Abstract
MHC class I-peptide tetrameric complexes (‘tetramers’) have revolutionized the study of antiviral CD8+ T cell responses. They allow accurate quantification of immune responses ex vivo independent of function, with high levels of sensitivity. They have revealed unexpectedly large frequencies of ‘memory’ T cell responses against viruses such as cytomegalovirus (CMV) and Epstein–Barr virus (EBV), and provided information about their phenotypic and functional variation. However, such studies have generally concentrated on limited numbers of individuals analysed in detail. To allow larger population-based studies, we devised a method for tetramer analysis using 50–100 microlitre blood volumes in a 96-well plate format. We adapted this method to study the effect of age on responses in a cohort of nearly 600 individuals to an immunodominant HLA-A2 restricted response to CMV pp65 (NLVPMVATV). We observed the phenomenon of steady ‘memory inflation’ with age, similar to recently observed longitudinal data from murine studies. These data show that tetramers can be used as population screening tools and could be used to study age-related, geographical or seasonal effects in a number of other viral infections.
Keywords: CD8+ T lymphocyte, CMV, memory, MHC class I, tetramer
INTRODUCTION
CD8+ T cell responses play a major role in the control of a number of intracellular pathogens. In murine systems there is data from studies of viruses such as LCMV, MCMV, influenza and pathogens such as Listeria [1]. In man, persistent virus infections such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), Epstein–Barr virus (EBV) and cytomegalovirus (CMV) have all been associated with an important role for CD8+ T cells [2,3]. There has recently been an explosion of data in this area since the advent of new technologies designed to study responses ex vivo. Two of these, ELISpot, which allows analysis of interferon-gamma producing, antigen-specific cells, and tetramer analysis, have proved particularly useful in human studies [4,5]. Tetramers were developed 7 years ago and since then have been used in a wide variety of analyses of dynamics, magnitude and phenotype of antiviral T cell responses [3,6]. While in principle tetramers provide ideal tools for large-scale studies, in practice these are often limited by the perceived requirement for large cell numbers for cell preparation, cryopreservation and tissue-typing. Thus most studies have concentrated on analysis of limited numbers of patients [7,8], providing great detail, often on longitudinal samples. However, because very low cell numbers can be used on flow cytometer-based assays for both tetramer studies and tissue-typing, these problems could be bypassed readily for large-scale studies.
Such issues are of particular relevance in studies of T cell responses to common viruses. One such example is CMV, a beta-herpesvirus which infects the majority of the world's population. While acute infection is often asymptomatic in adults, it can cause significant disease in the fetus. The virus sets up long-term persistence and reactivation can produce severe disease in those immunosuppressed through HIV or after transplantation. CMV possesses a range of immune evasion strategies, many of which are designed to evade CD8+ T cell responses, and many studies have shown strong CD8+ T cell ‘memory’ responses during long-term clinical latency [9–11]. It is likely that such responses are maintained through exposure to viral antigens during continuous reactivation. In a murine model of CMV (MCMV), CD8+ T cell responses to two major epitopes showed slow and continuous expansion over time (with evidence of re-exposure to viral peptides in lymphoid tissue), a process termed ‘memory inflation’ [12,13]. Long-term longitudinal studies in man are not feasible, but to address the issue we used a population-based approach to study the effect of age on CMV responses. Although large CMV-specific responses in the elderly have been noted previously, the overall relationship with age across a wide range has not been well-defined [14,15]. Therefore, to test the applicability of tetramers in a population-based setting and to analyse whether ‘memory inflation’ occurs with human CMV infection, we performed a large cross-sectional study.
METHODS
We performed a cross-sectional analysis of HCMV-specific CD8+ T cell responses in a random selection of 581 anonymous ethyline diamine tetra-acetic acid (EDTA) blood samples sent to a district general hospital. Ethical approval was obtained for the study from the local review board (COREC). During the period of study, no samples tested CMV IgM positive, indicative of acute disease within the study population. Samples of volume 50 ul were screened for the presence of HLA-A2 using a FITC-conjugated monoclonal antibody (One Lambda Inc., Canoga Park, CA, USA), followed by lysis using FACSlyse (BD Pharmingen, San Jose, CA, USA) according to the manufacturers’ instructions. Samples were then analysed directly by flow cytometric analysis (FACScan, BD Pharmingen). A total of 275 HLA-A2-positive samples were then tested for CD8+ T cell responses against an immunodominant epitope in HCMV tegument pp65 (NLVPMVATV) [9], using a combination of staining with an HLA-A2 peptide tetramer (37°C, 20 min produced as described previously [11,16]) followed by anti-CD8-PerCP (BD Pharmingen) for 20 min at 4°C; 50 µl samples were analysed using previously titrated quantities of tetramer and antibody in a 96-well plate format. Samples were lysed as above, and resuspended in 200 µl PBS/1% formaldehyde. Analysis was performed using CellQuest software and the percentage of CD8+ T cells staining with the tetramer was determined. A subset of the HLA-A2 positive samples were tested for CMV IgG using a commercial Latex assay (BD, Pharmingen).
