Abstract
To explore the role of the 10-kDa Mycobacterium tuberculosis-specific secreted antigen (MTSA-10 or CFP-10) in modulation of macrophage function, J774 macrophages were transfected stably with DNA encoding MTSA-10. Compared to normal or mock-transfected controls, MTSA-10-expressing macrophages had markedly lower levels of co-stimulatory molecule B7·1 on their surface, while the expression of B7·2 and ICAM-1 was not affected. MTSA-transfected cells also produced significantly less microbicidal free radical nitric oxide (NO) upon stimulation with interferon (IFN)-γ, lipopolysaccharide or M. tuberculosis cell lysate. Western blot analysis revealed the absence of tyrosine-phosphorylated protein slightly larger than 112 kDa in MTSA-transfected macrophages. Moreover, the treatment of control J774 cells with protein tyrosine kinase inhibitor genistein completely mimicked the effects of transfection with MTSA-10, selectively down-regulating NO and B7·1, but not B7·2 or ICAM-1 expression. The observed MTSA-10-mediated block of B7·1 expression and NO release might contribute to the suppression of antimycobacterial response in tuberculosis.
Keywords: B7·1, CFP-10, IFN-γ, M. tuberculosis, MTSA-10, macrophages, nitric oxide
INTRODUCTION
Mycobacterium tuberculosis is a highly successful intracellular pathogen well known for its ability to persist inside macrophages and cause chronic disease in susceptible individuals. The prevention of phagosome maturation into an acidic, hydrolytic compartment with microbicidal activity seems to present a major survival strategy of M. tuberculosis [1,2]. However, additional mechanisms for evading the immune response are also employed by mycobacteria, including down-regulation of the protective T cell response through interference with antigen processing and presentation, and the expression of co-stimulatory molecule B7 in infected macrophages [3–5]. An important feature of mycobacterial subversion of the host immune functions is also the induction of macrophage unresponsiveness to interferon (IFN)-γ [6,7], a T cell cytokine crucial for optimal macrophage activation and subsequent synthesis of bactericidal molecules such as oxygen and nitrogen radicals [8,9].
The proteins secreted actively by M. tuberculosis into the culture medium (culture filtrate proteins: CFP) represent possible candidates for mycobacterial down-regulation of macrophage function. Such a notion is based on the findings that only live, but not dead M. tuberculosis, can prevent phagosomal-lysosomal fusion and macrophage B7 expression [5,10,11]. Among CFP constituents is the recently discovered 10-kDa protein M. tuberculosis secreted antigen (MTSA-10), designated originally CFP-10 [12]. MTSA-10 is one of the major antigens recognized by M. tuberculosis-specific human T and B cells [13–17], and it induces strong delayed type hypersensitivity response in M. tuberculosis-infected guinea pigs [13,18,19]. MTSA-10 is missing in M. bovis BCG, and therefore represents an ideal candidate for diagnostic test that will discriminate between infected and BCG-vaccinated people [19,20]. However, as it is specific for M. tuberculosis, MTSA-10 might also contribute to its unique ability for down-regulation of macrophage activity and subsequent impairment of the protective T cell response.
We have reported recently that MTSA-10 can bind to the surface of macrophages and profoundly modulate their function, causing partial unresponsiveness to induction of nitric oxide (NO) release [21]. As a continuation of this line of research, the present study shows that MTSA-10-transfected J774 macrophages are less capable of NO production, as well as B7·1 expression, indicating that intracellular presence of MTSA-10 might also contribute to the loss of macrophage antimycobacterial function in tuberculosis.
