Abstract
Reconstitution of functional CD4+ T cell responsiveness to in vitro stimuli is associated with continuous highly active antiretroviral therapy (HAART). Thirty-six antiretroviral naive patients received HAART over 16 weeks. Antigen-specific, mitogen and interleukin (IL)-2 induced lymphocyte proliferative responses and specific IL-2 and IL-4 production were assessed at each time-point, together with quantification of HIV-1 RNA load and lymphocyte populations. Reconstitution of recall responses was limited largely to persistent antigens such as Herpes simplex virus and Candida, rather than to HIV-1 or neo-antigens. Recall antigens, mitogens and IL-2-induced renewed responses were associated with in-vitro production of IL-2, but not IL-4. Differential responsiveness to low versus high concentration IL-2 stimulus increases in a stepwise manner, suggesting normalization of IL-2 receptor expression and improved functionality. These increases in in-vitro proliferative responses thus probably reflect short lived effector clones, driven by ongoing antigenic stimulus associated with persisting long-term organisms. In this context non-responsiveness to HIV-1 antigens suggests ongoing HIV-1 specific clonal T cell anergy.
Keywords: interleukin-2, interleukin-4, highly active antiretroviral therapy (HAART), lymphocyte proliferation, T cell anergy
INTRODUCTION
Memory CD4+ helper T lymphocyte (HTL) responses to recall antigens such as tuberculin, tetanus toxoid and candida albicans are detected readily in HIV-1 infected individuals throughout the asymptomatic disease stage [1]. Depletion of the CD4+ T cell population to below 300 cells/µl of blood results in diminution of these responses, such that they become completely undetectable [2].
In contrast HIV-1 specific HTL responses are lost early, when the CD4+ T cell population is quantitatively intact [3]. Activation of naive HIV-1 specific HTL following initial infection and antigenic exposure may make these T cell clones primary targets for HIV-1 infection and subsequent lysis [4]. Deletion of HIV-1 specific clones through such a mechanism could explain the loss of these responses early in infection. However observations of non-proliferative HIV-1-specific HTL throughout infection suggest this is not the case [5,6]. Alternatively, clonal anergy may result from presentation of HIV-1 peptides to HTL by antigen-presenting cells (APC), in the context of inappropriate costimulation [7] or with immunosuppressive cytokine signals.
In addition to immunosuppressive/antiproliferative regulation of immune responses by type 2 HTL (TH2) and inflammatory/pro-proliferative regulation by TH1 cells, these two HTL phenotypes enable either regulation of humoral responses against extracellular pathogens (TH2) or of CD8+ cytotoxic T lymphocytes (CTL) against intracellular pathogens (TH1) [8]. CTL are the primary means for sterilization of viral infection [9]. A large body of evidence supports the role of CTL in controlling HIV and other immunodeficiency lentiviruses [10–17].
As a result of the competitive nature of TH1 and TH2 populations a chronic immune response can polarize to a type 1 or type 2 profile. During HIV-1 infection progressive loss of TH1 occurs, such that a TH2 skewed population predominates [18]. This shift in immune phenotype may play a substantial role in the immune system's loss of control of HIV-1 infection, facilitating clonal anergy of HIV-1 specific HTL and providing inadequate help for HIV-1 specific CTL. Such a deficit in the immune response appears to lead to an immature effector state of CTL [19], permitting ongoing viral activity [20].
HIV-1 specific HTL are the principle immune correlate of long-term control of vireamia and disease progression [3,21]. These responses are vigorous in long-term non-progressors (LTNP) [21,22] characterized by normal CD4+ T cell counts, low HIV-1 RNA load and clinical non-progression [23,24].
Highly active antiretroviral therapy (HAART) is successful in clearing free virus and reversing symptomology for the majority of treated individuals [25,26]. Improvement of some immunological parameters have been described [27] involving biphasic reconstitution of the peripheral CD4+ T cell population [28]. Restoration of in vitro functional HTL responses to anti-CD3 antibody stimulation as well as recall antigens such as cytomegalovirus (CMV), tuberculin and candida, has been reported over varying time courses [27–31]. However HIV-1-specific HTL responses remain negligible for chronically infected patients receiving HAART. The relationship between these renewed recall responses, their responsiveness to interleukin (IL)-2 and their type-1/type-2 profile requires clarification.
Here we report functional immunological findings of the effects of HAART in 36 patients treated for 4 months. Patients were assessed for mitogen and interleukin-2 responsiveness, lymphocyte proliferative responses (LPR) to an extensive range of antigens and assessment of IL-2 and IL-4 production.
