Abstract
Moderate hyperhomocysteinaemia is associated with atherosclerosis, thrombosis and also with stroke and dementia. Elevated homocysteine concentrations are related to deficiency of folate and also vitamin-B12, as these two vitamins are essential co-factors in the remethylation of homocysteine to methionine. A causal role of homocysteine in the pathogenesis of vascular disease has been discussed over years. Immune activation appears to be involved strongly in atherogenesis as well as in other diseases found to be associated with moderate hyperhomocysteinaemia. To study a possible influence of immune stimulation on homocysteine metabolism, in vitro experiments were performed using peripheral blood mononuclear cells upon stimulation with mitogens concanavalin A, phytohaemagglutinin and pokeweed mitogen. In stimulated cells a dose-dependent increase of homocysteine concentrations was found. When cells were kept in medium supplemented with methionine, homocysteine concentrations increased further, while supplementation with folate had only a slight effect. We conclude that in supernatants of stimulated peripheral blood mononuclear cells homocysteine is accumulating. T cell activation could be involved in the development of moderate hyperhomocysteinaemia.
Keywords: homocysteine, human peripheral blood mononuclear cells, immune activation, in vitro
INTRODUCTION
Moderate hyperhomocysteinaemia is found to be associated with cardiovascular disorders such as atherosclerosis and thromobosis and also with cerebrovascular diseases such as stroke and dementia [1,2]. Moderate hyperhomocysteinaemia has been attributed so far to deficiency of B-vitamins folate and B12 and also deficiencies of enzymes such as cystathionine-β-synthase or thermolabile methylentetrahydrofolate-reductase [3–6]. In agreement, blood homocysteine concentrations correlate inversely with concentrations of B-vitamins, preferentially with folate concentrations. Similarly, folate supplementation is able to reduce homocysteine concentrations in patients [7].
Based on results of in vivo and in vitro studies, a causal role of homocysteine in the development of cardiovascular disease and atherosclerosis has been discussed, suggesting that auto-oxidation of homocysteine may induce oxidative stress leading to endothelial cell injury [8,9]. Immune activation appears to be involved strongly in atherogenesis [10] as well as in other diseases found to be associated with moderate hyperhomocysteinaemia, e.g. autoimmune diseases such as rheumatoid arthritis and neurodegenerative disorders such as Alzheimer's disease. We studied the homocysteine metabolism of activated human peripheral blood mononuclear cells (PBMC) as an in vitro model of immune activation. Furthermore, the influence of folate and methionine on stimulation-induced homocysteine production was examined.
MATERIALS AND METHODS
Cell culture
PBMC were isolated from whole blood obtained from healthy voluntary blood donors by density centrifugation (Lymphoprep, Nycomed Pharma AS, Oslo, Norway). Cells were maintained in RPMI-1640 (PAA-Laboratories, Linz, Austria) supplemented with 10% heat-inactivated fetal calf serum (Biochrom, Berlin, Germany), 2 mm l-glutamine (Serva, Heidelberg, Germany) and 50 µg/ml gentamycin (Bio-Whitaker, Walkersville, MD, USA).
For stimulation cells were seeded at a density of 1 × 10 6/ml and stimulated with different concentrations of lectins concanavalin A (ConA, Sigma, Vienna, Austria), phytohaemagglutinin (PHA, Sigma) and pokeweed mitogen (PWM, Sigma) as well as with TNF-α (100–1000 U/ml; Strathmann Biotech, Hamburg, Germany), interferon-γ (250–1000 U/ml; Bioferon, Laupheim, Germany) and lipopolysaccharide (LPS; 0·01–1 µg/ml; Sigma). Cells were then incubated at 37°C in 5% CO2 for 72 h, and supernatants were harvested by centrifugation (1500 r.p.m., 4°C, 8 min) and frozen at − 20°C until measurement.
The effect of folate and methionine (Sigma, Vienna, Austria) and a combination of these two substances on homocysteine production of cells were studied employing different concentrations of these compounds.
Experiments were performed three times with duplicates of controls and stimulated cells.
Determination of homocysteine
Homocysteine was determined by high performance liquid chromatography (HPLC), as described previously [11].
Statistical analysis
For comparisons of grouped data the Mann–Whitney U-test was applied. P-values below 0·05 were considered to indicate significant differences.
RESULTS
Stimulation of peripheral blood mononuclear cells was performed with several stimuli at different concentrations. While TNF-α, interferon-γ and LPS showed no significant effects on peripheral blood mononuclear cells with regard to homocysteine production (Table 1), lectins concanavalin A (Con A), PHA and PWM exhibited not only mitogenic effects, they also induced formation of homocysteine.