MATERIALS AND METHODS
Reagents
Dulbeco's modified Eagle medium (DMEM), fetal calf serum (FCS) and lipofectin reagent were all from Invitrogen Life Technologies (Carlsbad, CA, USA). Retroviral pLNCX2 vector and PT67 cell line were from Clontech/BD Biosciences (Palo Alto, CA, USA). Griess reagent, murine recombinant IFN-γ, genistein, SB203580, G418, HRPO-conjugated antimouse or antirabbit IgG, as well as biotinylated antiphosphotyrosine (PT-66), antiphosphoserine (PSR-45) and antiphosphothreonine (PTR-8) antibodies were all purchased from Sigma (St Louis, MO, USA). FITC-labelled antimouse B7·1 and B7·2, antimouse CD32/CD16 (Fc block), biotinylated antimouse ICAM-1 and streptavidin-FITC were from BD Pharmingen (San Diego, CA, USA), while U0126 was obtained from Promega (Madison, WI, USA). Recombinant MTSA-10 was expressed in Escherichia coli [lipopolysaccharide (LPS) concentration <1 ng/mg of protein] as described previously [21], and used for immunizing the rabbits to obtain polyclonal anti-MTSA-10 antibodies. Whole cell lysate of M. tuberculosis was a kind gift from John Belisle of University of Colorado, Fort Collins, CO, USA.
Cloning and transfection
The open reading frame Rv3874 encoding MTSA-10 protein of M. tuberculosis was amplified by polymerase chain reaction (PCR) from the genomic DNA of a local clinical isolate using the following primers: forward, 5′-GCGGATCCCATGGCA GAGATGAAGACCG-3′] reverse, 5′-CCCAAGCTTGTCA GAAGCCATTTGCGAG-3′ (BamHI and HinDIII sites are underlined, respectively). The PCR product was directly cloned in the intermediate vector pGEM-T-Easy, and nucleotide sequence of the gene was validated (GenBank Accession number AF419854). The cloned MTSA-10 gene was then re-amplified using the following primers: forward, 5′-ATAAGAATGCGGC C G C A T G G C A G A G A T GAAGACCGATGCCGCTACC-3′] reverse, 5′-CCATCGATGTCAGTGGTGGTGGTGGTGGTG GAAGCCCATTTGCGAG-3′ (Not I and Cla I sites are underlined, respectively). Six copies of GTG codon were included in the reverse primer to facilitate incorporation of a 6xHis tag at the C-terminal of the transduced MTSA-10 protein. PCR product was again cloned in the pGEM-T-Easy, and nucleotide sequence of the gene was re-validated. Full length authentic gene was subcloned into pLNCX2 vector, designed for retroviral gene delivery and expression, and this construct was used for lipofection of the PT67 cell line according to the manufacturer's instructions. The supernatant from PT67 cells, containing infectious, replication-incompetent retroviral particles, was used to infect J774 cells and deliver the MTSA-10 gene. Two stably transfected J774 cell lines were selected on the basis of resistance to G418. Stable mock-transfected cell lines were generated after G418 selection of the cells transfected with the vector only.
Cell cultures
Mouse macrophage cell line J774·1 (ATCC) was maintained in HEPES-buffered DMEM supplemented with 10% FCS, sodium bicarbonate, glutamine, penicillin and streptomycin (culture medium). Mock-transfected and MTSA-transfected cell lines were maintained in culture medium containing 0·6 mg/ml of G418. After being detached by scraping, J774 cells were washed in culture medium and used for flow cytometry analysis. Alternatively, prior to flow cytometry cells were incubated in six-well plates (5 × 106 cells/well) for 24 h in 4 ml of culture medium containing IFN-γ (100 U/ml), recombinant MTSA-10 (50 µg/ml) or genistein (20 µg/ml), as described in the figure legends. For NO production, cells were seeded in triplicate in flat-bottomed 96-well plates (1 × 105 cells/well) in 200 µl of culture medium containing different agents, as described in the figure legends. After 24 h of incubation at 37OC in the humidified atmosphere with 5% CO2, cell culture supernatants were collected for determination of nitrite concentration.
Flow cytometry
For the flow cytometry analysis, J774 cells were washed twice with staining buffer (PBS with 0·5% bovine serum albumin (BSA) and 0·1% sodium azide), resuspended at 1 × 106 cells/100 µl, and incubated for 15 min with antimouse CD32/CD16 (Fc block). Without being washed, cells were then incubated for 40 min on ice with FITC-conjugated anti-B7·1 or anti-B7·2 or biotinylated anti-ICAM-1 antibody (1 : 1500 in staining buffer), followed by 1 h incubation with streptavidin-FITC (1 : 2000 in staining buffer) in the latter case. Cells were washed and analysed on FACScalibur (Becton Dickinson).
