Abstract
Evidence suggests that spironolactone, an aldosterone antagonist, has effects on many cell types independent of its binding to cytosolic mineralocorticoid receptors. We tested the effects of spironolactone on ex vivo-activated human blood leucocytes using gene expression analyses (GeneChip®, 12 000 genes) and enzyme immunoassay for quantitating secreted pro- and anti-inflammatory cytokines. Furthermore, to evaluate the safety and efficacy of spironolactone as an anti-inflammatory drug 21 patients with rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) or other arthritides were treated for up to 22 months with 1–3 mg/kg/day. Spironolactone, at in vivo attainable doses, markedly suppressed transcription of several proinflammatory cytokines and, accordingly, inhibited release of tumour necrosis factor, lymphotoxin, interferon-γ, granulocyte-macrophage colony-stimulating factor and interleukin 6 (70–90% inhibition). Release of these cytokines was also suppressed when testing whole blood from RA patients receiving 50 mg spironolactone twice daily, indicating that pharmaceutical use of the drug may suppress the release of inflammatory cytokines. Spironolactone therapy was generally well tolerated, although treatment had to be stopped in two adults on concomitant methotrexate therapy. Sixteen patients (76%) responded favourably. American College of Rheumatology criteria (ACR)20 or better was achieved in six of nine RA patients; four reached ACR70. Eight of nine JIA patients improved. In conclusion, spironolactone inhibits production of several proinflammatory cytokines considered to be of pathogenic importance in many immunoinflammatory diseases and shows positive effect in patients with chronic arthritis. Its effect as an anti-inflammatory drug should be explored, because prolonged spironolactone therapy is reasonably safe and economically attractive compared with many modern anti-inflammatory therapies.
Keywords: autoimmune diseases, clinical trial, gene array (GeneChip), inflammation
INTRODUCTION
Spironolactone (SPIR) is a competitive aldosterone receptor antagonist used for over 40 years to treat diseases associated with primary or secondary hyperaldosteronism [1]. SPIR, however, also binds to other steroid receptors, as evidenced by progestational and antiandrogenic side effects associated with long-term therapy, and as SPIR inhibits voltage-dependent Ca2+ channels, it may affect many cell types independent of its interaction with cytosolic mineralocorticoid receptors [1,2]. SPIR has been shown recently to improve endothelial dysfunction and increase survival in congestive heart failure [3,4].
Cytokines are small (glyco)proteins involved primarily in growth processes and in host responses to tissue injury, infections and many diseases mainly, but not invariably associated with immunoinflammation [5,6]. One of the clinically most important cytokines, tumour necrosis factor (TNF), is produced by macrophages and T-lymphocytes activated by a wide variety of stimuli, including endotoxin (lipopolysaccharide, LPS) and other microbial components, and various stress-related processes [7]. TNF recruits and activates inflammatory cells through increased production of chemokines and induction of adhesion molecules on endothelial cells and leucocytes. TNF also induces other cytokines with major roles in inflammation, for example interleukin (IL)-1α, IL-1β, IL-6, IL-18 and granulocyte-macrophage colony-stimulating factor (GM-CSF). The involvement of TNF as a pathogenic factor has been documented in several immunoinflammatory diseases, including arthritic diseases, inflammatory bowel diseases, type 1 diabetes mellitus, multiple sclerosis, and Guillain–Barré syndrome [7]. In patients with rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) and ankylosing spondylitis, for example, neutralizing anti-TNF antibodies and soluble TNF receptors are powerful means of controlling disease activity [8–12].
Other cytokines than TNF may be critically involved in the pathogenesis of arthritides. For example, IL-1 receptor antagonist (IL-1ra), which inhibits the effects of IL-1α/IL-1β, and neutralizing antibody to interferon-γ (IFN-γ) is effective in RA [13,14]. In addition, GM-CSF therapy worsens arthritis in patients with Felty's syndrome, and antibody to IL-6 receptor inhibits the development of collagen arthritis in monkeys [15–17].
Against this background, we investigated the effects of SPIR on in vitro production of cytokines. The use of massive gene expression analyses, combined with measurements of cytokines induced in and released from blood leucocytes, revealed a marked suppressive effect of the steroid on several proinflammatory cytokines. This finding prompted initiation of a phase II clinical trial of SPIR as an anti-inflammatory drug in patients with chronic arthritic diseases.
MATERIALS AND METHODS
Preparation and stimulation of human whole-blood and blood mononuclear cells
To mimic the in vivo conditions most effectively, whole-blood cultures were prepared from heparinized peripheral venous blood from healthy donors or from patients. The blood, diluted 1 : 4 with RPMI-1640, was cultured for 22 h in 10 ml polypropylene tubes (Nunc, Roskilde, Denmark) at 37°C in a humidified 5% CO2 atmosphere in the presence or absence of reagents as indicated.
