease activity at both locations. CD4+ T lymphocytes were the major source of IL-10 mRNA. IL-2, IFN-γ and TNF-α mRNA levels were decreased in colonic T lymphocytes, and virtually no IL-2, IFN-γ, TNF-α or TGF-β positive cells were detected in basal lymphoid aggregates. However, scattered IL-10 positive cells were found here. Lamina propria outside the aggregates contained IL-10-, IFN-γ, TNF-α and TGF-β but not IL-2 positive cells. T cells of UC patients did not express IL-4 or IL-5. Taken, together the data suggest a generalized activation of IL-10 producing CD4+ T cells along the intestine of UC patients. The local environment seems to determine the biological consequences of elevated IL-10.
Keywords: basal lymphoid aggregates, cytokine inflammatory bowel disease, intestinal T cells
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease restricted to the large bowel. A marked increase in the number of immune cells in the colonic mucosa and patchy destruction of the epithelium are typical features of the disease. There are indications that immune mechanisms play an important role in the pathogenesis of the disease. Autoantibodies directed against colon antigens have been demonstrated in serum and colonic mucosa of UC patients [1,2] and immunosuppressive drugs are effective in the treatment of the disease [3].
That T cell-mediated immune mechanisms play an important role in inflammatory bowel disease (IBD) has become evident from studies of genetically manipulated mice. Thus, T cell receptor (TCR)-α chain-, TCR-β chain-, MHC class II deficient mice and mice with aberrant T cell development due to transfection with human CD3e-chain followed by bone marrow reconstitution all develop intestinal inflammation. Moreover, disruption of genes for certain cytokines produced by T cells, e.g. interleukin-2 (IL-2), IL-10 and transforming growth factor-β (TGF-β), also causes bowel inflammation [4]. A role for the CD4 expressing T cell subset has been suggested in several mouse models for inflammatory bowel disease and is implicated most strongly by the fact that transfer of CD4+CD45RBhigh cells to SCID mice induces colitis [5]. The disease development in TCR-αβ and IL-10 deficient mice is dependent on the presence of commensal microbial flora in the gut [4,6].
The colonic lamina propria of UC patients is heavily infiltrated by lymphocytes. The majority of these lymphocytes are located in basal lymphoid aggregates, a microanatomical structure not found in normal colon [7]. The most prominent T cell subtype in the aggregates is a cell of the suppressive phenotype CD4+CD28–TCR-αβ+. Furthermore, activated γδ T cells constitute as much as 12% of the cells in the aggregates.
It is still unresolved whether a Th1- or Th2-type of immune response dominates in UC. Elevated local antibody production is seen in UC [1,8]. Two experimental colitis models resembling human UC were shown to display a Th2 cytokine profile [9,10]. However, Th2 cytokines have not been demonstrated conclusively in UC colon (reviewed in [11]). Crohn's disease (CD), the other major IBD in man, seems to be dominated by a Th1-type of immune response and CD patients have been treated successfully with monoclonal antibodies (MoAbs) directed against the Th1-cytokine tumour necrosis factor-α (TNF-α) [11,12].
The aim of this study was to determine the cytokine production by T lymphocytes in UC, to gain insights into the role played by local immune responses in the pathogenesis of the disease.
MATERIALS AND METHODS
Patients
Specimens were obtained from 44 patients suffering from UC. Twenty-six of these were endoscopic punch biopsies and 18 were colonic resection material. Twelve of the patients had inactive disease while the others had active disease with varying degrees of inflammation, as judged by clinical symptoms and routine histopathology. The duration of disease was less than 1 year for 11 patients while 19 patients had had UC for 10 years or more. Samples were from 29 male and 15 female patients 19–71 years old (mean 35 ± 13 years). Eleven patients had no medication the last 4 weeks prior to sampling, 20 received prednisolone alone or in combination with 5-aminosalicylic acid or azathioprine, whereas 13 patients received 5-aminosalicylic acid compounds only. Ileal and colonic punch biopsies were simultaneously collected from 20 patients. The ileal samples did not show any sign of inflammation.
Colon samples were also obtained from six patients with Crohn's colitis. Four samples were obtained by surgical resection and two were endoscopic punch biopsies. Two of the patients had active disease. Samples were from two male and four female patients 18–54 years old (mean 32 ± 14 years). One of the CD patients with active disease had no medication the last 4 weeks prior to sampling. The other CD patients all received azathioprine.
Control specimens from apparently normal human colon (n = 36) and ileum (n = 8) were from patients with no history of IBD. Specimens were obtained from patients undergoing bowel resection for cancer (colonic and rectal carcinoma, n= 28; adenocarcinoma in appendix, n= 1; tubulovillous adenoma, n= 2) or benign conditions (n = 3). Colonic surgical samples were from 21 male and 15 female patients 51–84 years old (mean 66 ± 10 years) and ileal samples were from four male and four female patients 39–78 years old (mean 62 ± 14 years). Colonic and ileal samples were from the same patient in seven cases. Control specimens were distal to any macroscopically detectable lesion. In addition, endoscopic punch biopsies of colonic tissue were obtained from patients undergoing investigation for non-inflammatory conditions (n = 2).
All patients undergoing bowel resection received a single i.v. dose of antibiotics 2 h prior to surgery according to preoperative standard procedure. None of the control patients were or had been subjected to radio- or chemotherapy, long-standing antibiotic medication or steroid treatment. This study was approved by the Ethical Committee at the Medical and Odontological Faculty of Umeå University Hospital and the patients gave their informed consent.