Nitrite measurement
Nitrite accumulation, an indicator of NO production, was measured using the Griess reagent [22]. Briefly, 50 µl aliquots of culture supernatants were mixed with an equal volume of Griess reagent and incubated at room temperature for 10 min. The absorbance at 540 nm was measured in an automated microplate reader. The nitrite concentration (µm) was calculated from a NaNO2 standard curve.
Western blot detection of intracellular phosphorylation and MTSA-10
Cells (1 × 107) were grown for 24 h in a 15-cm Petri dish, scraped gently, centrifuged, washed with cold PBS and lysed with ice-cold lysis buffer (40 mm TrisCl pH 7·3, 0·5 m NaCl, 1% Triton X-100, 0·2 mm sodium ortho-vanadate, 0·2 mm PMSF, 2 mm sodium azide). For the detection of p-tyr, p-ser or p-thr, the lysates were centrifuged, supernatants collected, and after protein estimation, mixed with 4× sample buffer (250 mm Tris pH 6·8, 8% SDS, 40% glycerol, 0·2% bromophenol blue, 4% β-mercaptoethanol), boiled and run at 12% polyacrylamide gel. Proteins were transferred by electrophoresis to nitrocellulose membrane in a transfer buffer (25 mm Tris, 190 mm glycine, 20% methanol). The membrane was washed with wash buffer (100 mm Tris pH 7·5, 0·9% NaCl, 0·1% Tween 20), blocked for 1 h with blocking buffer (1% BSA, 100 mm Tris pH 7·5, 0·9% NaCl, 0·1% Tween 20), and incubated for 1 h with the phospho-specific antibodies (1 : 5000) in the blocking buffer. After washing, the membrane was incubated for 1 h with the HRPO-conjugated antimouse IgG (1 : 2000) in wash buffer. The membrane was washed extensively and the blot was developed using DAB/NiCl2 visualization solution. For confirming the MTSA-10 expression in transfected cells, the 6xHis-tagged MTSA-10 was purified from cell lysates by affinity chromatography using Ni-NTA agarose. The eluted fraction was transferred to nitrocellulose membrane and probed with anti-MTSA-10 rabbit polyclonal antibody (1 : 1000), followed by antirabbit IgG/HRPO (1 : 2000). Final detection was performed with DAB/NiCl2 visualization solution.
Statistical analysis
Statistical significance of the difference between various treatments was analysed using one-way analysis of variance (anova), followed by post-hoc Student-Newman-Keuls test. A P-value less than 0·05 was considered to be significant.
RESULTS
B7·1 expression and NO release are impaired in MTSA-transfected macrophages
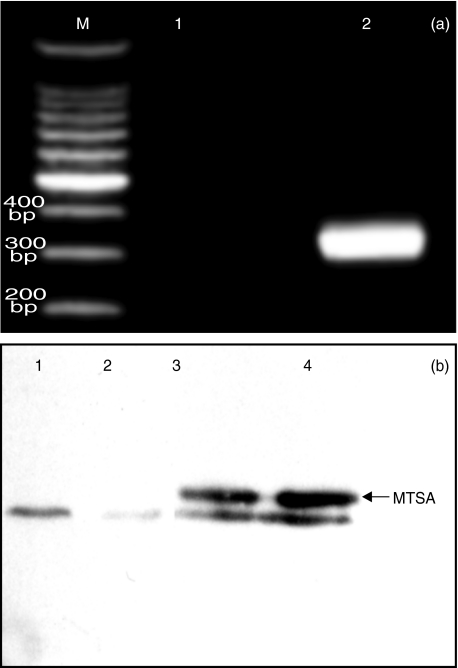
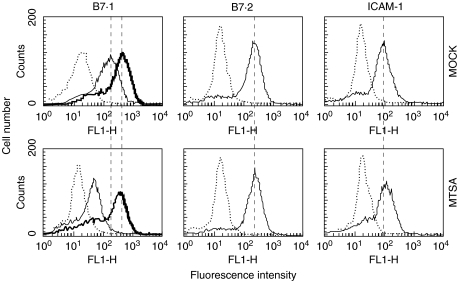
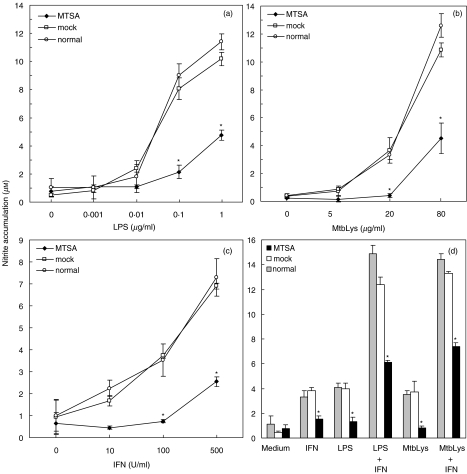
The presence of MTSA-10 gene and the protein in transfected cells was confirmed by PCR with MTSA-10-specific primers and Western blotting with MTSA-10-specific antibody, respectively (Fig. 1). J774 cells used in our study constitutively expressed high levels of B7·1, B7·2 and ICAM-1. The levels of B7·2 and ICAM-1 were comparable in MTSA-transfected cells and mock-transfected or untransfected controls (Fig. 2 and Table 1). Interestingly, B7·1 expression was significantly lower in MTSA-transfected cells, as demonstrated by reduced number of B7·1-positive cells, as well as decreased mean fluorescence intensity in MTSA-transfected