Table 1.
Homocysteine concentrations in supernatants of peripheral blood mononuclear cells stimulated with LPS, TNF-α and IFN-γ
| Homocysteine (µmol/l) (mean ± s.d., range in brackets) | |
|---|---|
| Unstimulated | 1·1 ± 0·2 (0·5–1·3) |
| LPS (0·01–1 µg) | 0·9 ± 0·1 (0·5–1·3) |
| TNF-α (100–1000 U/ml) | 0·8 ± 0·2 (0·6–1·1) |
| IFN-γ (250–1000 U/ml) | 1·1 ± 0·1 (0·7–1·3) |
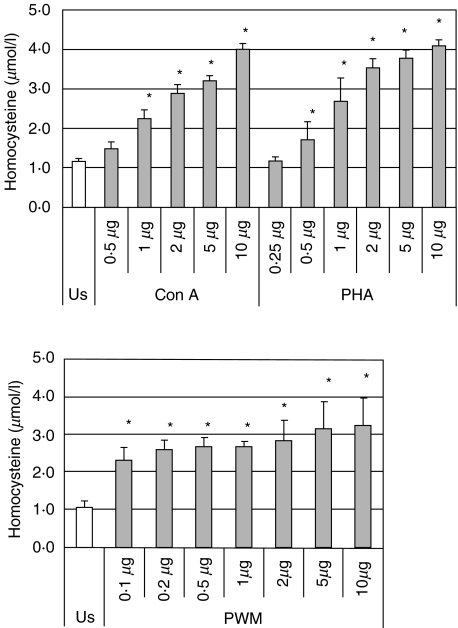
Peripheral blood mononuclear cells stimulated with Con A and PHA produced homocysteine in a dose-dependent manner; the highest homocysteine concentrations were reached with 10 µg/ml Con A or PHA (Fig. 1, showing concentrations dependency down to the first concentration not showing a significant difference to the unstimulated control). PWM also induced significant homocysteine production, but the effect of PWM was smaller in comparison with Con A and PHA. In addition, dose-dependency was weak within the concentration range of 0·1–10 µg/ml (Fig. 1).
Fig. 1.
Homocysteine concentrations (mean ± s.d.) in supernatants of unstimulated peripheral blood mononuclear cells and on stimulation with lectins concanavalin A (Con A), phytohaemagglutinin (PHA) and pokeweed mitogen (PWM). *P < 0·01 (compared to unstimulated controls cells, US).
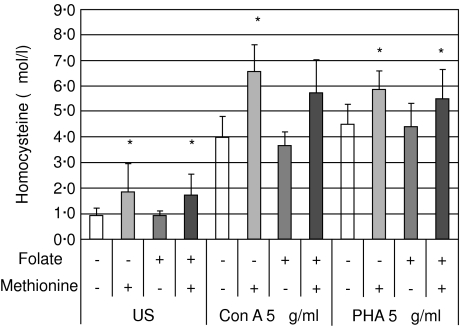
Media were supplemented with different folate and methionine concentrations. In media supplemented with 10 µmol/l folate and 500 µmol/l methionine, or a combination of these two compounds, differences with regard to homocysteine production could be monitored most effectively. Methionine supplementation further increased homocysteine production of cells stimulated, whereas additional folate had only a slight homocysteine-lowering effect (Fig. 2). Similar results were obtained in experiments performed with Con A and PHA.
Fig. 2.
Homocysteine concentrations (mean ± s.d.) in supernatants of unstimulated peripheral blood mononuclear cells (US) and of cells stimulated with concanavalin A (Con A) and phytohaemagglutinin (PHA) supplemented with methionine (500 µmol/l) and/or folate (10 µmol/l). *P < 0·01 (comparison between experiments with/without methionine supplementation).
DISCUSSION
In in vitro experiments homocysteine was found to accumulate in supernatants of stimulated peripheral blood mononuclear cells. While other stimuli such as TNF-α, interferon-γ or LPS had nearly no influence, homocysteine formation increased in PBMCs treated with mitogens Con A, PHA and PWM in a dose-dependent way. In contrast to treatment with cytokines and LPS, mitogens preferentially stimulate cell growth. Homocysteine production therefore seems to be associated with proliferative activity of PBMCs. Similarly, enhanced homocysteine production was demonstrated in tumour cells recently by Wu and coworkers [12], suggesting the use of homocysteine as tumour marker. Tumour cells proliferate spontaneously, which might be the reason for homocysteine formation. However, the fact that ‘normal’, i.e. non-malignant, cells also show an enhanced homocysteine–methionine metabolism when stimulated suggests a more general association between cell proliferation and homocysteine accumulation. Although it is difficult to extrapolate from the in vitro to the in vivo situation, it appears possible that during the immune response, proliferating immunocompetent cells could contribute substantially to the development of hyperhomocysteinaemia.