Human blood mononuclear cells (MNC) were prepared from healthy donors by density gradient centrifugation on Ficoll-Hypaque (Lymphoprep, Nycomed, Oslo, Norway). The cells, 1 × 106/ml, were suspended in RPMI-1640 containing 5% heat-inactivated, pooled normal human serum and cultured as mentioned above. For GeneChip experiments, 20 × 106 MNC were cultured in 10 ml RPMI-1640 containing 10% autologous human serum (see below).
Antigen non-specific induction of cytokine production from blood cells was achieved by addition of 0·1 µg/ml of LPS (Escherichia coli 055:B5; Difco Laboratories, Detroit, MI, USA) and 20 µg/ml of phytohaemagglutinin-P (PHA; Difco). SPIR and canrenoic acid were purchased from Sigma (St Louis, MO, USA), and aldosterone was purchased from Research Plus (Bayonne, NJ, USA). These hydrophobic reagents were dispersed by vigorous vortexing of a 1-m suspension in preheated (60°C) phosphate-buffered saline (PBS), with subsequent cooling to 37°C under repeated vortexing before the final dilutions in each experiment. The reagents were added at total (free and protein-bound) concentrations of 10 and 100 µm. LPS + PHA-stimulated controls received equal of drug vehicle (PBS) alone, and were processed in parallel. Cell viability was ascertained by >95% trypan blue exclusion after overnight incubation of activated blood mononuclear cells without/with up to 1000 µm levels of SPIR.
GeneChip investigations
The protocols from Affymetrix (Santa Clara, CA, USA) were followed as detailed previously [18]. Briefly, MNC, isolated and cultured as mentioned above, were preincubated with 100 µm of SPIR or vehicle alone (control). After 15 min, 0·1 µg/ml of LPS and 20 µg/ml of PHA were added. After 4 h at 37°C, the cells were isolated by centrifugation and total RNA was prepared and subsequently repurified. Synthesis of double-stranded cDNA using an oligo-dT primer with the T7 RNA polymerase recognition sequence appended was performed, followed by in vitro transcription/biotin labelling with T7 RNA polymerase using the BioArrayTM High YieldTM RNA Transcript Labelling Kit (Enzo Diagnostics, Farmingdale, NY, USA). After fragmenting the biotin-labelled transcripts, the hybridization mixture was spiked with Bio B, C, D and cre for control of arrays. The human genome U95Av2 chips were used for hybridization; each contains gene expression data for ≈12·000 human full-length genes. After 16 h, the chips were washed and the biotin groups reacted with streptavidin-coupled phycoerythrin (Molecular Probes, Eugene, OR, USA). Antibody enhancement was used during washing, and the GeneChips were scanned using a Hewlett Packard HP G2500A GeneArray scanner. The hybridization results were processed using the Microarray Suite MAS 5·0 software and analysed further using MicroDB 3·0 and DMT 3·0 software (Affymetrix).
Real time polymerase chain reaction (PCR)
The expression of six genes selected from the GeneChip experiment (TNF, lymphotoxin (LT)-α, IFN-γ, osteopontin, IL-1β and IL-6) was verified on RNA from other healthy individuals using real time PCR with CYBR Green technology and GAPDH as the housekeeping reference gene (ABI protocol 2001; Applied Biosystem, Foster City, CA, USA).
Quantification of cytokines
Human cytokines were measured by a double sandwich ELISA using either monospecific, polyclonal rabbit antibody or monoclonal antibodies to purified human recombinant cytokines [19]. Human IL-1β was measured by a triple sandwich ELISA which largely excludes quantification of bioinactive pro-IL-1β[20]. The sensitivity limits ranged from 0·2 to 30 pg/ml with the intra- and interassay coefficients of variation below 15%.
Patients
Nine patients with RA, one patient with ankylosing spondylitis and arthritis, one patient with Reiter's disease and severe plantar pustulosis, one patient with systemic lupus erythematosus (SLE) with arthritis and nine patients with JIA were treated in an open trial with SPIR 1–3 mg/kg/day. Each fulfilled the internationally accepted criteria for diagnosis [21–23]. The inclusion criterion was clinically overt inflammatory arthritis. Most patients suffered from long-term disease and were controlled insufficiently by conventional disease-modifying antirheumatic drugs (DMARDS). Patients receiving more than 15 mg prednisolone daily were excluded, as were patients with hypotension or renal insufficiency. Patients on DMARDS/glucocorticoids were required to be on stable medication for at least 1 month before and after initiation of SPIR therapy. Patients gave informed consent, and the study was approved by the regional ethics committees.