Antibodies and substrates
The anticytokine antibodies used in the study were: anti-IL-2 (clone 80-3418-01, mouse IgG1; Genzyme, Cambridge, MA, USA); anti-IL-4 (clone IL-4I(82), mouse IgG1), anti-IL-10 (clone JES3–9D7, rat IgG1, and clone JES3–19F1, rat IgG2a), anti-IFN-γ (clone 1-DIK, mouse IgG1) (all five from Mabtech, Nacka, Sweden); anti-IL-5 (clone TRFK5, rat IgG1), anti-IFN-γ (clone MMHG-1, mouse IgG1), TGF-β (clone TB21, mouse IgG1) (all three from Nordic Biosite, Täby, Sweden); anti-IL-4 (clone 25D2, rat IgG1; Endogen, Woburn, MA, USA); anti-TGF-β (IgG fraction of rabbit antiserum; R&D Systems, Abingdon, UK); anti-TNF-α (clone Mab1, mouse IgG1; Pharmingen, San Diego, CA, USA). Anti-cell-surface markers MoAbs used were: anti-CD3 (clone OKT-3, mouse IgG2a; ATCC, Rockville, MD, USA); anti-CD19 (clone HD37, mouse IgG1), anti-CD45 (a mixture of clones 2B11 and PD7/26, mouse IgG1), anti-CD68 (clone EBM11, mouse IgG1), epithelial cell marker anti-BerEP4 (clone BerEP4, mouse IgG1; all four from Dakopatts, Denmark). Control antibodies were: anti- Aspergillus niger glucose oxidase (clone DAK-GO1, mouse IgG1; Dakopatts), IgG fraction of normal rabbit serum (Dakopatts), and anti-dinitrophenyl (clone LO-DNP-1, rat IgG1; Serotec, Oxford, UK). Conjugates and substrates used in immunohistochemistry were horseradish peroxidase-conjugated F(ab′)2 fragments of sheep antimouse Ig (Amersham, Buckinghamshire, UK), biotinylated F(ab′)2 fragments of rabbit antirat IgG (Serotec), horseradish peroxidase-conjugated donkey antirabbit Ig (Amersham), 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, Stockholm, Sweden), 3′-amino-9-ethylcarbazole (AEC; The Binding Site, Birmingham, UK) and nitro-blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; Roche Molecular Biochemicals, Mannheim, Germany).
Paramagnetic beads used for cell fractionation were Dynabeads M-450 coupled with goat-antimouse IgG (Dynal, Oslo, Norway) and charged with anti-CD3 MoAb, anti-CD45 MoAbs or MoAb BerEP4 and Dynabeads M-450 directly coupled with anti-CD2, anti-CD4, anti-CD8 or anti-CD19 MoAb (Dynal).
Isolation of intestinal leucocytes
Lamina propria leucocytes (LPL) were isolated from surgical and biopsy samples as described previously [7,13]. Contaminating epithelial cells were removed by incubation with MoAb BerEP4 charged magnetic beads [13]. Unbound cells were subjected to positive selection using magnetic beads charged with MoAbs specific for CD2, CD3, CD4, CD8, CD19 or CD45 as described [14]. The weight of tissue collected as 10–20 punch biopsies varied between 280 and 570 mg (365 ± 85 mg and 340 ± 95 mg for colonic and ileal samples, respectively). In UC patients, the average number of isolated cells/g tissue was 4·3 × 105 (range 0·2–16 × 105) for colonic CD3+ LPL and 5·9 × 105 (range 0·7–7·5 × 105) for ileal CD3+ LPL.
Isolation of peripheral blood mononuclear cells (PBMC) and polyclonal T cell activation
PBMC were isolated from nine healthy adult blood donors (median age 36 years; range 26–48 years) by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation. PBMC were either frozen immediately or activated by incubation with the mitogenic anti-CD3 MoAb OKT3 (100 ng/ml) for 7 h at a concentration of 1 × 106 cells/ml in HEPES-buffered RPMI-1640 containing 0·4% human serum albumin and antibiotics, at 37°C in humid air with 5% CO2.
Immunohistochemistry and immunomorphometry
Fresh tissue samples were rinsed in cold 0·15 m phosphate buffered saline (PBS), snap-frozen in isopentane precooled in liquid nitrogen and stored at − 80°C. Staining for surface antigens and cytokines was performed using indirect immunoperoxidase staining technique for mouse MoAb and IgG fraction of rabbit antibodies [7] or the ABC-peroxidase technique (Dakopatts) for rat MoAbs. Saponin (0·1%) was included in incubation buffers for staining of cytokines in single- and double-staining protocols. Final concentrations of anticytokine MoAbs ranged between 5 and 25 µg/ml. Double staining was performed as follows. Sections were fixed in 2% paraformaldehyde and blocked with 0·02 m PBS (pH 7·2) containing 20% horse serum. Anti-IL-10 MoAb in combination with either anti-CD3, anti-CD19 or anti-CD68 MoAb was applied to the sections and incubated overnight at 4°C. Endogenous peroxidase activity was quenched with 1% H2O2 in methanol, followed by addition of avidin/biotin blocking solution (Dakopatts). Sections were incubated with secondary antibodies conjugated with horseradish peroxidase and biotin, followed by alkaline phosphatase conjugated strepavidin (Jackson Immunoresearch). AEC was used as substrate for peroxidase and NBT/BCIP for alkaline phosphatase. Sections incubated with isotype and concentration matched irrelevant MoAb and IgG fraction of rabbit preimmune serum, respectively, served as negative controls.
Morphometry analysis of cells in aggregates, in solitary follicles and in lamina propria outside aggregates and/or follicles of inflamed and control colon was performed by the lattice point method as described [7]. Eight to 15 randomly chosen ocular fields were counted. Morphometry analyses were performed independently by two people (S.M. and M.M.-W.Y.). Two different antibodies were used for IL-4, IL-10, interferon-γ (IFN-γ) and TGF-β staining. The results were identical for a particular cytokine, irrespective of which specific reagent was used.
Immunoflow cytometry
After selection procedures LPL and unbound cells were characterized either by single or dual colour immunoflow cytometry as described previously [7,13].
RNA preparation and qualitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from positively selected LPL subpopulations still attached to the magnetic beads and from residual non-bound LPL fractions as well as from freshly isolated and activated PBMC, as described previously [14]. Reverse transcription of RNA to cDNA and PCR amplification was performed using recombinant thermostable Thermus thermophilus DNA polymerase (P-E Biosystems, Norwalk, CT, USA) and specific primer pairs for IL-2, IL-4, IL-5, IL-10, IFN-γ, TNF-α, TGF-β1, β-actin and CD45 as described [13]. β-actin and CD45 mRNA served as controls for RNA quality. A pool of RNA from PBMC activated with anti-CD3 MoAb OKT3 for 4 h, 7 h and 20 h served as positive control in RT-PCR for all primers.
Real-time quantitative RT-PCR
Levels of mRNA for IL-2, IL-4, IL-10, IFN-γ, TNF-α, TGF-β1 and GAPDH were determined in real-time quantitative RT-PCR using the TaqMan EZ technique (P-E Applied Biosystems). Specific primer pairs are placed in different exons and used in combination with a 5′-fluorescent reporter dye-labelled internal probe hybridizing over the exon boundary. For sequences of primers and probes in cytokine mRNA assays see Forsberg et al. [15]. The emission from released reporter dye is monitored by ABI Prism 7700 Sequence Detection System (P-E Applied Biosystems). For each cytokine a specific RNA copy standard was prepared (see below). Determinations were carried out in triplicate and expressed as copies of mRNA/µl as determined from parallel RT-PCR of serial dilutions of the standard. All samples were analysed for their content of mRNA for the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), using the primers and probe supplied by the manufacturer (P-E Applied Biosystems). The amount of GAPDH/cell was not statistically different between the patient groups. Therefore, results are expressed as cytokine mRNA copies per GAPDH mRNA unit as an estimation of the average cytokine mRNA content per cell.
