Abstract
In humans, transmission of Helicobacter pylori is thought to occur largely during childhood. Infected mothers are generally considered to be the main source of the pathogen. However, little is known about when and how often maternal transmission of H. pylori occurs during childhood. In the present study, we examined these issues in an experimental murine model. Pregnant C57BL/6 mice, infected experimentally with H. pylori, delivered and nursed their litters. The stomachs of the infants were isolated and assessed for transmission of H. pylori. We also investigated the effect of systemic immunization using H. pylori antigen–aluminium hydroxide (AlOH) with regard to providing anti-H. pylori immunity and eradicating the maternally transmitted bacteria in infants. Polymerase chain reaction (PCR) was used to examine the presence of transmitted bacteria and their eradication. Maternal transmission of H. pylori varied widely during the nursing period, but almost all litters showed bacterial transmission at 2 weeks postpartum. Systemic immunization with bacterial antigen–AlOH eradicated the bacteria in most litters; this immunization induced a local decrease of Th2 cytokines and a local increase of Th1 cytokines in the gastric tissue, as determined by ELISA. Our results indicate that our H. pylori vaccine provides not only protection, but also eradication of the already transmitted H. pylori.
Keywords: aluminium hydroxide (AlOH), experimental murine model, Helicobacter pylori, maternal transmission, systemic immunization
INTRODUCTION
Helicobacter pylori is a Gram-negative, spiral-shaped, microaerophilic bacterium that infects the human gastric mucosa [1]. Chronic infection is thought to be associated with chronic active gastritis, peptic ulcer and gastric malignancies, such as mucosa-associated B cell lymphoma and adenocarcinoma [2]. In particular, this organism has been categorized as a class I carcinogen by the World Health Organization [3] and previous studies have confirmed that long-term infection with H. pylori induces adenocarcinoma in Mongolian gerbils [4,5].
Young children are particularly vulnerable to infection by transmission of H. pylori from their infected parents, especially infected mothers [6,7], and it is generally believed that such transmission is influenced by socio-economic status. However, little is known about how and when maternal transmission occurs during childhood, especially whether this occurs before or after weaning.
If prolonged and chronic H. pylori infection causes gastric cancer, an H. pylori vaccine should be targeted at children as a prophylaxis against cancer. Gottwein et al. [8] noted that prophylactic vaccination of infants might prevent H. pylori infection and the associated long-term consequences that occur in adults, and confirmed that systemic immunization with aluminium hydroxide (AlOH), already approved for human children, is effective at inducing protective anti-H. pylori immunity in mice [8]. However, these investigators did not examine the effects of transmission of H. pylori before immunization and did not determine whether such immunization can eradicate previously transmitted bacteria.
The present study was designed to examine the incidence and mechanisms of maternal transmission of H. pylori in an experimental murine model. Furthermore, we evaluated the effects of systemic immunization using AlOH to eradicate maternally transmitted H. pylori through the stimulation of production of cytokines by gastric tissues.
MATERIALS AND METHODS
Mice
Specific-pathogen-free (SPF) C57BL/6 mice were purchased from Seac Yoshitomi (Yoshitomi-cho, Fukuoka, Japan). Mice were housed under SPF conditions and provided with food and water ad libitum. All procedures described in the present study were conducted in accordance with the guidelines of the Ethical Committee for Animal Experiments of Oita Medical University, Oita, Japan.
Bacteria
H. pylori (Sydney strain 1) stocked in our laboratory was used for H. pylori infection and immunogen preparation. It was grown at 37°C for 48 h with continuous shaking in Brucella broth supplemented with 10% horse serum in microaerobic conditions (5% O2, 10% CO2, 85% N2). For quantitative analysis, H. pylori was cultured on M-BHM pylori agar medium (Nikken Biomedical Laboratory, Kyoto, Japan) under the same conditions for 5 or 6 days. The detection limit of the quantitative assay was 1 × 102 colony forming units (CFU)/g gastric tissue.
Mice were inoculated twice at 3-day intervals by oral administration of 1 × 108 CFU of H. pylori suspended in 0·5 ml of Brucella broth. At the final examination, the challenged mice were sacrificed and confirmed to be H. pylori-positive by culture of gastric tissue. H. pylori-negative mice were excluded from the following study.