RESULTS
Effect of SPIR on gene expression in blood mononuclear cells
To evaluate the sites of action of SPIR in maximally stimulated leucocytes, and to reveal cytokines or other molecules whose synthesis might be affected by the drug, we tested the in vitro effect of the drug on expression of approximately 12 000 genes in human blood MNC stimulated with both PHA and LPS. Almost 700 of these genes were scored as significantly inhibited, and approximately 600 were scored as significantly increased in activated cells cultured for 4 h with SPIR. Table 1 shows some of the results of one such experiment emphasizing the effects on cytokines. The most down-regulated of all genes was that encoding recombinant IFN-γ (top transcript); the most up-regulated gene is shown at the bottom.
Table 1.
Effect of spironolactone on gene expression in blood mononuclear cells
| A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|
| Before SPIR (control) > | After SPIR > | |||||||||
| Signal | Det. score | Det. P-value | Signal | Det. score | Det. P-value | Signle % of control | Change | Change P-value | Cytokine | Description |
| 225·5 | P | 0·00 | 19 | A | 0·16 | 8 | D | 1·00 | IFN-γ | Cluster Incl. X13274:Human mRNA for interferon IFN-γ |
| 5306·9 | P | 0·00 | 451·3 | P | 0·00 | 9 | D | 1·00 | IFN-γ + | M26683/Human interferon gamma treatment inducible mRNA |
| 566·7 | P | 0·00 | 57·2 | P | 0·01 | 10 | D | 1·00 | OPN | Cluster Incl. AF052124:Homo sapiens clone 23810 osteopontin mRNA |
| 92·4 | P | 0·00 | 10·3 | A | 0·72 | 11 | D | 1·00 | IFN-γ + | X02530/Human mRNA for gamma-interferon inducible early response gene |
| 309·1 | P | 0·00 | 46·1 | P | 0·00 | 15 | D | 1·00 | IFN-γ | J00219/HUMIFNG Human immune interferon (IFN-γ) gene |
| 920·9 | P | 0·00 | 140·3 | A | 0·11 | 15 | D | 1·00 | G-CSF | X03656/Human gene for granulocyte colony-stimulating factor (G-CSF) |
| 99·9 | P | 0·00 | 18·3 | A | 0·34 | 18 | D | 1·00 | FasL | D38122/Human mRNA for Fas ligand |
| 168·3 | P | 0·03 | 39·9 | A | 0·42 | 24 | D | 1·00 | GM-CSF | M13207/Human granulocyte-macrophage colony-stimulating factor (CSF1) gene |
| 152·5 | P | 0·00 | 46·8 | A | 0·32 | 31 | D | 1·00 | OSM | M27288/Human oncostatin M gene, exon 3 |
| 178·6 | P | 0·00 | 63·3 | P | 0·01 | 35 | D | 1·00 | OPN | J04765/Human osteopontin mRNA |
| 6255·7 | P | 0·00 | 3224 | P | 0·00 | 52 | D | 1·00 | IL-1ra | Cluster Incl. X52015:H.sapiens mRNA for interleukin-1 receptor antagonist |
| 25·5 | P | 0·03 | 13·2 | A | 0·25 | 52 | NC | 0·99 | IL-1a | M28983/Human interleukin 1 α (IL 1) mRNA |
| 6778·5 | P | 0·00 | 3760 | P | 0·00 | 55 | D | 1·00 | IL-6 | Cluster Incl. X04430:Human IFN-beta 2a mRNA |
| 1743·8 | P | 0·00 | 1046 | P | 0·00 | 60 | D | 1·00 | TNF | X02910/Human gene for tumour necrosis factor (TNF-α) |
| 746·2 | P | 0·00 | 461 | P | 0·00 | 62 | D | 1·00 | IFN+/IFI56 | M24594/Human interferon-inducible 56 Kd protein mRNA |
| 575·3 | P | 0·00 | 373·1 | P | 0·00 | 65 | D | 1·00 | IFN-γ +/IFI16 | M63838/Human interferon-γ induced protein (IFI 16) gene |
| 53·7 | P | 0·00 | e39 | A | 0·19 | 73 | D | 1·00 | IL-10 | U16720/Human interleukin 10 (IL10) gene |
| 1·5 | A | 0·93 | 1·1 | A | 0·96 | 73 | NC | 0·50 | IL-7 | J04156/Human interleukin 7 (IL-7) mRNA |
| 2713·6 | P | 0·00 | 2078 | P | 0·00 | 77 | D | 1·00 | IFN+/IFI17 | J04164/Human interferon-inducible protein 9–27 mRNA |
| 8463·7 | P | 0·00 | 6963 | P | 0·00 | 82 | D | 1·00 | IL-1β | Cluster Incl. M15330:Human interleukin 1-β (IL1B) mRNA |
| 99·4 | M | 0·05 | 86·4 | P | 0·02 | 87 | NC | 0·50 | pro-IL-13 | U31120/Human interleukin-13 (IL-13) precursor gene |