RESULTS
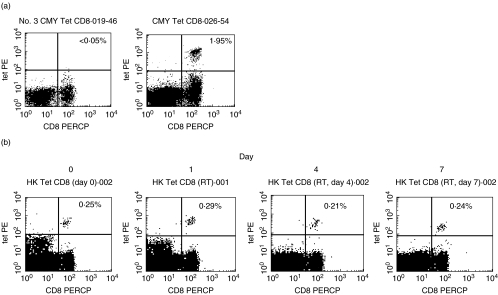
The technique of tetramer staining 50 µl samples in a 96-well plate format provided good quality staining (Fig. 1a). Analysis of HLA-A2 negative samples from healthy donors was used to provide a stringent cut-off for detection of 0·05% of CD8s (data not shown). Use of samples of volumes lower than 50 µl did not provide sufficiently large cell populations after washing and lysis for reliable tetramer analysis.
Fig. 1.
Tetramer staining for population studies. (a) Examples of staining from HLA-A2-positive subjects. The FACS plots are gated upon live lymphocyte populations from two donors, one negative (left) and one positive (right), for CMV responses. Proportions of positive CD8+ lymphocytes are shown in the upper right quadrant. (b) Effect of storage on tetramer staining. Samples were taken into EDTA and stored at room temperature. On day 0 and after 1, 4 and 7 days the blood was stained for CMV tetramer-positive cells as described in Methods. The proportions of positive lymphocytes are shown in the upper right quadrants. A day 10 sample showed much weaker staining intensity and lower frequency (0·14%) of positive cells.
We also performed analyses to determine whether storage of blood (which may be an inevitable part of the collection process in such population-based studies) affected the tetramer staining (Fig. 1b). We found that storage of EDTA blood at room temperature over a period of 1 week had little effect on the frequency of tetramer positive cells detected. Storage for longer periods or storage at either 4°C or at 37°C led to significant decreases in the staining detected (data not shown).
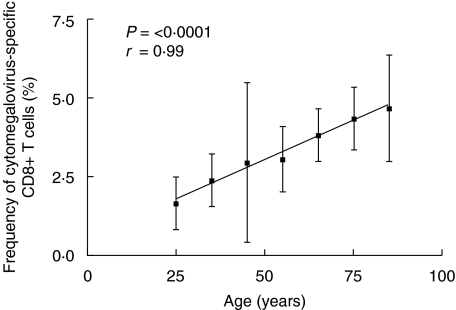
Having established the quality of the staining protocols we next analysed the frequency of tetramer positive cells in the CD8+ T cell compartment as a function of age. A strong association with age was shown (Table 1, Fig. 2). The frequency of those staining positive with tetramer showed age dependence, as expected, given the rising frequency of CMV seropositivity with age in western populations [17]. However, a striking increase was also seen in the proportion of any individual's CD8+ T cells which were responsive to this particular epitope. This rose continuously with age, in a linear fashion. The proportion of CD8+ T cells did not change significantly over time, and the proportion of tetramer-positive cells among live lymphocytes showed a similar gradual increase (Table 1).
Table 1.