RNA copy standard preparation
A pool of PBMC activated with anti-CD3 MoAb OKT3 for 4 h, 7 h and 20 h was used as starting material for copy standard preparations and for optimization of the assays (see above). RT-PCR products from the different cytokine RT-PCR assays were cloned and sequenced to ascertain proper sequence and used thereafter for preparation of RNA copy standards, as described previously [16].
Statistics
Mann–Whitney's ranking test was used for statistical analysis of differences between the groups in cytokine mRNA expression levels. Percentage of cytokine mRNA expressed in different cell types is given as mean ± 1 S.E. and Student's t-test was used in statistical analyses. Values obtained by immunomorphometry analyses are given as mean ± 1 S.D. and statistical analyses of differences in frequencies of cytokine positive cells between UC and normal colon were performed using Student's t-test. Analyses of correlation between mRNA levels of different cytokines were performed using Spearman's rank correlation test. Two-tailed analyses were used throughout.
RESULTS
Cytokine mRNA profile of colonic lamina propria T cells
Because the great majority of the T cells in UC colon are located in the lamina propria, particularly in the basal lymphoid aggregates, we isolated these cells from UC colon lamina propria by positive selection with anti-CD3 MoAb giving CD3+LPL. For comparison, CD3+LPL from control colon as well as from non-inflamed UC ileum and control ileum were also isolated. In the initial screening qualitative RT-PCR was used. Five to 13 samples were analysed for each cytokine mRNA. We noted that IFN-γ, TNF-α and TGF-β1 mRNA were detected in almost all samples of CD3+LPL, both from UC colon and control colon. In contrast, IL-4 and IL-5 mRNA were not found in any of the CD3+LPL samples. IL-10 mRNA was detected in most CD3+LPL samples from UC colon but not in CD3+LPL samples from control colon. The reverse was true for IL-2 mRNA, which was found only rarely in CD3+LPL samples from UC colon (2/13) but was frequent in CD3+LPL samples from control colon (7/9).
As a quality control we also analysed the CD3–/non-T cell or CD19+/B cell fractions from control and UC colon for IFN-γ and IL-2 mRNA. mRNA for these T cell-specific cytokines was not found in these fractions. Moreover, no CD3+ cells were detected in the unbound fraction by immunoflow cytometry. These results show that T cells were efficiently retrieved in the CD3+ fraction by the positive selection procedure.
IL-10 mRNA levels are increased in colonic lamina propria T cells of UC patients and varies with disease activity
Real-time quantitative RT-PCR assays were developed for cytokines with suggested importance as indicated by the cytokine mRNA screening, i.e. IL-2, IL-10, IFN-γ, and TNF-α, and in addition for IL-4 and TGF-β1. Cytokine and GAPDH mRNA levels were determined in freshly isolated colonic CD3+ LPL and the average cellular expression levels of cytokine mRNA were calculated as the ratio between the cytokine and GAPDH mRNA levels in the individual samples.
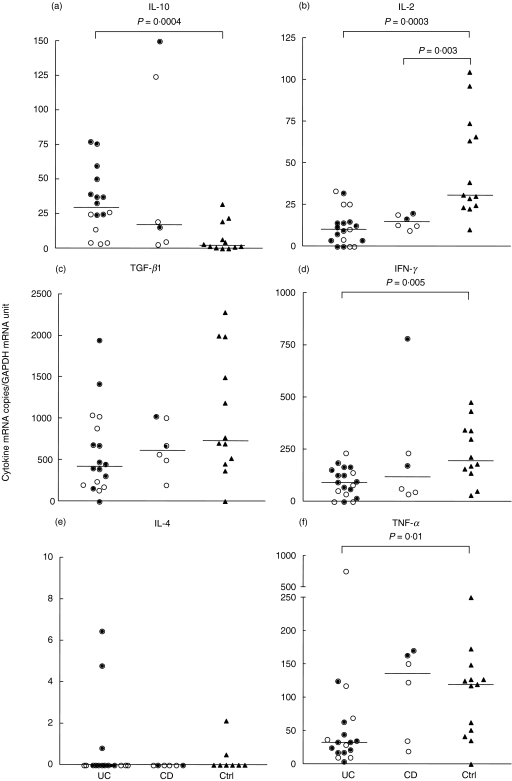
Almost all CD3+ LPL samples from control colon expressed detectable levels of IL-2, IFN-γ, TNF-α and TGF-β1 mRNA, while IL-10 was detected in 70% of analysed control samples, although mainly at low levels (Fig. 1). IL-4 was detected in only 20% of analysed control samples (Fig. 1). The level of IL-10 mRNA was increased significantly in colonic CD3+ LPL of UC patients compared to the corresponding cells of controls (Fig. 1a), with a 10-fold increment in the median value. In contrast, IL-2 mRNA levels in CD3+ LPL were significantly lower in UC (Fig. 1b), with a threefold decrease in the median value. Similarly, the mRNA levels for the proinflammatory cytokines IFN-γ and TNF-α were significantly lower in CD3+ LPL of UC patients compared to controls with 2·1- and 3·6-fold decreases in the median values, respectively (Figs 1d,f). The mRNA levels of TGF-β1 were not significantly different in UC patients compared to controls (Fig. 1c). IL-4 mRNA was detected only in occasional UC samples and occurred at the same frequency as in controls, i.e. in 20% of analysed samples (Fig. 1e).
Fig. 1.
IL-10 (a), IL-2 (b), TGF-β1 (c), IFN-γ(d), IL-4 (e) and TNF-α(f) mRNA levels in freshly isolated colonic lamina propria T lymphocytes, CD3+ LPL, of ulcerative colitis patients (UC), patients with Crohn's disease (CD) and control patients (Ctrl). The amounts of cytokine mRNA and GAPDH mRNA were determined using the quantitative TaqMan EZ real time quantitative RT-PCR technology. The average level of cytokine mRNA/T cell was calculated as the ratio between the concentration of cytokine mRNA copies and GAPDH mRNA units in each sample. For the different cytokines each circle or triangle represents the value from a single subject. Filled circles indicate samples from patients with active colitis. Open circles indicate samples from patients with inactive colitis. Triangles indicate samples from controls. Horizontal bars indicate medians. Statistically significant differences with P-values ≤ 0·01 are indicated. (Mann–Whitney's ranking test).