MATERIALS AND METHODS
Study design
Thirty-six ART naive, chronically infected HIV-1-infected individuals were enrolled with informed consent and ethics committee approval. After a 6-week analysis of baseline characteristics patients initiated HAART (at least one protease inhibitor or non-nucleoside reverse transcriptase inhibitor and two nucleoside analogues) at week 0 of the study. Blood samples were obtained at − 6, − 3, 0, 1, 2, 4, 8, 12 and 16 weeks from initiation of HAART for plasma HIV-1 RNA measurement, lymphocyte subset analysis and functional CD4+ T cell assays.
Separation of whole blood
Whole blood was collected into lithium heparin tubes (Becton Dickinson, Oxford, UK) for cell culture assays and into EDTA tubes (Becton Dickinson) for viral loads and flow cytometry. Plasma was removed after centrifugation. Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation and cultured in RPMI-1640 medium containing NaCO3, (Sigma Immunochemicals, Poole, Dorset, UK) with 100 IU/ml penicillin, 100 µg/ml streptomycin and 2 mm l-glutamine (Sigma) supplemented with 10% human AB plasma (Sigma).
Viral load
Plasma viral load was measured using the Versant HIV-1 RNA 3·0 branched DNA assay (Bayer PLC, Newbury, UK) with a lower limit of detection of 50 HIV-1 RNA copies/ml.
Lymphocyte subsets
Whole blood lymphocyte quantification was determined using murine antihuman monoclonal antibodies (MoAb) to CD3, CD4, CD8, CD56, CD19 and the pan-lymphocyte marker CD45 (Tetra One, Beckman Coulter, High Wycombe, UK) and were evaluated on an Epics XL-MCL (Beckman Coulter) flow cytometer.
Lymphocyte proliferative assays
LPR were carried out as described previously [32,33]. Results are presented as both counts per minute (cpm) and stimulation index (SI). Stimulation index was calculated as the experimental cpm/background cpm at each time-point. Correction for CD4+ T cell count was not made as there was no correlation between renewed LPR and changes in CD4+ T cell count (data not shown). A positive LPR is defined as an SI of 5 or greater and a cpm value of at least 2000.
Measurement of cytokine production
Cytokine bioassays were carried out as described previously [32,34] using IL-2 dependent (CTLL-2) and IL-4 dependent (CT.h4S) cell lines.
Phenotypic analysis of lymphocytes
Cryopreserved PMBC were thawed and incubated with a panel of murine antihuman MoAbs (all Beckman Coulter), for 30 min at 4°C. Directly conjugated antibodies used were: fluorescein isothiocyanate (FITC)-CD8; phycoerytherin (PE)-CD25 and CD122; and PE-cyanine (PC-5)-CD4, all used according to the manufacturer's instructions. Cells were washed and fixed in phosphate buffered saline (PBS) containing 2% paraformaldehyde (Sigma). On acquisition, a gate was set around the lymphocyte population on a forward scatter versus side-scatter dot plot, and 10 000 gated events collected for each sample. Data analysis was performed using CELLQuestTM Software (Becton Dickinson, Oxford, UK). Appropriate isotype matched controls were run in parallel for each sample.
Statistical analysis
Lymphocyte subset, viral load and LPR data were non-parametrically distributed. The Wilcoxon signed rank test was performed with a confidence interval of 99% on changes in lymphocytes subsets, HIV-1 RNA load and LPRs between weeks 0 and 16.
RESULTS
Effects of HAART on HIV-1 RNA load and lymphocyte subsets
Week 0 absolute CD4+ T cell counts and plasma HIV-1 RNA loads are described in Table 1, together with choice of HAART regimen per patient and baseline LPRs. No correlation was apparent between the ability to mount LPRs and baseline viral load or CD4+ T cell count. Mean HIV-1 RNA load at week 0 was 171 742 HIV-1 RNA copies/ml plasma (range 10 133–779 254). After 16 weeks of HAART plasma viraemia was suppressed to below the limit of detection (50 copies/ml) for the majority of patients P = <0·01 (Fig. 1a). Four patients did not achieve undetectable viral loads by week 16. HIV-1 RNA levels at week 16 for patients 5, 12, 14 and 21 were 559, 740, 127 and 62 copies/ml, respectively. In addition, two patients experienced viral rebounds at week 16. Patient 11 discontinued HAART at week 12 due to toxicity and experienced a rebound in HIV-1 RNA to 2091 copies/ml having not achieved an undetectable viral load beforehand. Patient 30 achieved successful suppression of viral load to below the limit of detection by week 8, although at week 16 HIV-1 RNA had risen to 47 161 copies/ml as a result of discontinuation of therapy at week 12. Week 16 data from patients 11 and 30 have not been included in the analysis.
Table 1.