Overall analysis of CD8+ T cell responses to CMV by age: 583 subjects were analysed initially, of whom 277 were A2-positive (47·5%). Of these 98/277 (35·4%) were tetramer-positive (i.e. tetramer/CD8 > 0·05%). The frequencies of those staining positive for CMV responses and of CD8+, Total Tet% and Tet/CD8+ are displayed according to age group
| Age (years) | 0–9 n = 5 | 10–19 n = 9 | 20–29 n = 28 | 30–39 n = 57 | 40–49 n = 23 | 50–59 n = 44 | 60–69 n = 39 | 70–79 n = 44 | 80–89 n = 26 | 90 + n = 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| CMV Tet + ve | 0 | 1 | 8 | 14 | 7 | 15 | 18 | 23 | 11 | 1 |
| (0%) | (11%) | (28·6%) | (24·6%) | (30·4%) | (34·1%) | (46·1%) | (50·0%) | (42·3%) | (50%) | |
| CD8(%) | 28·4 | 28·9 | 27·1 | 24·1 | 25·1 | 22·6 | 23·1 | 25·6 | 4·2 | |
| Tet (%) | 0·54 | 0·46 | 0·65 | 0·46 | 0·76 | 0·86 | 0·86 | 0·93 | 0·47 | |
| Tet/CD8 (%) | 1·87 | 1·66 | 2·40 | 2·95 | 3·06 | 3·81 | 4·33 | 4·66 | 10·6 |
Fig. 2.
CMV-specific T cell populations as a function of age. Mean frequencies of tetramer positive cells as a fraction of total CD8+ cells in individuals of different ages. Of 581 individuals screened, 275 were HLA-A2-positive. Only tetramer positive samples are included in the analysis (mean ± s.e.m. displayed in 10-year age groups). Staining using matched controls established a threshold of detection of 0·05%. Frequencies of those HLA-A2-positive individuals scoring tetramer-positive in each 10-year age group are shown in Table 1 − the highest and lowest 10-year age groups are not included due to lack of positive samples. The correlations shown are for the 10-year age groups included. Values for breakdown by individual year age groups showed P= 0·0014, r= 0·32.
DISCUSSION
We examined whether tetramers could be used in a large-scale study, and found that rapid high quality staining of large numbers of samples can be achieved. We focused on an HLA-A2 restricted response, as this is common, and HLA A2 status assessed readily with fluorescently labelled monoclonal antibodies and FACS analysis. In theory it would be possible to include the HLA-A2 typing step with the tetramer staining and therefore save further time and minimizing blood usage, although this would also increase the use of tetramer on mismatched samples. It is also possible to perform typing of multiple other alleles using similar monoclonal reagents, so in future such studies need not be restricted to A2. Given the small amounts of blood required, it would be possible to perform similar studies using only a fingerprick sample.
Using this technique we have demonstrated a continuous accumulation of pp65 specific T cells over a range of ages. This effect is similar to that seen in murine systems, where responses to two peptides studied increased gradually with age (‘memory inflation’) [12,13]. The rate of increase shown here, however, is very slow and on average doubles over several decades, so it is unlikely that such an effect would be seen clearly in even long-term longitudinal follow-up studies in man. In a subset of patients, we analysed the relationship between CMV seropositivity and tetramer positivity. We found that of 44 seropositive HLA-A2 positive patients, 34 had detectable tetramer clouds using the single pp65 tetramer (77%). For the remaining tetramer negative individuals it will be of interest in future studies to identify whether such people have large responses restricted by another HLA allele or directed at another gene product.
The most likely explanation for the accumulation seen is that HCMV infection, while apparently latent, undergoes reactivation continuously at a subclinical level [17]. Again, data from the murine model (MCMV) suggests strongly that viral genes are expressed [18–20]. This may not be true for all genes, so it will be of interest in future studies to analyse peptides derived from a variety of gene products with different expression profiles in latency. It is predicted that only a limited number of these might induce ‘inflationary’ responses. Studies of other infections will be of great interest, as to date most show either an apparent plateau (e.g. EBV [21,22]) or a decline to very low levels (e.g. influenza) [3,23]. Infections by other members of the herpesvirus family (herpes zoster, herpes simplex, HHV6 and EBV), which form complex, long-term relationships with their host, will be of particular interest.
The role of the tetramer positive cells in vivo is not entirely clear in relation to control of the virus. Because viral replication is not usually detected in blood, it is difficult to relate the size of the population to the viral load. It is possible that it relates to a gradually increasing but still undetectable viral load, or even to an oscillating low load [24]. This low-level tissue-based load is difficult to study in man, but as it is clear that the murine model reflects the human infection in this respect, future studies of MCMV over time might shed light on this issue.
The large CMV-specific populations which have been identified in the elderly have presumably been accumulating in these individuals over many decades. There is evidence that the presence of CMV may have a strong influence on the overall composition of the immune system in the elderly [14,15]. This study would suggest that any effect of CMV-driven expansion would be gradual, rather than a specific effect on the elderly.