| 9585·2 | P | 0·00 | 8471 | P | 0·00 | 88 | NC | 0·89 | pro-IL-1β | X04500/Human gene for prointerleukin 1 β |
| 23·5 | M | 0·05 | 21·2 | A | 0·07 | 90 | NC | 0·99 | IL-15 | U14407/Human interleukin 15 (IL15) mRNA |
| 8220·4 | P | 0·00 | 7441 | P | 0·00 | 91 | NC | 0·98 | IL-8 | M28130/Human interleukin 8 (IL8) gene |
| 50·2 | A | 0·30 | 46·5 | A | 0·18 | 93 | NC | 0·50 | LT-α | Cluster Incl. D12614:Human mRNA for lymphotoxin (TNF-β) |
| 69·9 | P | 0·03 | 74·3 | P | 0·01 | 106 | NC | 0·14 | IL-4 | M13982/Human interleukin 4 (IL-4) mRNA |
| 683·1 | P | 0·03 | 747·4 | P | 0·02 | 109 | NC | 0·50 | M-CSF | M37435/Human macrophage-specific colony-stimulating factor (CSF-1) mRNA |
| 1065·7 | P | 0·00 | 1215 | P | 0·00 | 114 | NC | 0·11 | TGF-β | M38449/Human transforming growth factor-β mRNA |
| 21 | A | 0·23 | 24·2 | A | 0·22 | 115 | NC | 0·26 | IL-5 | X04688/Human mRNA for T-cell replacing factor (interleukin-5) |
| 37·3 | A | 0·30 | 45·6 | A | 0·23 | 122 | NC | 0·50 | IL-3 | M20137/Human interleukin 3 (IL-3) mRNA |
| 2·2 | A | 0·98 | 3·3 | A | 0·99 | 150 | NC | 0·50 | IL-2 | M22005/Human interleukin 2 gene, clone pATtacIL-2C/2TT |
| 669·5 | P | 0·00 | 1135 | P | 0·00 | 169 | I | 0·00 | PF4 | M25897/Human platelet factor 4 (PF4) mRNA |
| 0·4 | A | 0·98 | 11·6 | A | 0·20 | 2900 | I | 0·00 | M73239/Human (clone SF1) hepatocyte growth factor (HGF) mRNA | |
Data were obtained with MNC from a healthy adult, stimulated with PHA + LPS before (control) and after addition of 100 µm of SPIR (free and protein-bound). Genes shown were selected from the ≈ 12 000 full-length genes represented on the human genome U95Av2 chip and the selection highlights the impact of SPIR on the expression of genes encoding cytokines. The data were sorted according to the signal change (column G), showing the most negatively affected genes at the top and the most enhanced genes at the bottom. Columns A and D show the signals before/after SPIR. Columns B and E show the detection scores before/after SPIR (A = absent, P = present, M = marginal). Columns C and F show the detection P-values before/after SPIR. Column G shows the signal changes in percentage of controls. Column H shows the change scores (D = decreased, NC = no change, I = increased). Column I shows the change in P-values, where values >0·95 indicate significant suppression and values <0·05 indicate significant enhancement of gene expression. Column J shows the cytokine acronyms, and column K shows the gene accession number/identifier. This study was repeated two times with essentially similar responses. FasL: Fas ligand, G-CSF: granulocyte colony-stimulating factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, IFN-γ: interferon γ (various IFN- and IFN-γ-induced genes are shown as IFN+ and IFNγ+, respectively), IL: interleukin, IL-1ra: IL-1 receptor antagonist, LT-α: lymphotoxin α, M-CSF: macrophage colony-stimulating factor, OPN: osteopontin, OSM: oncostatin M, PF4: platelet factor 4, TGF-β: transforming growth factor-β. Det.: detection.
Interestingly, the genes that were suppressed the most by SPIR included a number of genes encoding proinflammatory cytokines. Thus, the level of osteopontin mRNA decreased markedly after SPIR exposure as assessed by two different probe sets. Other negatively affected genes were those encoding IFN-γ, Fas ligand (FasL), GM-CSF, oncostatin M and IL-6. The TNF gene was also significantly down-regulated, whereas LT-α was scored as unchanged (only small amounts of LT-α transcripts were produced after culture for 4 h). Transcripts of a number of IFN-γ-induced genes also decreased significantly, a further indicator of the biological effect of SPIR-induced suppression of the IFN-γ gene.