Adult-adult transmission of H. pylori
Each animal group consisted of six mice. One mouse in each group was challenged with H. pylori, housed separately for 4 weeks and then co-raised with five unchallenged mice in a laboratory cage. Mice were sacrificed after co-habitation for 3 weeks. The stomach of each mouse was dissected out and homogenized in 0·5 ml sterile saline.
Maternal transmission of H. pylori
One week after challenge with H. pylori, three female infected mice and one male mouse in each group were transferred to one cage for mating. As soon as a female mouse was confirmed to be pregnant, one was separated from the group and cared for until delivery. After birth at full term, the families were divided into four maternal-transmission groups and three immunization groups. In the maternal-transmission groups, the mother and litters in each group were sacrificed at 2 days, 1 week, 2 weeks and 3 weeks postpartum. The gastric specimen was isolated as described above. In the immunization groups, litters were weaned after 3 weeks of breastfeeding, and then the mother was sacrificed. In the negative control group, the litters were nursed by an uninfected mother mouse, and they were sacrificed 3 weeks postpartum.
Isolation of H. pylori
Aliquots (100 µl) of homogenate were cultured on M-BHM pylori agar medium plates and the plates were incubated under the aforementioned conditions. Isolated colonies of H. pylori were examined by a standard RUT assay as described previously [9] to confirm H. pylori infection. The remainder of the homogenate was used for the following polymerase chain reaction (PCR) procedure.
PCR
Bacterial DNAs were extracted from the above homogenate by the SDS-proteinase K method using cetyltrimethylammonium bromide (CTAB) [10]. The PCR primers used in the present study were derived from the Ure A and 26 kDa genes (Table 1), both of which have been used previously [11–13]. Taq polymerase (1·25 U; Promega, Madison, WI, USA) was added and mixed to give a final 50-µl reaction mixture. PCR (first and nested) was performed with an automatic thermal cycler (PC-700, Astec Inc., Fukuoka, Japan) (Table 2). The PCR products were visualized by gel electrophoresis. Mice were considered to have been colonized with H. pylori via transmission when PCR (by at least one kind of primer) or culture was positive.
Table 1.
PCR primers used in the present study and derived from the following species-specific genes
| Ure A gene | ||
| First primer | Sense | 5′-ATATTATGGAAGAAGCGAGAGC-3′ |
| Antisense | 5′-ATGGAAGTGTGAGCCGATTTG-3′ | |
| Second primer | Sense | 5′-CATGAAGTGGGTATTGAAGC-3′ |
| Antisense | 5′-AAGTGTGAGCCGATTTGAACC-3′ | |
| 26 KDa gene | ||
| First primer | Sense | 5′-TGGCGTGTCTATTGACAGCGAGC-3′ |
| Antisense | 5′-CCTGCTGGGCATACTTCACCATG-3′ | |
| Second primer | Sense | (same as the first sense primer) |
| Antisense | 5′-GATCACTGCATGTCTTACTTTCATGTTTTT-3′ | |
Table 2.
Amplification cycles in the first and second PCR using the primers listed in Table 1
| First PCR | Second PCR | ||
|---|---|---|---|
| Ure A gene | |||
| First cycle | First cycle | ||
| 94°C | 2·0 min | 94°C | 2·0 min |
| 40 cycles | 40 cycles | ||
| 94°C | 1·0 min | 94°C | 1·0 min |
| 54°C | 1·5 min | 58°C | 1·5 min |
| 72°C | 1·5 min | 72°C | 1·5 min |
| Final cycle | Final cycle | ||
| 72°C | 5·0 min | 72°C | 5·0 min |
| 26 KDa gene | |||
| First cycle | First cycle | ||
| 94°C | 2·0 min | 94°C | 2·0 min |
| 40 cycles | 40 cycles | ||
| 94°C | 1·0 min | 94°C | 1·0 min |
| 54°C | 1·5 min | 56°C | 1·5 min |
| 72°C | 1·5 min | 72°C | 1·5 min |
| Final cycle | Final cycle | ||
| 72°C | 5·0 min | 72°C | 5·0 min |
Immunization
H. pylori antigen (Hp), which we have demonstrated to be an effective experimental vaccine antigen by the oral route, was generated in our laboratory, as described previously [14]. Ovalbumin (OVA) (Sigma Chemical Co., St Louis, MO, USA) was used as a control antigen. Aluminium hydroxide (AlOH) (Imject Alum, Pierce, IL, USA) was used as the adjuvant. Antigen (100 µg) in aqueous solution (50 µl), mixed with 50 µl of adjuvant, was injected intraperitoneally. Hp–AlOH was injected into half of the litters from each family in the immunization groups, while OVA–AlOH was injected into the remaining members. The injection was performed twice: at 3 and 4 weeks of age. In addition, one control group that was not vaccinated was included for cytokine measurements.