macrophages (Fig. 2 and Table 1). The expression of B7·1 was increased further in the presence of macrophage-activating cytokine IFN-γ (Fig. 2). However, the difference in B7·1 expression observed in resting cells was lost upon IFN-γ stimulation, which up-regulated B7·1 levels to a similar extent in both MTSA-transfected and control cells (Fig. 2). Basal production of NO in J774 cell cultures, measured as nitrite accumulation, was below the limit of detection (2 µm). A significant NO release was observed readily upon the addition of LPS, M. tuberculosis cell lysate or IFN-γ (Fig. 3). The production of NO in activated J774 cells was mediated by inducible NOS (iNOS) isoform, as it was totally abolished by specific iNOS inhibitor aminoguanidine (not shown). Regardless of the stimuli used, MTSA-transfected macrophages displayed consistently a decreased ability for NO synthesis when compared with untransfected or mock-transfected counterparts, which produced similar amounts of NO (Fig. 3a-c). Furthermore, although the addition of IFN-γ synergistically increased LPS- or M. tuberculosis lysate-triggered NO production in both MTSA-transfected and control macrophages, MTSA-transfected cells still produced significantly less NO upon combined stimulation (Fig. 3). Therefore, exogenous IFN-γ could surmount completely the defect in B7·1 expression, but not NO production in MTSA-transfected cells.
Fig. 1.
Detection of MTSA-10 DNA and protein in MTSA-transfected J774 cells. (a) A PCR with MTSA-10-specific primers was performed with DNA from mock-transfected (line 1) and MTSA-transfected cells (line 2). The expected size of MTSA-10 band was 346 bp (M represents DNA molecular weight marker). (b) Cell lysates of mock-transfected (lanes 1 and 2) and MTSA-transfected cells (lanes 3 and 4) were run on SDS-PAGE and, after Ni-NTA extraction and Western blotting, probed with anti-MTSA-10 followed by peroxidase-labelled secondary antibody. The arrow indicates the recombinant MTSA-10 protein band of the expected size of 10 kDa (a distinct band below the one corresponding to MTSA-10 is a consequence of nonspecific antibody binding, and it was regularly observed in either MTSA-transfected, normal or mock-transfected cells).
Fig. 2.
B7·1 expression on MTSA-10-transfected macrophages. Constitutive (thin line) or IFN-γ-induced (thick line on B7·1) expression of B7·1, B7·2 or ICAM-1 in MTSA-transfected or mock-transfected J774 cells was determined by flow cytometry, as described in Materials and Methods (dotted line represents isotype control staining). Results from the representative of four separate experiments are presented.
Table 1.
Intracellular MTSA-10 down-regulates B7·1 expression on J774 cells. Constitutive expression of B7·1, B7·2 or ICAM-1 in MTSA-transfected, mock-transfected or normal J774 cells was determined by flow cytometry. Results are presented as mean values ± s.d. from four independent experiments
| Normal cells | Mock-transfected | MTSA-transfected | |
|---|---|---|---|
| % of B7·1+ cells | 77 ± 14 | 71 ± 16 | 47 ± 11* |
| Mean fluorescence | 195 ± 40 | 180 ± 28 | 104 ± 17* |
P < 0·05: normal and mock-transfected cells.