Transcripts of other proinflammatory cytokines, notably IL-1α and IL-1β, did not change (using probe sets for IL-1α and the IL-1β precursor) or decreased only slightly (mature IL-1β). It is noteworthy that the genes encoding the anti-inflammatory cytokines IL-4 and transforming growth factor β (TGF-β) were unaffected, whereas the levels of IL-1ra and IL-10 transcripts decreased slightly.
Similar results were obtained when the GeneChip experiment was repeated twice using MNC from different healthy individuals and GeneChip U133A interrogating approximately 35 000 transcripts.
When RNA samples from cells of other individuals were tested by real time PCR, the data obtained for all the selected cytokines (TNF, LT-α, IFN-γ, osteopontin, IL-1β, IL-6) were in complete agreement with the GeneChip results, except that SPIR diminished the level of LT-α mRNA (data not shown).
Effect of SPIR on cytokine production
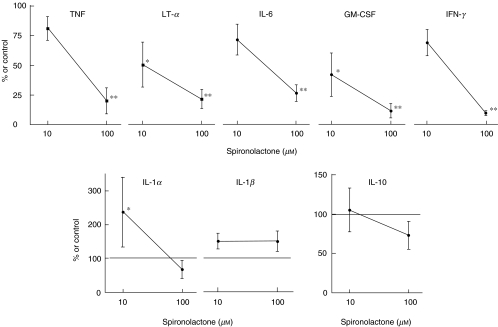
We next tested the effect on the in vitro production/release of cytokines. Figure 1 shows the results from whole-blood cultures, where SPIR significantly (70–90%) suppressed release of cytokines whose genes were also inhibited by the drug. In contrast, the release of IL-1β, a monocyte/macrophage-derived proinflammatory cytokine, was not inhibited, showing that the suppressive function cannot be ascribed to an overall cytotoxic effect of the drug; this was also supported by viability studies.
Fig. 1.
Effect of spironolactone on whole-blood cytokine production. Human whole blood was diluted to 20% v/v with medium RPMI-1640 and incubated with drugs, or with medium alone. After 22 h, the supernatants were isolated and tested by ELISA for cytokines. Results are shown as percentage of controls cultured in parallel with PHA + LPS, but without drug (mean ± s.e.m., n = 6). Asterixes mark values significantly different from those of controls (*P < 0·05 and **P < 0·01, Student's t-test). The levels of cytokines in the controls were IL-1α: 348 ± 128, IL-1β: 2588 ± 1019, TNF: 1063 ± 201, LT-α: 592 ± 228, IFN-γ: 2550 ± 357, GM-CSF: 2175 ± 685, IL-6: 10 490 ± 2737, IL-10: 97 ± 24 (pg/ml, mean ± s.e.m., n = 6).
SPIR caused a similar pattern of response when testing the release of the same cytokines from human blood MNC isolated as described for the GeneChip studies (data not shown).
Effect of aldosterone and a major SPIR metabolite on cytokine production
Because SPIR and its major non-sulphated metabolite, canrenone, both function as competitive aldosterone antagonists at the level of cytosolic and nuclear mineralocorticoid receptors [24], we tested whether canrenone and/or aldosterone had effects similar to SPIR. Canrenone, up to 100 µm, failed to affect the release of the cytokines shown in Fig. 1 to be suppressed by SPIR; this was seen when using whole blood as well as MNC cultures. Aldosterone, had no effect up to 10 µm (≈ 10 000 times the physiological level). Neither of these compounds affected suppression of cytokine production afforded by 10 µm SPIR. This suggests that SPIR-induced inhibition of production of proinflammatory cytokines is unrelated to its effect on mineralocorticoid receptors.
Effect of SPIR therapy on cytokine production ex vivo
It is unknown whether the in vitro effects of SPIR on cytokine production depend solely on the free, non-protein-bound fraction of the drug and, hence, whether the SPIR levels used in the in vitro studies might have pharmaceutical relevance. For this reason, it was important to ascertain whether blood leucocytes isolated from individuals receiving conventional therapeutic doses of SPIR were prevented from producing proinflammatory cytokines ex vivo. As shown in Table 2, leucocytes from two patients suffering from RA, who had been treated with SPIR 50 mg twice daily for 2 weeks, produced less TNF, LT-α, IFN-γ, GM-CSF and IL-6 compared with leucocytes isolated from the same patients immediately before SPIR therapy.
Table 2.