Determination of vaccine efficacy
Mice were sacrificed 2 weeks after the last vaccination. One half of the stomach, to be prepared for cytokine measurement, was frozen immediately in liquid nitrogen. The other half, to be prepared for the determination of vaccine efficacy, was treated in a manner similar to that described in the section ‘Isolation of H. pylori’. Eradication of H. pylori was considered successful when both PCR and culture were negative.
Cytokine measurement
The concentrations of interleukin [IL]-4, IL-10, interferon [IFN]-γ and IL-12 in the gastric tissue of the above mice were measured by enzyme-linked immunosorbent assay (ELISA). First, each frozen specimen was homogenized with 2 ml of phosphate-buffered saline (PBS) containing 0·1% sodium dodecylsulphate (SDS) and 0·1% Tween 20 [15]. After overnight storage at −20°C, the sample was centrifuged at 400 g for 15 min at 4°C. The supernatant was analysed with mouse IFN-γ ELISA kit (MedSystems Diagnostics GmbH, Vienna, Austria) and the OptEIATM set for mouse IL-4, IL-10, and IL-12 (BD Biosciences Pharmingen, San Diego, CA, USA). The results were expressed as concentration per mg protein. Protein levels were measured using Dc protein assay reagents (Bio-Rad Technologies, Richmond, CA, USA).
Statistical analysis
Statistical analysis was carried out using Statview software. The χ2 test was used to assess differences in the transmission rate between maternal-transmission groups. The eradication rate in the Hp–AlOH-vaccinated group was compared to the OVA–AlOH-vaccinated group using the Fisher's exact test. The unpaired t-test was used to analyse differences in cytokine production. A P-value of <0·05 was considered to be statistically significant.
RESULTS
The mouse strain C57BL/6 has often been used as a murine model of H. pylori immunization. In the present study, we used the same mouse strain not only as a vaccination but also as a transmission model. First, we investigated the stage at which maternal transmission occurs, and compared its frequency with that of adult–adult transmission. While culture of the bacterium is one of the gold standards in the diagnosis of H. pylori infection, in the present study it was not considered to be ideal for determination of H. pylori transmission for two reasons: (i) only small amounts of bacteria were suspected to colonize the stomach and (ii) the detection limit of the quantitative culture assay was 1 × 102 CFU/g gastric tissue. Therefore, we also used the PCR method along with the culture method to detect small numbers of H. pylori. Maternal transmission was examined at 2 days, 1 week, 2 weeks and 3 weeks postpartum (corresponding to colostrums, transitional milk, mature milk and weaning stage, respectively). Each stage group was composed of three or four families.
The frequency of maternal transmission fluctuated during the nursing period, but it was noteworthy that H. pylori was detected in almost all (15/16) 2-week-old-mice (Table 3). The transmission rate at 2 weeks postpartum was significantly higher than at 1 week postpartum (P < 0·001). Furthermore, the rate at 3 weeks postpartum (44%) was significantly lower than at 2 weeks postpartum (P = 0·002), but the 3-week rate tended to be higher than the adult–adult transmission rate after the same co-habitation period (20%), albeit statistically insignificant (P = 0·16). H. pylori was not detected in any litters of the negative control group.
Table 3.