When UC patients were subdivided with regard to disease activity, significantly higher IL-10 mRNA levels were observed in CD3+ LPL of patients with active inflammation (P = 0·003; Fig. 1a, filled circles). The median IL-10 mRNA copies/GAPDH mRNA unit values for CD3+ LPL of patients with active disease was approximately four times higher than that of patients with inactive disease (open circles; 39 versus nine copies/GAPDH unit, respectively), which in turn was higher than in controls (median: 3 IL-10 copies/GAPDH unit). CD3+ LPL from areas with high and low inflammatory activity was analysed from one UC patient (data not included in Fig. 1a). Interestingly, in this patient the IL-10 mRNA expression level was markedly higher in the active area (38 IL-10 copies/GAPDH unit) compared to the inactive area (0·1 IL-10 copies/GAPDH unit). No significant correlation between mRNA levels and disease activity was observed when the other cytokines were analysed in the same way. However, within the group of UC patients with active disease there was an inverse relationship between the IL-10 and the IFN-γ levels (n = 9, P= 0·04, r=− 0·7).
The observed differences in cytokine mRNA levels between CD3+ LPL in UC and controls were consistent when UC patients with and without medication were analysed separately. Furthermore, the same significant changes in cytokine mRNA expression were observed when CD3+ LPL isolated from endoscopic punch biopsies and from surgical samples were analysed separately in UC and controls.
Colonic T cells from six CD colitis patients were studied. Similar to colonic T cells of UC patients the IL-2 mRNA levels were decreased compared to controls (Fig. 1b) and two of the samples had high IL-10 mRNA levels. In contrast to UC, there appears not to be a decrease in the levels of IFN-γ and TNF-α. Instead, the levels of mRNA for IFN-γ and TNF-α, as well as for TGF-β1 and IL-4 in T cells, did not differ from the levels of corresponding cytokines in controls (Fig. 1c–f).
CD4+ cells are the main producers of IL-10, TGF-β1, IFN-γ and TNF-α in the colonic lamina propria of UC patients
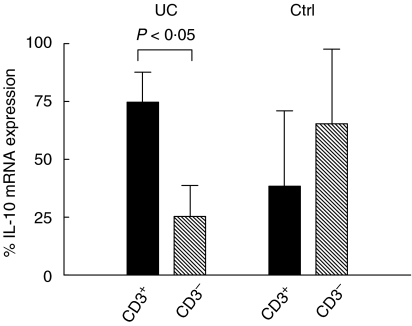
T cells, B cells and macrophages are the main producers of IL-10. To determine the relative contribution by intestinal T cells, IL-10 mRNA levels were determined in colonic T cells (CD3+ LPL) and non-T cell leucocytes (CD3– LPL) from UC and control patients. In UC, T cells accounted for ≈ 75% of all the IL-10 mRNA, which was significantly more than non-T cells (Fig. 2). Non-T cells accounted for most of the IL-10 mRNA in controls (Fig. 2). In further experiments total LPL preparations were subjected to sequential positive selection with anti-CD4 MoAb charged beads yielding CD4+ cells. Unbound cells were treated further with anti-CD8 MoAb charged beads yielding CD8+CD4– cells. Residual unbound cells were treated with anti-CD2 MoAb charged beads yielding CD4CD8 double-negative T cells (CD2+DN cells). As much as 90% of the IL-10 mRNA was expressed by the CD4+ cells in UC samples (Table 1). CD4+ cells were also the main source for TGF-β1, IFN-γ and TNF-α mRNA in UC (Table 1). CD8+ cells were the second largest source, while the CD4CD8 double-negative T cells expressed only low levels of all four cytokines (Table 1). In controls, the comparatively low levels of IL-10 mRNA in T cells were also expressed predominantly by CD4+ cells (Table 1). Interestingly, CD4+ cells of controls contributed with 12% of the IFN-γ mRNA compared to about 50% in UC. Most IL-2 mRNA was expressed in CD4+ cells (69 ± 9%) of controls. IL-2 levels in colonic T cell subpopulations of UC were too low to allow calculations.
Fig. 2.
Relative contribution by T cells and non-T cell leucocytes to IL-10 mRNA in the colonic lamina propria of UC patients and controls. T cells were positively selected from freshly isolated colonic LPL of four UC patients and three controls (Ctrl) and IL-10 mRNA amounts were determined in bound (CD3+; black columns) and unbound (CD3–; hatched columns) LPL. The amount of IL-10 mRNA was determined as described in Fig. 1 and percentage IL-10 mRNA expression was calculated for the individual samples as [amount of IL-10 mRNA in the indicated cell type/(amount of IL-10 mRNA in CD3+ LPL + CD3– LPL)] × 100. Columns indicate mean ± 1 S.D. Student's t-test was used for statistical analysis.
Table 1.
Relative contribution of cytokine mRNA expressed by different lamina propria T lymphocyte subsets in UC and control colon
| % of cytokine mRNA expressed by indicated T cell subtype | ||||||
|---|---|---|---|---|---|---|
| UC patients | Controls | |||||
| Cytokine | CD4+† | CD8+CD4– | CD2+ DN | CD4+ | CD8+CD4– | CD2+ DN |
| IL-10 | 91 ± 5‡ | 10 ± 5 | <1 | 87 ± 10 | 12 ± 10 | <1 |
| TGF-β1 | 59 ± 10 | 37 ± 11 | 5 ± 4 | 48 ± 11 | 47 ± 12 | 12 ± 2 |
| IFN-γ | 53 ± 16* | 46 ± 17 | 1 ± 1 | 12 ± 6 | 82 ± 12 | 17 ± 15 |
| TNF-α | 74 ± 18 | 25 ± 19 | 1 ± 1 | 59 ± 13 | 40 ± 13 | 3 ± 1 |
T cell subset. Freshly isolated LPL from UC and control patients were subjected to sequential positive selection by treatment with magnetic beads coupled with anti-CD4 MoAb (CD4+), followed by beads with anti-CD8 MoAb (CD8+CD4–) and finally beads with anti-CD2 MoAb (CD2+ DN).
In the individual samples the percentage cytokine mRNA expressed by a particular T cell subset was calculated for each cytokine as [amount of cytokine mRNA in the indicated cell type/(amount of cytokine mRNA in CD4+ LPL + CD8+CD4– LPL + CD2+DN LPL)] × 100. Results are given as mean ± 1 S.E. of four independent experiments for UC and control patients, respectively.
P-value < 0·05 compared to CD4+ cells in controls (Student's t–test).