Effect of spironolactone therapy on whole-blood cytokine production ex vivo
| Patient 1 | Patient 2 | ||||
|---|---|---|---|---|---|
| Cytokine | Before SPIR (control) | During SPIR therapy | Before SPIR (control) | During SPIR therapy | % of control mean |
| TNF | 1110 | 130 | 1850 | 650 | 23 |
| LT-α | 170 | 48 | 270 | 54 | 24 |
| IFN-γ | 4300 | 1140 | 1230 | 700 | 42 |
| GM-CSF | 3460 | 2740 | 1090 | 610 | 68 |
| IL-6 | 22 350 | 9700 | 14 200 | 4355 | 37 |
| IL-1α | 425 | 440 | 530 | 530 | 102 |
| IL-1β | 1490 | 1450 | 2300 | 2530 | 104 |
Diluted whole-blood cultures from two RA patients were used, one prepared from before start of spironolactone (SPIR) therapy, and the other after 14 days of treatment with SPIR tablets 50 mg × 2 daily. The supernatant cytokine levels are shown as pg/ml.
Effect of SPIR therapy in patients with arthritides
To evaluate whether SPIR therapy might have anti-inflammatory potential in patients suffering from immunoinflammatory disorders, a phase II study was carried out in patients with chronic arthritic diseases largely unaffected by conventional therapies.
As shown in Table 3, 16 of 21 arthritis patients (76%) responded favourably. American College of Rheumatology criteria (ACR)20 [21] or better was achieved in six of nine RA patients (67%), in some cases within 2–4 weeks of therapy, and ACR70 was achieved in four (44%). Interestingly, a patient with Reiter's disease experienced complete clearing of pustules affecting the soles. A patient with long-standing ankylosing spondylitis failed to improve even when given 200 mg SPIR/day. Eight of nine patients with JIA (89%) improved according to the Giannini criteria: at least 30% improvement from baseline in three of six defined clinical variables, with no more than one of the remaining variables worsening by over 30% [23]. One of these patients, number 14, stopped therapy with methotrexate on entering the study, but the dose of prednisolone was augmented in the second week of the trial, making it difficult to assess the efficacy of SPIR in this case.
Table 3.
Effect of spironolactone therapy in patients with chronic arthritic diseases
| n | Mean age years (range) | Disease duration years (range) | Concomitant DMARDS/glucocorticoid (n) | SPIR therapy (n) | Baseline activity (range) | Outcomea (n;%) | Side effects (n) | |
|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis | 9 | 57 (43–77) | 11 (2–30) | Leflunomide (2)Methotrexate (3)Anti-IL-6R,MRA/CHARISMA (1)Prednisolone (3) | 50 mg × 2 (7)100 mg × 2 (2)for up to 1 yearb | Tender joints: 4–23Swollen joints: 1–11Pain score: 19–80Patient GA: 16–98Physician GA: 9–76Phys.disab. 0·25–2·4 | ACR70 (4; 44%)ACR30 (1; 11%)ACR20 (1; 11%)< ACR20 (1; 11%)Withdrawn (2; 22%)(hyperkalaemia) | Hyperkalaemia (3)Dizziness (2) |
| Other arthritides in adults One had severe palmoplantar pustulosis | 3 | 54 (32–74) | 14 (8–30) | Azathioprine (1)Prednisolone (1) | 50 mg × 2 (1)100 mg × 2 (2)for up to 8 months | Tender joints: 3–5Swollen joints: 6–13Pain score: 23–30Patient GA: 24–71Physician GA: 20–44Phys.disab. 0·25–1·35 | ACR70 (2; 67%)< ACR20 (1; 33%)Clearing of pustules | Disturbance of menstrual cycle (1) |
| Juvenile idiopathic arthrits | 9 | 12 (9–19) | 6 (1–9) | Methotrexate (7)Anti-TNF, etanercept (2)Prednisolone (7) | 2–3 mg/kg/day for up to 5 months (9) | Tender joints: 2–21Pain score: 10–80Patient GA: 10–60Physician GA: 30–80CHAQ: 0·2–1·2 | Improved (8; 89%)Unchanged (1; 11%) | Disturbance of menstrual cycle (1) |
Outcome was evaluated by ACR (American College of Rheumatology) criteria and, for patients with JIA, by the Giannini criteria, see text.
2 and 3 weeks, respectively, in the two withdrawn patients. CHAQ: Children's Health Assessment Questionnaire, DMARDS: disease-modifying antirheumatic drugs, GA: global assessment, n: number of patients.
SPIR was generally well tolerated, although the drug was withdrawn from two RA patients on concomitant methotrexate therapy. Hyperkalaemia (serum potassium >5 mm) was seen in four adult patients (33%). Three had only slightly elevated serum potassium levels; two of these experienced ACR70 responses and had no subjective side-effects and one relapsed after an initial ACR20 response. One patient had pronounced hyperkalaemia and was discontinued after only 2 weeks of therapy. Two female patients complained of disturbed menstruational cycle. One patient was treated with SPIR for 22 months without detectable side effects.