Transmission rate of H. pylori in respective transmission groups
| PCR | ||||||
|---|---|---|---|---|---|---|
| Group | Co-raising period | Culture | Ure A | 26 KDa | Totald | Pe |
| MTa (0) | 2 days | 0/19 | 12/19 | 0/19 | 12/19 |
 f f
|
| MT (1) | 1 week | 0/25 | 9/25 | 0/25 | 9/25 |
 g g
|
| MT (2) | 2 weeks | 0/16 | 15/16 | 13/16 | 15/16 |
 h h
|
| MT (3) | 3 weeks | 0/18 | 4/18 | 6/18 | 8/18 |
 i i
|
| ATb | 3 weeks | 0/20 | 2/20 | 4/20 | 4/20 | |
| NCc | 3 weeks | 0/7 | 0/7 | 0/7 | 0/7 | |
MT, maternal transmission group.
AT, adult-adult transmission group.
NC, negative control group.
Mice with positive transmission of H. pylori as confirmed by culture or PCR (Ure A or 26 KDa).
Differences in the transmission rate between MT groups were evaluated by the χ2 test. Differences in the transmission rate between MT (3) group and AT group were evaluated by Fisher's exact test.
P = 0·074;
P < 0·001;
P = 0·002;
P = 0·16.
Because these experiments suggested frequent maternal transmission, we next determined whether systemic immunization with AlOH, which has been approved for use in human children, is efficacious in treating these litters. Gottwein et al. [8] showed that immunization of C57BL/6 mice with this adjuvant can confer protection against H. pylori infection of the gastric mucosa, and that immunity with this adjuvant can induce splenocytes in these mice to specifically produce Th2 cytokines (IL-5), as measured by ELISPOT assay. In our next series of experiments, we tested whether immunization using this adjuvant could eradicate already transmitted H. pylori. We systemically immunized C57BL/6 litters, weaned from H. pylori-infected mothers, with H. pylori antigens emulsified in AlOH. Two weeks later, the presence or absence of H. pylori was examined by culture and PCR. It was found that systemic immunization with AlOH resulted in significant eradication of the pathogen, as determined by the almost complete absence of H. pylori (Table 4). While six of eight control mice (75%) continued to exhibit gastric colonization with H. pylori, eight of nine mice (89%) immunized with Hp–AlOH showed eradication of the pathogen. The difference in the cure rate between the Hp–AlOH immunized and OVA–AlOH immunized litters was significant (P = 0·015).
Table 4.
Detection rate of H. pylori in immunization groups
| PCR | ||||||
|---|---|---|---|---|---|---|
| Group | Immunizationa | Culture | Ure A | 26 KDa | Totale | Pf |
| A | Hpb: AlOHd | 0/9 | 1/9 | 0/9 | 1/9 | 6/8 g g
|
| B | OVAc: AlOH | 0/8 | 6/8 | 2/8 | ||
A total of 100 µg of Hp or OVA with AlOH, as specified;
Hp, Helicobacter pylori antigen;
OVA, ovalbumin;
AlOH, aluminium hydroxide.
Eradication was defined as the complete absence of detectable bacteria. Two weeks after the second vaccination, litters of the immunization groups were examined for the eradication of H. pylori by culture and PCR.
Differences in postimmunization detection rates were evaluated by Fisher's exact test.
P = 0·015.
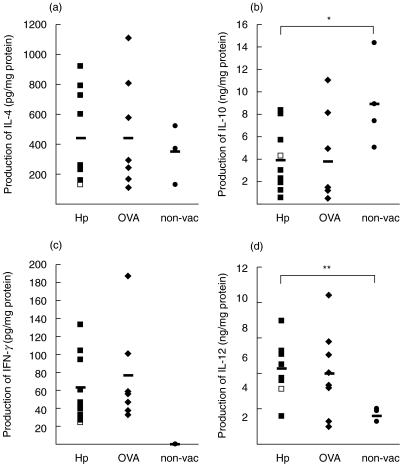
To explore the mechanism(s) for the anti-H. pylori effects of the Hp–AlOH vaccine, we measured the production of Th1 (IFN-γ, IL-12) and Th2 cytokines (IL-4, IL-10) by gastric tissue using ELISA (Fig. 1) and evaluated the balance of Th1 and Th2 immunity in the stomach, but not in the spleen. There was no significant difference in the production of IL-4 and IFN-γ between Hp–AlOH-vaccinated, OVA–AlOH-vaccinated and non-vaccinated mice, although the production of IFN-γ in Hp–AlOH-vaccinated mice tended to be higher than in the non-vaccinated mice (P = 0·079). In contrast, the production of IL-10 in Hp–AlOH-vaccinated mice was significantly lower than in non-vaccinated mice (P = 0·026), and production of IL-12 in Hp–AlOH-vaccinated mice was significantly higher than in non-vaccinated mice (P = 0·0067), although the production of both cytokines in OVA–AlOH-vaccinated mice was almost similar to that in Hp–AlOH-vaccinated mice. Furthermore, only one mouse (Fig. 1, open square) failed to show complete eradication of H. pylori by Hp–AlOH-immunization and, interestingly, production of IL-4 and IFN-γ in this mouse was the lowest among the Hp–AlOH-immunized mice. Thus, in the present study, a specific induction of Th2 immunity by vaccination with AlOH, as detected by Gottwein et al. [8] using the ELISPOT assay, was not observed when using ELISA and gastric tissues here. In contrast, immunization with AlOH resulted in induction of Th1 immunity in the gastric tissue.