Fig. 3.
NO production in MTSA-10-transfected macrophages. MTSA-transfected, mock-transfected or normal J774 cells were incubated with various concentrations of (a) LPS, (b) M. tuberculosis cell lysate (MtbLys) or (c) IFN-γ. (d) Alternatively, cells were stimulated with LPS (100 ng/ml) or MtbLys (20 µg/ml), in the presence or absence of IFN-γ (100 U/ml). Nitrite accumulation, as a marker of NO production, was assessed after 24 h of incubation. Results from the representative of four independent experiments are presented as mean ± s.d. of triplicate observations (*P < 0·05 refers to mock-transfected and normal cells).
Down-regulation of macrophage B7·1 expression and NO release are not mediated by exogenous MTSA-10
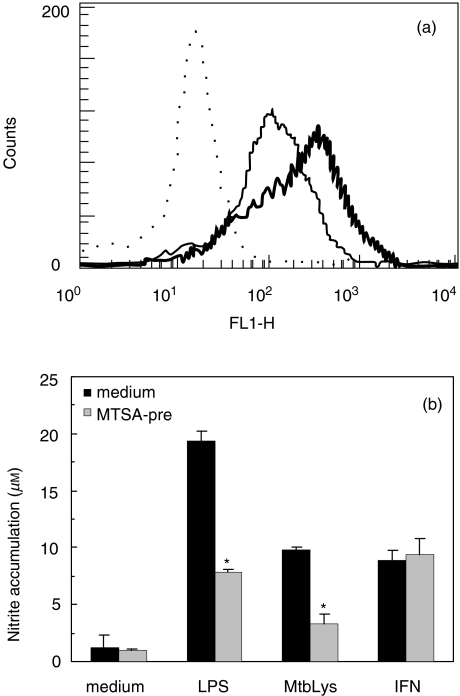
Although we were unable to demonstrate the presence of MTSA-10 in the culture supernatants of MTSA-transfected J774 cells (not shown), there was still a possibility that low amounts of secreted or membrane-bound MTSA-10 might account for the effects seen in transfected cells. We therefore investigated the effect of exogenous MTSA-10 on B7·1 expression and NO production in J774 macrophages. Contrary to the results obtained with transfected cells, exogenous MTSA-10 up-regulated B7·1 levels readily on the surface of control J774 cells (Fig. 4A). On the other hand, 18 h pretreatment with MTSA-10 rendered J774 cells significantly less responsive to subsequent induction of NO release by LPS or M. tuberculosis cell lysate, thus resembling the situation observed in MTSA-transfected cells (Fig. 4b). However, while MTSA-transfected cells exhibited reduced ability for NO release upon stimulation with IFN-γ alone (Fig. 3a), the NO production elicited by IFN-γ was completely refractory to MTSA-10 pretreatment (Fig. 4b). Furthermore, conditioned medium from the cultures of MTSA-transfected cells was completely unable to mimic MTSA-10-mediated inhibition of macrophage NO synthesis (data not shown). It appears therefore that neither B7·1 down-regulation nor impaired NO release in MTSA-transfected macrophages were mediated by MTSA-10 that was membrane-bound or possibly secreted from the transfected cells.
Fig. 4.
Effect of exogenous MTSA-10 on macrophage expression of B7·1 and NO release. (a) Normal J774 cells were incubated for 24 h without (thin line) or with (thick line) recombinant MTSA-10 (50 µg/ml) and B7·1 expression was determined by flow cytometry (dotted line represents isotype control). (b) Following 18 h preincubation with or without MTSA-10 (50 µg/ml), normal J774 cells were washed extensively and stimulated for NO production with LPS (1 µg/ml), M. tuberculosis cell lysate (MtbLys; 50 µg/ml) or IFN-γ (500 U/ml). Nitrite accumulation in cell culture supernatants was determined after additional 24 h of incubation. Results from the representative of four separate experiments are presented as mean ± s.d. of triplicate observations.