Raised homocysteine production in vitro may indicate incomplete remethylation of homocysteine to methionine. Proliferating cells require methionine, because it is one of the most important methylgroup donors for the methylation of nucleic acids. In contrast to several tumour cell lines that are auxotroph for methionine [13], normal cells do not generally have any problem in obtaining methionine from homocysteine. It appears plausible that proliferating cells have an enhanced demand for methionine, on one hand, and folate on the other hand. In quickly proliferating cells, within the redox-recycling of essential co-factors some folate might be wasted and could impair remethylation reaction. Interestingly, in our experiments with methionine supplementation homocysteine accumulation was enhanced further.
Folic acid is involved in methylgroup metabolism as well as in DNA synthesis and repair, which provides an appropriate explanation for the various effects of folate deficiency besides hyperhomocysteinaemia: inhibition of cellular proliferation, cell cycle alterations, chromosome instability and even cell death [14,15]. In experiments performed previously it has been demonstrated that in lymphocytes grown in medium containing no or low folate concentrations DNA strand breakage and uracil misincorporation increased in a time- and dosage-dependent manner [16]. While PHA- or interleukin-stimulated cells showed high proliferation rates, cells incubated in folate-deficient medium did not grow and DNA strand breakage as well as uracil misincorporation were increased. Furthermore, unstimulated lymphocytes cultured in folate-containing medium were able to compensate for most of the damage induced by hydrogen peroxide while cells deprived of folate were unable to prevent DNA damage sufficiently. In accordance with these findings, declining folate concentrations developing by stimulation of lymphocyte proliferation could be responsible for the enhanced formation of homocysteine. Folate depletion could therefore also serve as an explanation for hyperhomocysteinaemia in cancer patients, as due to the rapid proliferation of malignant cells folic acid concentrations, e.g. in lung cancer, cells were found to be lower than in unaffected tissue. The suggested role of homocysteine as a potential tumour marker [12,16] could therefore reflect, on one hand, tumour progression, and on the other hand, the enhanced consumption of folate by proliferating tumour cells.
Activated macrophages appear to be involved critically in atherogenesis. Reactive oxygen species (ROS) are also produced in scope with immune activation by stimulated macrophages to eliminate pathogens. As in states of chronic immune stimulation, the production of ROS is supposed to play an important role, e.g. in atherosclerosis, but in cancer and dementia [17–19] ROS could also be responsible for the enhanced oxidation of antioxidants. In this way development of hyperhomocysteinaemia may relate to macrophage activation. The interaction between different immunocompetent cells, e.g. T cells stimulating macrophages to produce ROS by producing various cytokines, could be crucial in this process. In situations of immune activation, the enhanced production of ROS influences the redox balance of cells strongly by leading to the oxidation of antioxidant substances and afterwards to the oxidation of other oxidation-sensitive substances such as methylene-tetrahydrofolate, the active form of folate participating in the remethylation of homocysteine to methionine.
An association between hyperhomocysteinaemia and increased concentrations of immune activation marker neopterin has been described previously in several clinical conditions such as disturbed glucose metabolism, pre-eclampsia or Parkinson's disease [20–22]. Increased amounts of neopterin are produced by human monocyte-derived macrophages on stimulation with interferon-γ in scope [23]. Recently, higher neopterin concentrations in carotid atherosclerosis were reported to be related to innate immune mechanisms via toll-like receptor 4 polymorphism [24]. Thus, the coincidence of increased neopterin and homocysteine concentrations suggests a relationship between activated macrophages and the accumulation of homocysteine.
B-vitamins tetrahydrofolate and vitamin B12 are known to be easily oxidized [25]; oxidative stress derived from immune activation would provide an appropriate explanation for increased homocysteine levels resulting from the enhanced oxidation of these two essential co-factors in the remethylation of homocysteine to methionine. Therefore, the question arises as to whether moderate hyperhomocysteinaemia in cardiovascular disorders and other diseases may be secondary and represent a consequence of another primary event, namely immune system activation.
Acknowledgments
This work was supported by the Austrian Funds ‘Zur Förderung der wissenschaftlichen Forschung’, project 14942 and by the Austrian Federal Ministry of Social Affairs and Generations.