Fig. 1.
Production of (a) interleukin (IL)-4, (b) IL-10, (c) interferon (IFN)-γ and (d) IL-12 in the gastric tissue of H. pylori-A1OH-inmmunized (Hp, ▪), OVA-A1OH-immunized (OVA, ⋄) and non-vaccinated (non-vac, •) mice. Open squares (□): a single mouse that failed to show a positive response to H. pylori-A1OH immunization. Horizontal bars indicate mean value of each cytokine. *P = 0·026; **P = 0·0067.
DISCUSSION
The mouse is not the natural host for H. pylori so the results obtained in this study may not apply to humans. On the other hand, data from human children are limited, because most H. pylori-infected children have no symptoms and it is difficult for a paediatrician to examine such asymptomatic children invasively. In the present study, we analysed the stomachs of many infant mice directly and we believe that it is meaningful to use our results to speculate when H. pylori infection occurs in human children.
The five major findings of the present study are the following: (1) maternal transmission of H. pylori is almost complete during the breastfeeding period; (2) the incidence of maternal transmission of H. pylori varied during the nursing period; (3) the incidence of maternal transmission tended to be higher than that of adult–adult transmission; (4) H. pylori vaccine not only protected against infection but also eradicated already transmitted bacteria; (5) parenteral immunization with AlOH resulted in a lowered production of Th2 cytokines and a higher production of Th1 cytokines in the stomach.
The results of the maternal-transmission experiment indicated that maternal transmission of H. pylori was almost complete at a certain stage ( mature milk) of the breastfeeding period. Recent epidemiological studies in humans suggest that the acquisition of H. pylori occurs during childhood. For example, Rothenbacher et al. [6] reported that H. pylori acquisition seems to occur mainly between the first and second year of life: that is, after the age of weaning. This conclusion conflicts with our results, although our study was conducted in a mouse model, not in humans. We consider that the cause of this discrepancy is as follows: Rothenbacher et al. [6] applied an H. pylori antigen enzyme immunoassay using stool samples (HpSA test; Meridian Diagnostic Inc.) to define current infection status, while we used a PCR method and gastric tissue for the same purpose. Although the HpSA test is thought to have higher sensitivity, especially in young children, than serological methods [16,17], our method is more sensitive for the detection of bacteria. On the other hand, a number of studies have indicated that lactoferrin [18], sialyllactose [19], oligosaccharides [19] and secretory IgA antibody [20], present naturally in milk, have a bacteriostatic effect on H. pylori infection. Thus, we hypothesize that maternal transmission is often established within the breastfeeding period, but the bacterial burden is below the level of detection of the HpSA test because of the bacteriostatic effect of milk. Presumably in humans, especially individuals who live in better sanitary conditions, maternal transmission before the weaning period is not as frequent as in mice. However, in the presence of poor sanitary conditions a more frequent maternal transmission is likely to occur during the nursing period.
Our results also showed that the maternal-transmission rate of H. pylori fluctuated during the observation period, that is it decreased from 2 days to 1 week postpartum (albeit insignificantly), increased from 1 to 2 weeks postpartum and decreased from 2 to 3 weeks postpartum. We think that the first decrease is due to spontaneous eradication of H. pylori by the increased immunity of the neonatal stomach. The middle increase was probably due to the longer period of maternal–neonate contact, while the last decrease could be explained by the reduced maternal–newborn contact during the weaning stage combined with spontaneous eradication of H. pylori. Perri et al. [21] reported that eradication of H. pylori occurs spontaneously during human childhood, and that H. pylori infection shows a fluctuating pattern. Our results are in agreement with this report.