Protein phosphorylation pattern differs in normal and MTSA-10-transfected cells
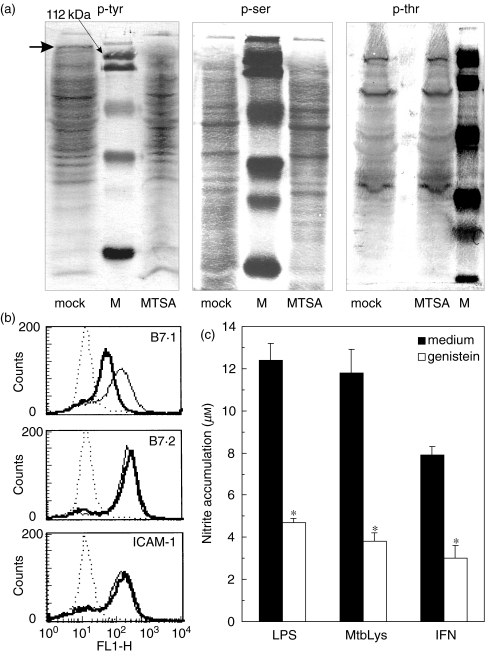
To obtain some insight into the intracellular events that might be affected by macrophage transfection with MTSA-10, we assessed tyrosine and serine/threonine phosphorylation of the proteins in MTSA-transfected cells. Interestingly, a different pattern of tyrosine phosphorylation was observed in mock- and MTSA-transfected macrophages, with a band slightly larger than 112 kDa clearly missing in the lysates of the latter cells (Fig. 5a). Although some less prominent differences in the tyr/ser/thr phosphorylation pattern are also evident in Fig. 5a, only the absence of phosphorylated 112-kDa protein was observed consistently in several experiments. Furthermore, the treatment of control cells with PTK inhibitor genistein down-regulated both NO release and constitutive B7·1 expression, without significantly affecting the levels of B7·2 or ICAM-1 (Figs 5b,c). On the other hand, although U0126 and SB203580, the inhibitors of ser/thr kinases p38 MAPK and p42/44 MAPK, respectively, markedly reduced IFN-γ + LPS-induced NO production in J774 cells, they failed completely to affect constitutive B7·1 expression (data not shown). These data indicate that impaired tyrosine phosphorylation or reduced expression of the putative 112-kDa intracellular protein might be involved in the defective B7·1 expression and NO release in MTSA-transfected cells.
Fig. 5.
PTK inhibition mimics the effects of MTSA-10 transfection on macrophage expression of B7·1 and NO release. (a) Intracellular phosphorylation of tyrosine, serine or threonine was compared in MTSA-transfected and mock-transfected cells, using specific antibodies as described in Materials and methods (the arrow in the ‘mock’ line indicates p-tyr band that is absent in the ‘MTSA’ line; M: protein weight marker). The representative blots from three independent experiments are shown. (b) The expression of B7·1, B7·2 or ICAM-1 on normal J774 cells was determined after 24 h of incubation without (thin line) or with (thick line) PTK inhibitor genistein (20 µg/ml) (dotted line represents isotype control). (c) Normal J774 cells were stimulated for NO production with LPS (1 µg/ml), M. tuberculosis cell lysate (MtbLys; 50 µg/ml) or IFN-γ (500 U/ml), in the absence or presence of genistein (20 µg/ml). Nitrite accumulation was measured after 24 h of incubation and data are presented as mean values ± s.d. of triplicate observations. (b, c) Results from the representative of three independent experiments are shown.
DISCUSSION
The present study shows for the first time that transfection with M. tuberculosis secreted protein MTSA-10 can reduce macrophage ability for B7·1 expression and production of microbicidal free radical NO. However, to apply any relevance to these findings, one must postulate intracytoplasmic localization of MTSA-10 within infected macrophages. It has been proposed that viable mycobacteria might have the ability to facilitate transit of macromolecules between the vacuolar and cytosolic compartments of infected cells [23]. Moreover, the existence of MTSA-10-specific CD8+ T cells in infected individuals [16] indicates that this mycobacterial protein can indeed gain access to macrophage cytoplasm, which might enable its interference with macrophage function.