Cytokine mRNA expression in ileal T cells of UC patients
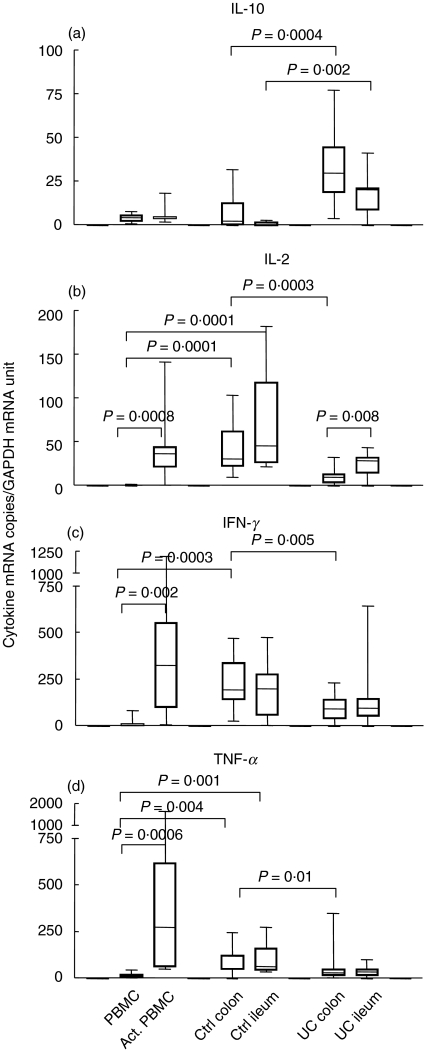
To investigate whether the abnormal cytokine mRNA expression pattern noted above was limited to T cells of inflamed colon in UC patients, we also analysed lamina propria T cells from their non-inflamed ileum. Colonic and ileal biopsies were collected at the same occasion from 11 UC patients. For comparison we also analysed cytokine mRNA expression in CD3+ LPL of control ileum and in PBMC before and after activation with anti-CD3 MoAb. Figure 3 shows the results for IL-10, IL-2, IFN-γ and TNF-α. The results may be summarized as follows: (1) there is no difference between lamina propria T cells from control colon and control ileum with respect to expression levels of mRNA for IL-10, IL-2, IFN-γ, TNF-α and TGF-β1 (the latter not shown); (2) lamina propria T cells in normal intestine express high levels of IFN-γ, TNF-α and IL-2 compared to freshly isolated PBMC. However, IL-10 mRNA levels are low both in CD3+ LPL from normal intestine and PBMC. (3) The IFN-γ, TNF-α and IL-2 mRNA levels in normal intestinal CD3+ LPL correspond to those of anti-CD3 activated PBMC, e.g. of activated blood T cells; (4) as noted earlier and also shown here, IL-10 mRNA levels were highly increased in CD3+ LPL from UC colon compared to CD3+ LPL from control colon. For IL-2, IFN-γ and TNF-α the reverse was true. Comparing CD3+ LPL from UC ileum with CD3+ LPL from control ileum revealed that IL-10 mRNA levels are also significantly higher in UC (Fig. 3a), while there is no difference between ileal T cells of the two groups with respect to expression levels of IL-2, IFN-γ and TNF-α mRNA (Fig. 3b–d). (5) Finally, when CD3+ LPL from UC colon were compared with CD3+ LPL from UC ileum we found that the mRNA levels for IL-10, IFN-γ and TNF-α were the same in the two cell populations while the IL-2 mRNA level was clearly decreased in the T cells from the diseased UC colon.
Fig. 3.
IL-10 (a), IL-2 (b), IFN-γ(c) and TNF-α(d) mRNA levels in freshly isolated mononuclear cells from blood (PBMC), PBMC activated for 7 h with anti-CD3 MoAb (Act. PBMC) and freshly isolated lamina propria T lymphocytes, CD3+ LPL, from colon and ileum of control patients (Ctrl colon, Ctrl ileum) and of ulcerative colitis patients (UC colon, UC ileum). The cytokine mRNA levels were determined as described in Fig. 1. Whiskers indicate the range, boxes the 25th to 75th percentile and horizontal bars inside boxes the median values. Presented results are based on analysis of seven to nine PBMC samples, seven to nine samples of activated PBMC, 12 or 13 control colon samples, eight control ileum samples, 16–19 UC colon samples and 14–17 UC ileum samples for each cytokine. Statistically significant differences with P-values ≤ 0·01 are indicated. (Mann–Whitney's ranking test).
Cytokines mRNA levels in ileal CD3+ LPL did not correlate to medication.
Lymphocytes producing down-regulatory cytokines are present in the basal aggregates of UC colon
The frequencies and distributions of cells stained for IL-10, TGF-β, IL-2, IFN-γ, TNF-α, IL-4 and IL-5 protein/peptide were studied by immunomorphometry in UC and control colon. Cells located in basal lymphoid aggregates and in lamina propria outside the aggregates and follicles were analysed separately in UC samples. These microanatomical compartments were compared, respectively, to solitary follicles and to lamina propria outside the follicles in control colon. The basal lymphoid aggregates of UC occupy approximately 25% of lamina propria and are composed of densely packed T and B lymphocytes, whereas monocytes and granulocytes are generally not found [7]. Cells stained for IL-10 and TGF-β were present in the aggregates. IL-10 positive cells constituted up to 1·5% of the cells and were scattered throughout the aggregates (Table 2,Fig. 4b). The majority of the IL-10 positive cells were CD3 positive (Fig. 5b) and very few B cells were IL-10 positive (data not shown). The TGF-β positive cells were less frequent and were located often in the outer rim of the aggregates. A few TNF-α positive cells were also present and exhibited similar distribution to TGF-β positive cells (Table 2 and not shown). Virtually no IL-2, IL-4, IL-5 or IFN-γ positive cells were detected (Table 2, Figs 4c,d). Solitary follicles of normal colon were essentially devoid of cells expressing any of the seven cytokines studied here (Table 2).
Table 2.