The results of our control studies, i.e. adult–adult transmission studies, did not indicate any significant difference in the rates of adult–adult transmission and maternal transmission. It is possible that these results were due to the experimental design: we compared the rates in the two groups three weeks after co-habitation, i.e. at the time of reduced maternal transmission. However, the high maternal-transmission rate at 2 weeks postpartum (94%) suggests that the incidence of mother–child transmission could be higher, at least occasionally, than that of adult–adult transmission. Rothenbacher et al. [7] reported that infected parents, especially infected mothers, play a key role in the transmission of H. pylori within families. Maternal contact behaviour during the breastfeeding period may be responsible for the high frequency of maternal transmission.
It is not known whether infant mice develop gastric inflammation, and this is technically difficult to investigate because the stomachs of infant mice are too small to prepare histological specimens. We have some preliminary data on the histology of infant mice right after weaning, i.e. 3 weeks postpartum mice, and they show no inflammation. We conjecture that infant mice (<3-week mice) also do not have gastritis.
The above results indicate that an H. pylori vaccine should be considered not only as protective against H. pylori infection, but also able to eradicate the already transmitted pathogen. Our vaccination studies showed that systemic immunization with AlOH resulted in eradication of maternally-transmitted H. pylori. These results are compatible with those of Gottwein et al. [8], who reported that AlOH-induced systemic immunity could induce protective immunity in mice. In this regard, AlOH is the only adjuvant available for use in human children, and it is a very promising vaccine for this situation. However, we have reported recently that vaccination using AlOH did not reduce bacterial density in H. pylori-infected adult mice that had been inoculated with a bacterial suspension and confirmed the presence of H. pylori in cultured tissues from these mice [22]. The same result was also noted in young mice in preliminary studies conducted in our laboratories (unpublished results). Considered together, these results suggest that, with regard to eradication of H. pylori, the AlOH vaccine is useful only for eliminating a very low load of the bacteria. Furthermore, Lee et al. [23] reported that monkeys receiving only systemic immunization of urease plus AlOH failed to respond with respect to protection against the infection, compared with sham-immunized controls. The mechanisms underlying the complex relationship between host and bacteria may function differently in mice and primates. Thus, several questions remain unanswered regarding the effects of the AlOH vaccine, emphasizing the need for further studies before widespread use of this vaccine.
Finally, we examined the mechanisms underlying the beneficial effects of the vaccine seen in our mice. Gottwein et al. [8] conjectured that the local release of Th2 cytokines in the stomach after parenteral immunization with AlOH might be effective in recruiting or activating the effector cells that eliminate the bacteria. However, they did not evaluate the dynamics of cytokine production in the stomach, although they did investigate the profile of cytokines released by splenocytes. In our study, parenteral immunization with AlOH induced a local decrease of the Th2 cytokine IL-10 and local increase of the Th1 cytokine IL-12 in the stomach. These results were quite the opposite to the speculation of Gottwein et al. [8]. Our results also indicated that the polarized Th1 immunity was not antigen-specific. One mouse immunized with Hp–AlOH that failed to show eradication of H. pylori showed the lowest production of IL-4 and IFN-γ, which are downstream cytokines of IL-10 and IL-12, respectively. These results suggest that antigen-specific immunity followed by minimum production of both types of downstream cytokines in the stomach may play an important role in the eradication of H. pylori, while a change in the local cytokine profile induced by immunization with AlOH is not very important.
In conclusion, the present study provides new and important information on maternal transmission of H. pylori and systemic immunity with AlOH. Because acquisition of H. pylori infection during childhood seems to be a critical risk factor for the later development of gastric cancer, the elucidation of the mechanisms involved in transmission of H. pylori during childhood and subsequent improvement of the vaccine that can provide both protective and therapeutic immunity against H. pylori are essential.