The interaction of co-stimulatory molecules B7·1 and B7·2 on antigen-presenting cells with CD28 on T cell membrane is necessary for optimal activation of antigen-recognizing T lymphocytes. The data accumulated in recent years indicate the ability of these co-stimulatory molecules to promote distinct functional T cell responses. Although different outcomes have been observed in various experimental systems, B7·1 seems predominantly responsible for development of Th1-like cells that fight intracellular pathogens, while B7·2 promotes mainly the expansion of Th2 lymphocytes involved in the induction of humoral response against multicellular parasites [24–29]. In tuberculosis, IFN-γ-secreting Th1 cells are required for optimal macrophage activation and clearance of mycobacteria, as demonstrated by the severe, disseminated disease in IFN-γ knockout mice [30,31]. The reduction of macrophage B7·1 expression by MTSA-10, reported in the present study, could therefore contribute to reducing the host capacity for development of the protective Th1 response. Our data also show that a signature Th1 cytokine IFN-γ can overcome the defect in B7·1 expression in MTSA-10-transfected macrophages. However, if sufficient amounts of IFN-γ are lacking in the early phase of the infection, deficient expression of B7·1 might lead to a vicious circle in which the resulting low Th1 response will not be able to countervail MTSA-10-mediated down-regulation of B7·1 levels in infected macrophages. In contrast with data obtained from the transfected cells, exogenously applied MTSA-10 readily augmented the expression of B7·1 on J774 macrophages. Because the presence of antibodies against MTSA-10 in tuberculosis patients [13,14] indicates that this secreted protein might also be present in the extracellular compartment, the overall effect of MTSA-10 on B7·1 levels in infected macrophages would depend presumably on the balance between opposing actions of extracellular and intracellular MTSA-10. It should be noted, however, that the possible role of B7·1 down-regulation in tuberculosis progression, suggested by in vitro experiments with mouse macrophages [5], is still to be investigated in human disease.
While a large amount of data, including those describing higher susceptibility to infection in iNOS inhibitor-treated or iNOS-knockout animals [32,33], support the role for iNOS-derived NO in mycobacterial clearance in mice, the results obtained in humans seem more controversial. Nevertheless, monocytes from tuberculosis patients express iNOS [34], and human monocytes and macrophages displayed NO-dependent mycobacterial killing, at least in some in vitro studies [35,36]. The results of the present work indicate that intracellular presence of MTSA-10 might reduce antimycobacterial capacity of infected macrophages through interference with their NO synthesis. We have observed that MTSA-10 pretreatment renders J774 less responsive for subsequent NO induction by LPS or M. tuberculosis cell lysate [21 and the present study]. However, it seems that the impaired NO production in transfected cells might be an intrinsic defect, rather than caused by macrophage recognition of secreted or membrane-bound MTSA-10. Such an assumption is supported by the failure of the supernatants from transfected cells to affect NO release in normal macrophages, as well as by finding that IFN-γ could overcome readily the deficiency in NO production in MTSA-pretreated [21], but not in MTSA-transfected cells (the present study). These data imply that both extracellular and intracellular MTSA-10 might co-operate in obstructing NO synthesis in infected macrophages, thus promoting the survival of ingested mycobacteria.
Finally, a different pattern of protein phosphorylation was observed in control versus MTSA-transfected cells, with the absence in the latter cells of tyrosine-phosphorylated protein of approximately 112 kDa as the most prominent distinction. Interestingly, the inhibition of protein tyrosine kinase, but not serine/threonine kinase, mimicked completely the effects on B7·1, B7·2, ICAM-1 expression and NO release seen in MTSA-transfected cells. Although far from providing a formal proof, these data suggest indirectly a possible link between impaired tyrosine phosphorylation and reduced B7·1 expression and NO synthesis in MTSA-transfected macrophages. While this hypothesis remains to be tested, the experiments aimed at investigating whether MTSA-10 might act as a phosphatase or inhibit the expression of the putative 112-kDa protein are currently under way in our laboratory.
Taken together, the results of our previous [21] and present reports indicate that down-regulation of B7·1 and NO levels by MTSA-10 might participate in dampening host antimycobacterial response. As different mycobacterial products, such as lipoarabinomannan or 19-kDa lipoprotein [3,37,38], might be involved in down-modulation of the macrophage function, it would be interesting to explore possible co-operation between MTSA-10 and these molecules. Nevertheless, to establish the exact role of this secreted protein in modulation of the immune response in tuberculosis is dependent upon in vivo studies involving MTSA-10-negative M. tuberculosis mutants.