Frequency of cytokine positive cells in colonic tissue of ulcerative colitis (UC) and control patients as determined by immunomorphometry
| % positive cells in | |||||||
|---|---|---|---|---|---|---|---|
| Aggregates/follicles‡ | Lamina propria* | ||||||
| Cytokine | UC | Control | Significancea | UC | Control | Significance | n |
| IL-10 | 0·9 ± 0·4† | 0·1 ± 0·1 | P < 0·001 | 2·1 ± 1·0 | 0·5 ± 0·3 | P < 0·01 | 6 |
| TGF-β | 0·6 ± 0·2 | 0·2 ± 0·3 | NS | 4·2 ± 0·4 | 4·5 ± 0·3 | NS | 4 |
| IL-2 | <0·1 | <0·1 | NS | 0·2 ± 0·2 | 5·5 ± 0·3 | P < 0·001 | 6 |
| IFN-γ | <0·1 | <0·1 | NS | 6·6 ± 2·6 | 3·8 ± 0·4 | NS | 4 |
| TNF-α | 0·5 ± 0·1 | 0·1 ± 0·1 | P < 0·001 | 2·8 ± 0·6 | 3·1 ± 0·2 | NS | 5 |
| IL-4 | 0·2 ± 0·2 | <0·1 | NS | 0·6 ± 0·3 | 0·3 ± 0·3 | NS | 6 |
| IL-5 | 0·2 ± 0·1 | <0·1 | P < 0·01 | 0·7 ± 0·3 | 0·1 ± 0·1 | P < 0·01 | 6 |
Basal lymphoid aggregates were counted in UC colon and solitary follicles in control colon.
Lamina propria is defined as the area between the epithelium and mucosa muscularis, excluding basal lymphoid aggregates and solitary follicles in UC colon and excluding solitary follicles in control colon.
Statistical analysis comparing UC and control colon was performed using two-tailed Student's t-test. NS = not significant.
Frequencies of cytokine positive cells is expressed as percentage stained cells of all cells in the indicated compartment (mean ± 1 S.D.).
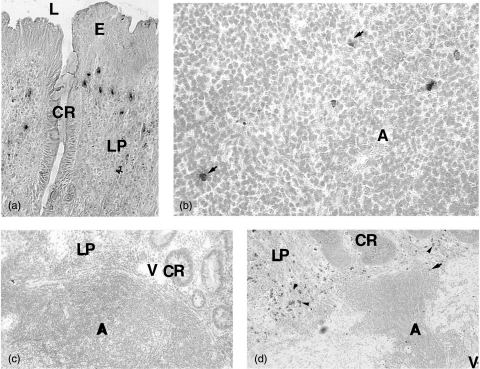
Fig. 4.
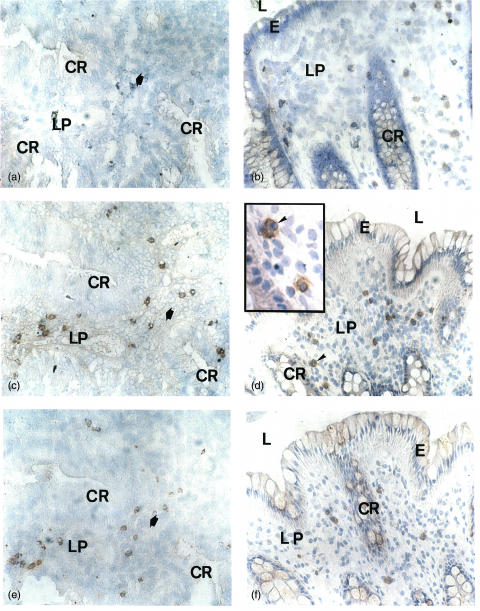
Immunohistochemical staining of IL-10 (a, b), IL-2 (c) and IFN-γ (d) in the colonic mucosa of UC patients. (a) Several IL-10 positive cells are present in the lamina propria. (b) Scattered IL-10 stained cells can be seen in the lymphoid aggregate (arrows). (c) No IL-2 stained cells are found in any compartment. (d) Only one IFN-γ positive cell (arrow) can be seen in the lymphoid aggregate while several IFN-γ positive cells are present in lamina propria outside the aggregate (arrow heads). A = lymphoid aggregate; CR = crypt; E = epithelium; L = lumen; LP = lamina propria; V = blood vessel. Original magnification ×55 in (a); ×220 in (b); ×55 in (c, d).
Fig. 5.
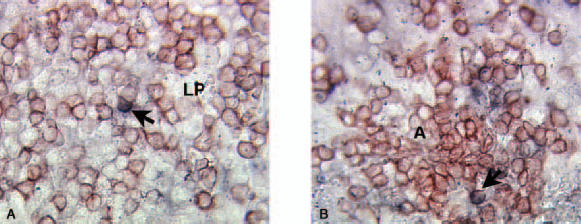
Immunohistochemical double staining of IL-10 (blue) and CD3 (red) in the colonic mucosa of UC patient. (a) A double positive cell (thick arrow) in the lamina propria outside basal lymphoid aggregate among several single stained CD3+ cells. (b) A double positive cell (thick arrow) in the T cell area of a basal lymphoid aggregate. For abbreviations see Fig. 4. Original magnification ×220.
The frequencies of cytokine positive cells in the lamina propria outside aggregates and follicles were high compared to those of cytokine positive cells within these structures (Table 2, Fig. 4d). However, IL-2 was different because IL-2 positive cells were virtually absent in the entire colonic mucosa of UC but constituted on average as much as 5·5% of the lamina propria cells in controls (Table 2, Figs 4c and 6a,b). In contrast, the frequency of IL-10 positive cells was significantly higher in UC compared to control colon. On average 2·1% of the lamina propria cells in UC were stained by anti-IL-10 MoAb, while only a few IL-10 positive cells were seen in control colon (Table 2, Fig. 4a). Most of these IL-10 positive cells in UC were stained by anti-CD3 MoAb (Fig. 5a). IFN-γ, TNF-α and TGF-β positive cells were numerous in lamina propria of both UC and control colon and constituted up to 10% of the cells (Table 2, Fig. 6c–f). IL-4 positive cells were infrequent and only found in occasional samples with no difference between UC and control colon (Table 2). IL-5 positive cells were scarce in control colon but present, although at a low frequency, in UC (<1%; Table 2). Epithelial cells were not positive for any of the cytokines analysed either in UC or in control colon (Figs 4 and 6 and data not shown).
Fig. 6.
Immunohistochemical staining for IL-2 (a, b), TGF-β (c, d) and IFN-γ (e, f) in the colonic mucosa of an UC patient (a, c, e) and a control patient (b, d, f); (a), (c) and (e) show sequential sections of UC. The bold arrow indicates an equivalent position in the sections. For abbreviations see Fig. 4. Counterstained with Mayer's haematoxylin. Original magnification ×51 in (a, b, c, d, e, f), and ×128 in the insert in (d).