Acknowledgments
This study was supported in part by Grants-in-Aid no. 13226104 (AN) and no. 14021102 (AN) from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–5. [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–9. [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. P. 188, IARC. 1994. p. 61. [PMC free article] [PubMed]
- 4.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–8. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 5.Honda S, Fujioka T, Tokieda M, et al. Development of Helicobacter pylori-induced gastric carcinoma in mongolian gerbils. Cancer Res. 1998;58:4255–9. [PubMed] [Google Scholar]
- 6.Rothenbacher D, Inceoglu J, Bode G, Brenner H. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J Pediatr. 2000;136:744–8. [PubMed] [Google Scholar]
- 7.Rothenbacher D, Inceoglu J, Bode G, et al. Helicobacter pylori among preschool children and their parents: evidence of parent-child transmission. J Infect Dis. 1999;179:398–402. doi: 10.1086/314595. [DOI] [PubMed] [Google Scholar]
- 8.Gottwein JM, Blanchard TG, Targoni OS, et al. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete freund's adjuvant by systemic immunization. J Infect Dis. 2001;184:308–14. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 9.Weltzin R, Kleanthous H, Guirakhoo F, Monath TP, Lee CK. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–6. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson K. Preparation of genomic DNA from bacteria, UNIT2.4. In: Ausubel FM, Brent R, Kingston RE, et al., editors. Current Protocols in Molecular Biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1999. [Google Scholar]
- 11.Yoshimatsu T, Shirai M, Nagata K, Okita K, Nakazawa T. Transmission of Helicobacter pylori from challenged to nonchallenged nude mice kept in a single cage. Dig Dis Sci. 2000;45:1747–53. doi: 10.1023/a:1005586312582. [DOI] [PubMed] [Google Scholar]
- 12.Makristathis A, Pasching E, Schütze K, et al. Detection of Helicobacter pylori in stool specimens by PCR and antigen enzyme immunoassay. J Clin Microbiol. 1998;36:2772–4. doi: 10.1128/jcm.36.9.2772-2774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellini L, Marzio L, Ferrero G, et al. Transmission of Helicobacter pylori in an animal model. Dig Dis Sci. 2001;46:62–8. doi: 10.1023/a:1005605724271. [DOI] [PubMed] [Google Scholar]
- 14.Goto T, Nishizono A, Fujioka T, et al. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531–9. doi: 10.1128/iai.67.5.2531-2539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto N, Sakagami T, Fukuda Y, et al. Influence of Helicobacter pylori infection on development of stress-induced gastric mucosal injury. J Gastroenterol. 2000;35:332–40. doi: 10.1007/s005350050357. [DOI] [PubMed] [Google Scholar]
- 16.Oderda G, Rapa A, Ronchi B, et al. Detection of Helicobacter pylori in stool specimens by non-invasive antigen enzyme immunoassay in children: multicentre Italian study. Br Med J. 2000;320:347–8. doi: 10.1136/bmj.320.7231.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuda M, Miyashiro E, Koike M, et al. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr Int. 2002;44:387–90. doi: 10.1046/j.1442-200x.2002.01585.x. [DOI] [PubMed] [Google Scholar]
- 18.Dial EJ, Hall LR, Serna H, et al. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig Dis Sci. 1998;43:2750–6. doi: 10.1023/a:1026675916421. [DOI] [PubMed] [Google Scholar]
- 19.Mysore JV, Wigginton T, Simon PM, et al. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel anti-adhesion compound. Gastroenterology. 1999;117:1316–25. doi: 10.1016/s0016-5085(99)70282-9. [DOI] [PubMed] [Google Scholar]
- 20.Thomas JE, Austin S, Dale A, et al. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet. 1993;342:121. doi: 10.1016/0140-6736(93)91327-i. [DOI] [PubMed] [Google Scholar]
- 21.Perri F, Pastore M, Clemente R, et al. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J Pediatr Gastroenterol Nutr. 1998;27:181–3. doi: 10.1097/00005176-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Yamashiro T, Minoura T, et al. Evaluation of therapeutic efficacy of adjuvant Helicobacter pylori whole cell sonicate in mice with chronic H. pylori infection. Microbiol Immunol. 2002;46:613–20. doi: 10.1111/j.1348-0421.2002.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee CK, Soike K, Giannasca P, et al. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–82. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]