DISCUSSION
SPIR is administered orally with dosages to adults from 25 to 200 mg/day, but up to 400 mg/day may be given for 3–4 weeks as a diagnostic measure of primary hyperaldosteronism. Absorption from the intestinal tract is almost complete, especially if administered with food intake, and SPIR and its immediate metabolite, 7α-thiomethylspirolactone, reach serum levels of 1–2 µm 1–4 h after intake [25]. Because more than 90% of the drug and its immediate metabolites are bound to plasma proteins, total (free and protein-bound) SPIR concentrations of 10–100 µm were considered relevant for the ex vivo studies.
The suppressive effect of these doses of the drug on the in vitro transcription of several proinflammatory cytokines, including TNF, IFN-γ and GM-CSF, with modest effects on anti-inflammatory cytokines, suggested that SPIR may indeed have anti-inflammatory potential. This was corroborated through direct quantification of cytokine release from LPS- and PHA-stimulated blood monocytes and B and T lymphocytes, because the drug inhibited release of several of the proinflammatory cytokines whose transcription was shown to be affected. This pattern of response was also seen when testing blood cells from RA patients receiving SPIR, indicating that cytokine-suppressive levels of the drug may indeed be achieved by administration of conventional doses of SPIR.
The major non-sulphated metabolite of SPIR, canrenone, had no effect on cytokine production. Because canrenone has retained an aldosterone antagonistic effect at the receptor level, these findings indicate that the cytokine-suppressive function of SPIR is unrelated to its ability to interact with mineralocorticoid receptors. This is supported by the finding that aldosterone itself also failed to affect the release of the proinflammatory cytokines listed in Table 2. In this context, it is noteworthy that aldosterone appears to inhibit the release of the anti-inflammatory cytokine IL-1ra, an effect counteracted by SPIR [26].
The observed suppressive effects of SPIR on several proinflammatory cytokines is likely to be of clinical interest. For example, some of the symptoms of autoimmune diseases, tissue transplantations, graft-versus-host disease, heart failure, asthma, sepsis and reactions to tissue injury, including complications to surgery, have been attributed to TNF. Direct involvement of TNF as a pathogenic factor has indeed been documented in several immunoinflammatory diseases, including RA, JIA, psoriasis with or without arthritis, inflammatory bowel diseases and atherosclerosis [5,6,11,27–29].
In RA and, most probably, in JIA, TNF is near the beginning stage of a sequential induction cascade that involves up-regulation of other proinflammatory cytokines such as IL-1, IL-18 and GM-CSF [30]; several of these have critical roles in arthritic diseases [13,15,17]. It is likely that other effector mechanisms contribute to the inflammatory activities attributed to TNF, for example recruitment and activation of inflammatory cells through increased production of chemokines, induction of adhesion molecules on endothelial cells and blood leucocytes and activation of chondrocytes, osteoclasts and sy-novial cells. Other cytokines than TNF and the TNF-induced cytokines may also be involved in the pathogenesis of RA. IFN-γ, for example, is up-regulated in synovial T-lymphocytes in RA and enhances IL-12 production in rheumatoid synovial cells, and monoclonal antibody to IFN-γ is effective even when administered to patients with treatment-resistant forms of RA [14].
Because the in vitro production of almost all cytokines considered of pathogenic importance in RA and JIA was suppressed by SPIR, we initiated an open-label study of safety and efficacy parameters in patients with therapy resistant arthritic diseases. The result of this first clinical study of SPIR as an anti-inflammatory drug shows that the drug was reasonably well tolerated and that most patients improved in clinical assessments of disease activity. Many of these patients had previous or current unfavourable responses to disease-modifying antirheumatic drugs and were considered candidates for anti-TNF therapies [8–11].
Although inhibition of the function of a single cytokine may be therapeutically effective, as in the case of anti-TNF therapies in arthritis patients, it is likely that prolonged and simultaneous inhibition of several pathogenically important cytokines may prove superior in many diseases, particularly if suppression is exerted at the level of production rather than function of these cytokines. This, however, is difficult to achieve, and modern biologicals suffer from several major limitations in this regard. These frequently include a narrow spectrum of activity, a need for repeated parenteral administration, a limited half-life in vivo, development of neutralizing antibodies with prolonged therapy and huge costs for the individual and society.
Against this background, the implications of the present study for future research are important and should include phase III clinical trials of SPIR or SPIR derivatives for the treatment of patients with a range of immunoinflammatory diseases. The drug not only inhibits production of several cytokines considered to be of pathogenic importance in many diseases, but decades of clinical experience suggest that SPIR may be administered for prolonged periods and hence provide an economically attractive contribution to modern anti-inflammatory therapies.