Baseline values for all 36 patients studied showing baseline characteristics. Values attributed to magnitude of LPR are representative of the scale of results in cpm for each antigen where –= cpm <200, +/– = cpm 200–2000 or >2000 and SI < 5, + = cpm 2000–10 000 and SI = >5, + + = cpm 10 000–20 000 and + + + = cpm > 20 000. The results shown here represent the highest proliferative response to any stimuli in the different categories of antigens/mitogens shown below at any of the three pre-HAART time-points
| Week 0 surrogate markers | Pre-HAART lymphocyte proliferative responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Plasma viral load | Absolute CD4 count | HIV | Recall | Mitogen | IL-2 (20 U/ml) | IL-2 (100 U/ml) | HAART regimen |
| 1 | 59 097 | 341 | – | – | – | – | – | AZT/3TC/IDV |
| 2 | 24 590 | 257 | – | – | – | – | – | d4T/3TC/NVF |
| 3 | 194 215 | 76 | – | + + + | + + + | – | – | d4T/ddI/SQV/RTV |
| 4 | 21 382 | 474 | +/– | + + + | + + + | + + + | + + + | d4T/3TC/NVF |
| 5 | 779 254 | 89 | +/– | +/– | + | – | +/– | d4T/ddI/NVF |
| 6 | 138 244 | 397 | +/– | + + | + + + | + + + | + + + | d4T/ddI/NVF |
| 7 | 141 169 | 278 | +/– | + + | + + + | + | + + | AZT/3TC/EFV |
| 8 | 39 917 | 551 | – | – | – | – | – | d4T/ddI/NVF |
| 9 | 84 603 | 298 | – | – | + – | – | – | d4T/ddI/NVF |
| 10 | 27 248 | 415 | – | – | – | – | + + | d4T/3TC/NVF |
| 11 | 174 786 | 381 | – | – | – | – | +/– | d4T/ddI/NVF |
| 12 | 514 975 | 13 | – | – | – | – | +/– | d4T/ddI/NVF |
| 13 | 331 292 | 486 | – | – | + + | + + + | + + | d4T/3TC/SQV/RTV |
| 14 | 182 718 | 420 | – | – | – | – | + + + | d4T/ddI/SQVSG |
| 15 | 22 706 | 471 | – | – | – | – | + + | d4T/3TC/SQVSG |
| 16 | 144 979 | 358 | – | – | + | – | + + + | d4T/3TC/IND/RTV |
| 17 | 64 004 | 209 | – | +/– | – | – | + + + | d4T/3TC/NVF |
| 18 | 10 133 | 233 | +/– | + + | + + + | + + + | + + | d4T/3TC/SQVSG |
| 19 | 202 760 | 179 | +/– | +/– | + + + | – | + + | d4T/3TC/RTV/SQV |
| 20 | 2 157 | 542 | – | + + | + + + | + | + + + | d4T/3TC/EFV |
| 21 | 228 941 | 284 | – | – | +/– | – | +/– | d4T/3TC/EFV |
| 22 | 115 439 | 337 | +/– | + + | + + + | + + + | + + + | AZT/3TC/EFV |
| 23 | 165 378 | 333 | +/– | + + + | + + + | + + | + + + | d4T/3TC/EFV |
| 24 | 76 430 | 279 | + + | + + | + + + | + + | + + + | d4T/3TC/EFV |
| 25 | 475 018 | 290 | + | + + | + + + | – | – | d4T/3TC/EFV |
| 26 | 500 000 | 8 | – | + + | + + | +/– | + + | d4T/3TC/EFV |
| 27 | 71 707 | 83 | +/– | +/– | + + | + + | + + | d4T/3TC/EFV |
| 28 | 109 235 | 127 | +/– | +/– | + + | +/– | + + | AZT/3TC/EFV |
| 29 | 17 878 | 230 | + + | + + | + + + | + + + | + + + | AZT/3TC/EFV |
| 30 | 45 632 | 252 | +/– | + + | + + + | + + + | + + + | AZT/3TC/EFV |
| 31 | 122 159 | 412 | +/– | + | + + | + | + + | AZT/3TC/EFV |
| 32 | 500 000 | 91 | +/– | +/– | + + | – | – | AZT/3TC/NVP |
| 33 | 12 280 | 272 | +/– | + + | + + + | + | + + | d4T/3TC/EFV |
| 34 | 62 473 | 175 | +/– | + + | + + + | + + | + + | AZT/3TC/EFV |
| 35 | 134 818 | 397 | +/– | + + | + + + | + + | + + | AZT/3TC/EFV |
| 36 | 92 795 | 239 | +/– | + | + + | + + + | + + + | AZT/3TC/NVP |
AZT, zidovudine; 3TC, lamivudine; d4T, stavudine; ddI, didanosine; IDV, indinavir; NVF, nelfinavir; SQV, saquinavir; RTV, ritonavir; SQVSG, soft gel saquinavir; EFV, efavirenz; NVP, nevirapine.