Acknowledgments
The study was funded partially by grant BT/PR/2423/Medical/13/087/2001 from the Department of Biotechnology, Government of India, New Delhi. V. T. was an ICGEB Post-Doctoral Fellow and G. B. S. was a CSIR Junior Research Fellow. The authors wish to thank John Belisle (University of Colorado, Fort Collins, CO, USA) for the kind gift of M. tuberculosis cell lysate.
REFERENCES
- 1.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–77. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 2.Pieters J, Gatfield J. Hijacking the host: survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 2002;10:142–6. doi: 10.1016/s0966-842x(02)02305-3. [DOI] [PubMed] [Google Scholar]
- 3.Noss EH, Pai RK, Sellati TJ, et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–8. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 4.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 5.Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–24. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 6.Douvas GS, Looker DL, Vatter AE, Crowle AJ. Interferon-γ activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–906. [PubMed] [Google Scholar]
- 8.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol. 2000;3:35–42. doi: 10.1016/s1369-5274(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 10.Malik ZA, Iyer SS, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J Immunol. 2001;166:3392–401. doi: 10.4049/jimmunol.166.5.3392. [DOI] [PubMed] [Google Scholar]
- 11.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–70. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 13.Brusasca PN, Colangeli R, Lyashchenko KP, et al. Immunological characterization of antigens encoded by the RD1 region of the Mycobacterium tuberculosis genome. Scand J Immunol. 2001;54:448–52. doi: 10.1046/j.1365-3083.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- 14.Dillon DC, Alderson MR, Day CH, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285–90. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 16.Lewinsohn DM, Zhu L, Madison VJ, et al. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol. 2001;166:439–46. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 17.Skjot RL, Oettinger T, Rosenkrands I, et al. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun. 2000;68:214–20. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colangeli R, Spencer JS, Bifani P, et al. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect Immun. 2000;68:990–3. doi: 10.1128/iai.68.2.990-993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immunol. 2000;7:155–60. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arend SM, Andersen P, van Meijgaarden E, et al. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–4. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 21.Trajkovic V, Singh G, Singh B, Singh S, Sharma P. Effect of Mycobacterium tuberculosis-specific 10-kilodalton antigen on macrophage release of tumor necrosis factor-α and nitric oxide. Infect Immun. 2002;70:6558–66. doi: 10.1128/IAI.70.12.6558-6566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Teitelbaum R, Cammer M, Maitland ML, Freitag NE, Condeelis J, Bloom BR. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc Natl Acad Sci USA. 1999;96:15190–5. doi: 10.1073/pnas.96.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 co-stimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 25.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical co-stimulatory signals, since B7-2 but not B7-1 preferentially co-stimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 26.Racke MK, Scott DE, Quigley L, et al. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranger AM, Das MP, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of IL-4-producing T cells. Int Immunol. 1996;8:1549–60. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 28.Brown JA, Titus RG, Nabavi N, Glimcher LH. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–8. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 29.Keane-Myers AM, Gause WC, Finkelman FD, Xhou XD, Wills-Karp M. Development of murine allergic asthma is dependent upon B7-2 co-stimulation. J Immunol. 1998;160:1036–43. [PubMed] [Google Scholar]
- 30.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CH, Lin HC, Liu CY, et al. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2001;5:283–91. [PubMed] [Google Scholar]
- 35.Jagannath C, Actor JK, Hunter RL., Jr Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide. 1998;2:174–86. doi: 10.1006/niox.1998.9999. [DOI] [PubMed] [Google Scholar]
- 36.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knutson KL, Hmama Z, Herrera-Velit P, Rochford R, Reiner NE. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J Biol Chem. 1998;273:645–52. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- 38.Post FA, Manca C, Neyrolles O, Ryffel B, Young DB, Kaplan G. Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits Mycobacterium smegmatis-induced cytokine production by human macrophages in vitro. Infect Immun. 2001;69:1433–9. doi: 10.1128/IAI.69.3.1433-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]