Overall, the study shows a new potential use of tetramers which to date have been used only in studies of relatively limited numbers of individuals. Use of such reagents in a whole blood-based, 96-well plate format for rapid processing could take them closer to clinical applications, where such tests have so far remained very much research tools. Because the samples may be stored under appropriate conditions and batched, this could in future be an efficient way of performing studies of T cell-based immunity in a range of settings including treatment or vaccination of viral infections [25] or tumours [26] and transplantation [16,27].
Acknowledgments
This work was sponsored by the Wellcome Trust. Ana Vargas Cuero is supported by the MRC, the Catholic University of Guayacil and the Fundacyt, Ecuador. We are grateful to Rodney Phillips for help with the CMV studies, and Michaela Lucas and Ellie Barnes for assistance on the FACS. Thanks also must go to the haematology and virology departments of the John Radcliffe Hospital for their co-operation.
REFERENCES
- 1.Masopust D, Vezys V, Marzo A, et al. Preferential localisation of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 2.Appay V, Dunbar P, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 3.Klenerman P, Cerunulo V, Dunbar P. Tracking T cells with tetramers: new tales from new tools. Nature Rev Immunol. 2002;2:263–72. doi: 10.1038/nri777. [DOI] [PubMed] [Google Scholar]
- 4.Lalvani A, Brookes R, Hambleton S, et al. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman J, Moss PAH, Goulder P, et al. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–6. [Google Scholar]
- 6.Lechner F, Cuero AL, Kantzanou M, et al. Studies of human antiviral CD8+ lymphocytes using class I peptide tetramers. Rev Med Virol. 2001;11:11–22. doi: 10.1002/rmv.295. [DOI] [PubMed] [Google Scholar]
- 7.Ogg G, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1 specific CTL and plasma load of HIV-1. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 8.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang EC, Moss PA, Frodsham P, et al. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155:5046–56. [PubMed] [Google Scholar]
- 11.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karrer U, Sierro S, Wagner M, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–9. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 13.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L (lo) memory-effector pool during latent murine cytomegalovirus infection of the lungs. J virol. 2000;74:11495–503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan N, Shariff NCM, Bruton R, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 15.Olsson J, Wikby A, Johansson B, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 16.Hassan-Walker AF, Vargas Cuero AL, Mattes FM, et al. CD8+ cytotoxic lymphocyte responses against cytomegalovirus after liver transplantation: correlation with time from transplant to receipt of tacrolimus. J Infect Dis. 2001;183:835–43. doi: 10.1086/319260. [DOI] [PubMed] [Google Scholar]
- 17.Mocarski E, Courcelle C. Cytomegaloviruses and their replication. In: Knipe D, Howley P, editors. Fields virology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. pp. 2629–73. [Google Scholar]
- 18.Henry S, Hamilton J. Detection of murine cytomegalovirus immediate early 1 transcripts in the spleens of latently infected mice. J Infect Dis. 1993;167:950–4. doi: 10.1093/infdis/167.4.950. [DOI] [PubMed] [Google Scholar]
- 19.Hummel M, Zhang Z, Yan S, et al. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J Virol. 2001;75:4814–22. doi: 10.1128/JVI.75.10.4814-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzimek N, Dreis D, Schmalz S, et al. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J Virol. 2001;75:2692–705. doi: 10.1128/JVI.75.6.2692-2705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callan M, Tan L, Annels N, et al. Direct visualisation of antigen specific CD8+ T cells during the primary immune response to EBV in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callan MF, Annels N, Steven N, et al. T cell selection during the evolution of CD8+ T cell memory in vivo. Eur J Immunol. 1998;28:4382–90. doi: 10.1002/(SICI)1521-4141(199812)28:12<4382::AID-IMMU4382>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar P, Ogg G, Chen J, et al. Direct isolation, phenotyping and cloning of low frequency antigen-specific CTL from peripheral blood. Current Biol. 1998;8:413–6. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 24.Luzyanina T, Engelborghs KES, Klenerman P, Bocharov G. Low level viral persistence after infection with LCMV: a quantitative insight through numerical bifurcation analysis. Math Biosci. 2001;173:1–23. doi: 10.1016/s0025-5564(01)00072-4. [DOI] [PubMed] [Google Scholar]
- 25.Ogg GS, Jin X, Bonhoeffer S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar PR, Smith CL, Chao D, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165:6644–52. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 27.Li CR, Greenberg PD, Gilbert MJ, et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–9. [PubMed] [Google Scholar]