DISCUSSION
We noted three major differences between intestinal T lymphocytes of UC patients and controls with regard to cytokine production. First, IL-10 mRNA levels were increased significantly in UC patients, and IL-10 mRNA levels correlated with disease activity. Notably, this was seen both in lamina propria T cells from the diseased colon and from the apparently normal ileum. IL-10 positive cells were scattered in the basal lymphoid aggregates in UC colon and the number of IL-10 positive cells was increased in the lamina propria outside these aggregates. CD4 positive T cells were responsible for almost all of the IL-10 production in UC while the small amounts of IL-10 present in normal colon appeared to be produced mainly by other leucocytes than T cells. Secondly, IL-2 production was decreased significantly in UC colon samples measured both as IL-2 mRNA expression and as frequency of cells stained for IL-2 protein. In fact, almost no IL-2 positive cells were detected in the entire colonic mucosa in UC. Thirdly, the average contents of IFN-γ and TNF-α mRNA/T cell were reduced significantly in UC colon compared to control colon. This reduction was, to a large extent, due to the fact that almost none of the T cells in the aggregates expressed these two cytokines.
To the best of our knowledge, this is the first study in which the cytokine mRNA expression in isolated intestinal T cells of UC patients has been determined in the absence of in vitro stimulation using quantitative real-time RT-PCR technology. There was good correlation between the results at the mRNA and protein levels. In addition, identical results in mRNA analyses were obtained using biopsies and resected intestine involving somewhat different isolation procedures. These findings suggest strongly that the methods used to isolate T cells here give representative cell recoveries and do not affect the level of cytokine mRNA expression. It may be argued that medication given to the patients could influence the cytokine expression in T cells. However, subgroup analyses of UC patients did not show any correlation between cytokine mRNA levels and drug treatment. Furthermore, IL-2 mRNA expression levels of colonic and ileal T cells of the same individual were different, arguing against general effects on cytokine production by medication. Thus, we are confident that these data reflect ongoing T cell responses in the intestinal mucosa of UC patients. Although the number of CD colitis samples is too limited to allow firm conclusions about local T cell responses in the mucosa of this disease, the results are consistent with a Th1-type of response [11].
Our finding of increased IL-10 expression in UC is in line with the results of Niessner and Volk [17], who showed increased IL-10 mRNA levels in crude extracts of colon biopsies from UC patients, and of Kucharzik and colleagues [18], who reported elevated serum levels of IL-10 in active UC. Moreover, in vitro activation of human intestinal T cells induces IL-10 production in lamina propria lymphocytes [19].
IL-10 is a pleiotropic cytokine involved in both cell-mediated and humoral immune responses. When added together with IL-2, it was shown to increase the cytolytic potential of virus specific cytotoxic T lymphocytes [20]. Conversely, when added together with soluble CD40-ligand it has the capacity to promote differentiation of B cells, particularly memory B cells, into plasma cells in vitro[21]. Furthermore, IL-10 has both anti-inflammatory and proinflammatory effects. Anti-inflammatory effects include induction and maintenance of oral tolerance [22] and preventing colitis in SCID mice reconstituted with CD4+CD45RBhigh T cells [23]. Anti-inflammatory effects of IL-10 seem to be executed through inhibiting the production of proinflammatory cytokines by T cells and macrophages [24,25]. IL-10 is also a growth factor for down-regulatory Tr1 cells [26]. Proinflammatory actions of IL-10 have been reported both in man [20,27] and mice [28,29]. In this context it is interesting to note that two large clinical trials with IL-10 treatment of CD patients showed limited therapeutic effects and bell-shaped dose–response curves [30,31]. Recently, it was reported that patients treated with high doses of rIL-10 showed induced IFN-γ production and IFN-γ-mediated macrophage activation [32]. Moreover, Colpaert and coworkers showed that IL-10 might induce a proinflammatory response rather than inhibition of intestinal LPL of CD patients [33]. Thus, the consequences of IL-10 production seem to be influenced strongly by the local milieu, the type of target cell as well as the concentration.
T cells in UC patients expressed high levels of IL-10 both in small and large intestine, although inflammation was restricted to the large intestine. It is conceivable that the small intestine, the suggested site for induction and maintenance of oral tolerance, is adapted to withstand high local concentrations of IL-10. However, high levels of this immunoregulatory cytokine may not be tolerated in colon, with its dense resident microflora. Colonic T cells produce IL-2, IFN-γ and TNF-α in the normal situation, indicating that these cytokines are required to keep the homeostasis with the commensal microflora. The levels of all three cytokines were significantly reduced in UC colon, which is most probably a consequence of down-regulatory actions by IL-10. Low IL-2 expression was restricted to colonic T cells in UC patients. Low IL-2 production was also seen in CD colitis and may be a general feature of chronic inflammation in the gastrointestinal tract. This notion is supported by previous reports on low IL-2 levels in the colonic mucosa of IBD patients [17,34,35].
Although Th1 cytokines were decreased in UC, there was no corresponding increase in the mRNA levels of the Th2 cytokines IL-4 and IL-5 in colonic T cells. Similarly, at the protein level we found only occasional IL-4 positive cells and no difference between UC and control colon was detected. These results are in line with previous reports [36,37]. For IL-5 there was a statistically significant difference between UC and controls, although the number of positive cells was very low (0·7% for UC). However, these cells are probably not T cells. Indeed, Lorentz and colleagues [38] demonstrated recently that mast cells and eosinophils are the main sources for IL-5 in UC. Thus, we do not find evidence for a Th2 response in UC patients as suggested by Fuss and colleagues [39], but questioned by MacDonald and colleagues [11]. What, then, is the source of IL-10? Because we have shown that CD4+ T cells produce almost all IL-10 in UC the probable source is regulatory T cells (Tr1), which are known to be induced in the presence of IL-10 and to produce high levels of IL-10 and low levels of IL-2 and IL-4 [26]. Indeed, cells with suppressor/regulatory phenotype (CD4+CD28– and/or CD25+) are present in inflamed mucosa of UC patients ([7] and our unpublished data).
We found that TGF-β1 is produced at unchanged levels in UC and that CD4+ cells are also a major source for this cytokine. Thus, it is probable that activated Tr1 cells producing both IL-10 and TGF-β1 are present in UC. It is interesting to note that human colonic mucosa was shown to harbour Tr1 cells specific for the intestinal commensal Escherichia coli[40]. Furthermore, it was reported recently that in vitro stimulation with intestinal microbial antigens induced higher IL-10 secretion by colonic LPL from IL-2–/– mice with colitis compared to the corresponding cells in wild-type mice, indicative of an enhanced Tr1 response in this IBD model [41]. IL-10 and TGF-β1 complement each other in down-regulation of immune reactions and IL-10 was shown to restore TGF-β1 responsiveness in activated T cells by induction of TGF-β receptor type II [26,42]. Failure of TGF-β1 to attenuate the inflammatory reaction could be due to aberrations in the TGF-β intracellular signalling pathway in the responding colonic T cells. Indeed, decreased phosphorylation of Smad3, an important component for TGF-β signal transduction, was reported in UC. Moreover, T cells of UC exhibited increased expression of Smad7, an inhibitory component of this pathway [43].