REFERENCES
- 1.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Bell IR, Edman JS, Selhub J, et al. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr Scand. 1992;86:386–90. doi: 10.1111/j.1600-0447.1992.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 3.Lentz SR. Homocysteine and vascular dysfunction. Life Sci. 1997;61:1205–11. doi: 10.1016/s0024-3205(97)00392-5. [DOI] [PubMed] [Google Scholar]
- 4.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 5.Bottiglieri T, Parnetti L, Arning E, et al. Plasma total homocysteine levels and the C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene: a study in an Italian population with dementia. Mech Ageing Dev. 2001;122:2013–23. doi: 10.1016/s0047-6374(01)00307-4. [DOI] [PubMed] [Google Scholar]
- 6.Lentz SR, Erger RA, Dayal S, et al. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H970–5. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 7.Ubbink JB, van der Vermaak WJMA, Becker PJ, Delport R, Potgieter HC. Vitamin requirements for the treatment of hyperhomocysteinemia in humans. J Nutr. 1994;124:1927–33. doi: 10.1093/jn/124.10.1927. [DOI] [PubMed] [Google Scholar]
- 8.Dudman NP, Hicks C, Wang J, Wilcken DE. Human arterial endothelial cell detachment in vitro: its promotion by homocysteine and cysteine. Atherosclerosis. 1991;91:77–83. doi: 10.1016/0021-9150(91)90189-a. [DOI] [PubMed] [Google Scholar]
- 9.Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980;18:113–21. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 11.Frick B, Schröcksnadel K, Neurauter G, Wirleitner B, Artner-Dworzak E, Fuchs D. Rapid measurement of total plasma homocysteine by HPLC. Clin Chim Acta. 2003;331:19–23. doi: 10.1016/s0009-8981(03)00076-7. [DOI] [PubMed] [Google Scholar]
- 12.Wu LL, Wu JT. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002;322:21–8. doi: 10.1016/s0009-8981(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RM. Altered methionine metabolism and transmethylation in cancer. Anticancer Res. 1985;5:1–30. [PubMed] [Google Scholar]
- 14.Fenech M. The role of folic acid and vitamin B12 in genomic stability of human cells. Mutat Res. 2001;475:57–67. doi: 10.1016/s0027-5107(01)00079-3. [DOI] [PubMed] [Google Scholar]
- 15.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998;12:1491–7. [PubMed] [Google Scholar]
- 16.Sun CF, Haven TR, Wu TL, Tsao KC, Wu JT. Serum total homocysteine increases with the rapid proliferation rate of tumor cells and decline upon cell death: a potential new tumor marker. Clin Chim Acta. 2002;321:55–62. doi: 10.1016/s0009-8981(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 17.Iuliano L. The oxidant stress hypothesis of atherogenesis. Lipids. 2001;36:S41–4. doi: 10.1007/s11745-001-0680-1. [DOI] [PubMed] [Google Scholar]
- 18.Tuppo EE, Forman LJ. Free radical oxidative damage and Alzheimer's disease. J Am Osteopath Assoc. 2001;101:S11–5. [PubMed] [Google Scholar]
- 19.Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med. 2002;33:192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 20.Gottsater A, Anwaar I, Eriksson KF, Mattiasson I, Lindgarde F. Homocysteine is related to neopterin and endothelin-1 in plasma of subjects with disturbed glucose metabolism and reference subjects. Angiology. 2000;51:489–97. doi: 10.1177/000331970005100606. [DOI] [PubMed] [Google Scholar]
- 21.Powers RW, Evans RW, Majors AK, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998;179:1605–11. doi: 10.1016/s0002-9378(98)70033-x. [DOI] [PubMed] [Google Scholar]
- 22.Widner B, Leblhuber F, Frick B, Laich A, Artner-Dworzak E, Fuchs D. Moderate hyperhomocysteinaemia and immune activation in Parkinson's disease. J Neural Transm. 2002;109:181–9. doi: 10.1007/s00702-002-0758-8. [DOI] [PubMed] [Google Scholar]
- 23.Widner B, Enzinger C, Laich A, Wirleitner B, Fuchs D. Hyperhomocysteinemia, pteridines and oxidative stress. Curr Drug Metab. 2002;3:225–32. doi: 10.2174/1389200024605091. [DOI] [PubMed] [Google Scholar]
- 24.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs D, Jaeger M, Widner B, Wirleitner B, Artner-Dworzak E, Leblhuber F. Is hyperhomocysteinemia due to the oxidative depletion of folate rather than to insufficient dietary intake? Clin Chem Lab Med. 2001;39:691–4. doi: 10.1515/CCLM.2001.113. [DOI] [PubMed] [Google Scholar]