Acknowledgments
The excellent work of Marianna Thomsen and Lone Bredahl is gratefully acknowledged. The study was supported by the Danish Rheumatism Association and The Danish Biotechnology Program.
REFERENCES
- 1.Doggrell SA, Brown L. The spironolactone renaissance. Expert Opin Invest Drugs. 2001;10:943–54. doi: 10.1517/13543784.10.5.943. [DOI] [PubMed] [Google Scholar]
- 2.Sorrentino R, Autore G, Cirino G, et al. Effect of spironolactone and its metabolites on contractile property of isolated rat aorta rings. J Cardiovasc Pharmacol. 2000;36:230–5. doi: 10.1097/00005344-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. Randomized Aldactone Evaluation Study Investigators. [DOI] [PubMed] [Google Scholar]
- 4.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES) Rales Investigators Circulation. 2000;102:2700–6. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M, Brennan FM, Maini R. Cytokines in autoimmune disorders. Int Rev Immunol. 1998;17:217–28. doi: 10.3109/08830189809084493. [DOI] [PubMed] [Google Scholar]
- 6.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 7.O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nature Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 8.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–10. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 9.Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 10.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 11.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. Pediatric Rheumatology Collaborative Study Group. [DOI] [PubMed] [Google Scholar]
- 12.Gorman JD, Sack KE, Davis JC., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–56. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 13.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Sigidin YA, Loukina GV, Skurkovich B, et al. Randomized, double-blind trial of anti-interferon-γ antibodies in rheumatoid arthritis. Scand J Rheumatol. 2001;30:203–7. doi: 10.1080/030097401316909530. [DOI] [PubMed] [Google Scholar]
- 15.Hazenberg BP, Van Leeuwen MA, Van Rijswijk MH, et al. Correction of granulocytopenia in Felty's syndrome by granulocyte-macrophage colony-stimulating factor. Simultaneous induction of interleukin-6 release and flare-up of the arthritis. Blood. 1989;74:2769–70. [PubMed] [Google Scholar]
- 16.Mihara M, Kotoh M, Nishimoto N, et al. Humanized antibody to human interleukin-6 receptor inhibits the development of collagen arthritis in cynomolgus monkeys. Clin Immunol. 2001;98:319–26. doi: 10.1006/clim.2000.4989. [DOI] [PubMed] [Google Scholar]
- 17.Cook AD, Braine EL, Campbell IK, et al. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3:293–8. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieneck K, Bovin LF, Josefsen K, et al. Massive parallel gene expression profiling of RINm5F pancreatic islet β-cells challenged with interleukin-1β. APMIS. 2000;108:855–72. doi: 10.1111/j.1600-0463.2000.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 19.Hansen MB, Svenson M, Bendtzen K. Human anti-interleukin 1α antibodies. Immunol Lett. 1991;30:133–40. doi: 10.1016/0165-2478(91)90102-g. [DOI] [PubMed] [Google Scholar]
- 20.Herzyk DJ, Berger AE, Allen JN, et al. Sandwich ELISA formats designed to detect 17 kDa IL-1β significantly underestimate 35 kDa IL-1β. J Immunol Meth. 1992;148:243–54. doi: 10.1016/0022-1759(92)90178-v. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [Letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Los LE, Colby HD. Binding of spironolactone metabolites in vivo to renal mineralocorticoid receptors in guinea pigs. Pharmacology. 1994;48:86–92. doi: 10.1159/000139166. [DOI] [PubMed] [Google Scholar]
- 25.Overdiek HW, Merkus FW. The metabolism and biopharmaceutics of spironolactone in man. Rev Drug Metab Drug Interact. 1987;5:273–302. doi: 10.1515/dmdi.1987.5.4.273. [DOI] [PubMed] [Google Scholar]
- 26.Sauer J, Castren M, Hopfner U, et al. Inhibition of lipopolysaccharide-induced monocyte interleukin-1 receptor antagonist synthesis by cortisol: involvement of the mineralocorticoid receptor. J Clin Endocrinol Metab. 1996;81:73–9. doi: 10.1210/jcem.81.1.8550797. [DOI] [PubMed] [Google Scholar]
- 27.van Deventer SJ. Review article: targeting TNFα as a key cytokine in the inflammatory processes of Crohn's disease − the mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999;13(Suppl. 4):3–8. doi: 10.1046/j.1365-2036.1999.00024.x. ; discussion 38. [DOI] [PubMed] [Google Scholar]
- 28.Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 30.Feldmann M. Pathogenesis of arthritis: recent research progress. Nat Immunol. 2001;2:771–3. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]