Taken together, these results suggest that the particular environment in UC colon with lack of major Th2 cytokines, decreased Th1 activity and aberrant TGF-β-mediated regulation pave the way for the proinflammatory actions of IL-10. The high levels of IL-10 may also promote plasma cell differentiation and thereby contribute to the production of autoantibodies seen in UC. Although the initial events leading to IL-10 production may be a response to inflammation and aimed at down-regulation, it may instead exacerbate the disease in this milieu. The demonstration of high levels of STAT-3 in UC support the notion of IL-10 mediated signalling in the inflamed tissue [44].
Acknowledgments
This work was supported by grants from the Swedish Natural Science Research Council (no. B 650–19981072, M.-L.H.), the County of Västerbotten (S.H. and Å.D.), the Swedish Cancer Foundation (S.H.), the Swedish Medical Research Council (no. 06X-09945–10A, S.H. and no. 72X-14060, Å.D.) and Ihre's Fund (Å.D.). The skilful technical assistance of Elisabeth Granström, Anne Israelsson, Katarina Omholt and Marianne Sjöstedt is gratefully acknowledged. We also wish to express our sincere gratitude to Dr Vladimir Baranov for stimulating discussions throughout this work and for critical reading of the manuscript.
REFERENCES
- 1.Fiocchi C. Inflammatory bowel disease. Etiol Pathogen Gastroenterol. 1998;115:182–505. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–71. doi: 10.1016/0016-5085(90)90343-y. [DOI] [PubMed] [Google Scholar]
- 3.Kozarek RA. Review article: immunosuppressive therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:117–23. doi: 10.1111/j.1365-2036.1993.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 4.Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–78. doi: 10.1002/jlb.67.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 Scid mice. Int Immunol. 1993;11:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 6.Sellon RK, Tonkongy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung MMW, Melgar S, Baranov V, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-γδ expression. Gut. 2000;47:215–27. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–39. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189:1169–79. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald TT, Monteleone G, Pender SFL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 12.Targan SR, Hanauer SB, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor-α for Crohn's disease. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 13.Lundqvist C, Hammarström M-L, Athlin L, Hammarström S. Isolation of functionally active intraepithelial lymphocytes and enterocytes from human small and large intestine. J Immunol Meth. 1992;152:253–63. doi: 10.1016/0022-1759(92)90147-l. [DOI] [PubMed] [Google Scholar]
- 14.Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström ML. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–12. [PubMed] [Google Scholar]
- 15.Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarström S, Hammarström ML. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology. 2002;123:667–78. doi: 10.1053/gast.2002.35355. [DOI] [PubMed] [Google Scholar]
- 16.Fahlgren A, Hammarström S, Danielsson Å, Hammarström ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131:90–101. doi: 10.1046/j.1365-2249.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neissner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucharzik T, Stoll R, Lügering N, Domschke W. Circulating anti-inflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;100:452–6. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braunstein J, Qiao L, Autschbach F, Schürmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–20. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santin AD, Hermonat PL, Ravaggi A, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8+ cytotoxic T lymphocytes. J Virol. 2000;74:4729–37. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–9. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- 22.Strobel S, Mowat AMcI. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 23.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1003. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 25.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 26.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa Y, Becker G, Stinn JL, Shimizu K, Libby P, Mitchell RN. Interleukin-10 (IL-10) augments allograft arterial disease. Paradoxical effects of IL-10 in vivo. Am J Pathol. 1999;155:1929–39. doi: 10.1016/S0002-9440(10)65512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wogensen L, Huang X, Sarvetnick N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin-10 in the islets of Langerhans. J Exp Med. 1993;178:175–85. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balasa B, Sarvetnick N. The paradoxical effects of interleukin 10 in the immunoregulation of autoimmune diabetes. J Autoimmun. 1996;9:283–6. doi: 10.1006/jaut.1996.0036. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber S, Fedorak RN, Haagen Nielsen O, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Gastroenterology. 2000;119:1461–72. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 31.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. Gastroenterology. 2000;119:1473–82. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 32.Tilg H, van Montfrans C, van den Ende A, et al. Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon γ. Gut. 2002;50:191–5. doi: 10.1136/gut.50.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colpaert S, Vanstraelen K, Liu Z, et al. Decreased lamina propria effector cell responsiveness to interleukin-10 in ileal Crohn's disease. Clin Immunol. 2002;102:68–76. doi: 10.1006/clim.2001.5149. [DOI] [PubMed] [Google Scholar]
- 34.Kusugami K, Matsuura T, West GA, Youngman KR, Rachmilewitz D, Fiocchi C. Loss of interleukin-2-producing intestinal CD4+ T cells in inflammatory bowel disease. Gastroenterology. 1991;101:1594–605. doi: 10.1016/0016-5085(91)90397-4. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima K, West GA, Fiocchi C. Adequacy of mucosal biopsies for evaluation of intestinal cytokine-specific mRNA. Comparative study of RT-PCR in biopsies and isolated cells from normal and inflamed intestine. Dig Dis Sci. 1995;40:1498–505. doi: 10.1007/BF02285198. [DOI] [PubMed] [Google Scholar]
- 36.Karttunnen R, Breese EJ, Walker-Smith JA, MacDonald TT. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994;47:1015–8. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West GA, Matsuura T, Levine AD, Klein JS, Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–95. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 38.Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496–503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ whereas ulcerative colitis manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 40.Khoo UY, Proctor IE, Macpherson AJS. CD4+ T cell down-regulation in human intestinal mucosa. Evidence for intestinal tolerance to luminal bacterial antigens. J Immunol. 1997;158:3626–44. [PubMed] [Google Scholar]
- 41.Waidmann M, Allemand Y, Lehmann J, et al. Microflora reactive IL-10 producing regulatory T cells are present in the colon of IL-2 deficient mice but lack efficacious inhibition of IFN-γ and TNF-α production. Gut. 2002;50:170–9. doi: 10.1136/gut.50.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cottrez F, Groux H. Regulation of TGF-β response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–8. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 43.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Inv. 2001;108:601–9. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Susuki A, Hanada T, Mitsuyama K, et al. CIS3/SOC3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]