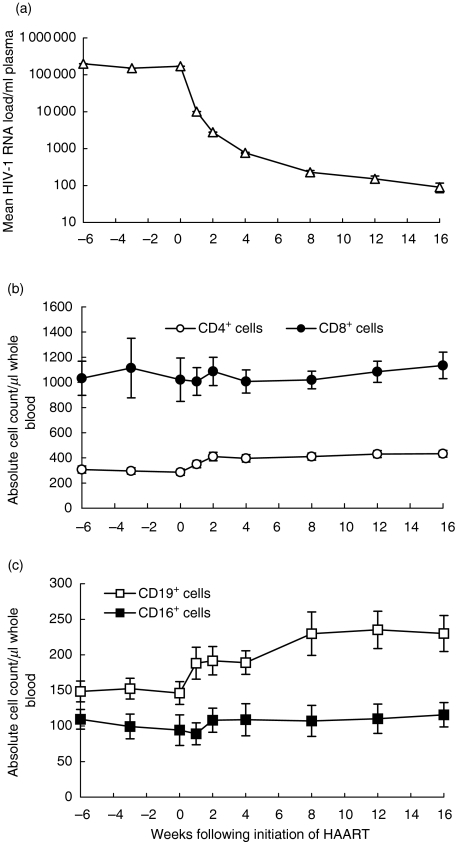
Fig. 1.
(a) Change in mean HIV-1 RNA load, (b) mean absolute CD4+ and CD8+ T cell counts and (c) mean CD19+ and CD56+ absolute cell counts for 36 patients undergoing initiation of HAART over 16 weeks of follow-up (not including patients 11 and 30 at week 16) with standard errors.
Mean CD4+ T cell counts rose significantly from 286 cells/µl of whole blood at week 0 (range 13–551) to 433 cells/µl at week 16 (range 165–742), P < 0·01 (Fig. 1b). A biphasic pattern occurs with an initial rapid increase over the first 2 weeks, followed by a slow gradual increase, confirming previous findings [28]. Over the first 16 weeks of HAART a non-significant increase in the absolute CD8+ T cell count was observed (Fig. 1b).
During this first phase of lymphocyte repopulation in the periphery following initiation of HAART, at which time the largest increase in CD4+ T cells occurred, there was also an increase in the absolute CD19+ B-cell count, P < 0·001 (Fig. 1c). Mean absolute CD56+ natural killer (NK) cells also increased gradually but failed to become significant, P = 0·183 (Fig. 1c).
Integrity of lymphocyte proliferative responses at baseline for HAART
Analysis of antigen specific LPR reveals that before HAART only 50% of this cohort demonstrated a positive response to recall stimuli (Table 1). IL-2 responsiveness at this time was dependent on the concentration of IL-2 used, as high concentration (100 U/ml) IL-2 induced cell proliferation in 69% of these individuals, while low concentration (20 U/ml) induced responses in only 47%. The mitogens phytohaemagglutinin (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM) induced responses in only 69% of patients at baseline (Table 1). Only three patients, 24, 25 and 29, demonstrated a baseline response to one or more of the HIV-1 antigens tested: gp120; p24; or nef. Patient 25 responded weakly to nef, patient 24 responded strongly to p24 with an SI of 41 (9157 cpm) and moderately well to gp120, and patient 29 responded strongly to gp120 with an SI of 187 (8799 cpm) and moderately to p24.
Lymphocyte proliferative responses following initiation of HAART
Functional T cell analysis revealed dramatic changes in responsiveness to recall (not HIV-1) and mitogenic stimuli following initiation of HAART. Considerable fluctuations in LPR that are difficult to explain are apparent in many individuals, particularly in cases where patients were able to demonstrate positive recall specific LPR at baseline (Table 1). However, for those patients who did not demonstrate such positive LPR before HAART (n = 18, 50%) responses increased substantially after initiation of HAART. Two patients, 3 and 8 (6%), were an exception, failing to generate recall or mitogenic responses at any time-point. In addition patients 21, 27, 32 and 35 (11%) demonstrated responses almost exclusively to mitogen after initiation of HAART.
Among those patients that developed new recall antigen specific LPR following initiation of HAART, a further pattern is evident. Fourteen patients (38% of all patients) clearly showed an ability to respond to high concentration IL-2 for several time-points before renewed responses to recall antigens became apparent. In nine of these 14 patients [64% of the ‘baseline non-responsive’ subgroup (these patients are non-responsive to recall antigens at baseline for HAART), 25% of the whole cohort] such responses to high concentration IL-2 were not evident before initiation of HAART. Therefore, in 25% of all patients a renewed response to high concentration IL-2 appears to be a prerequisite to renewed recall responses.
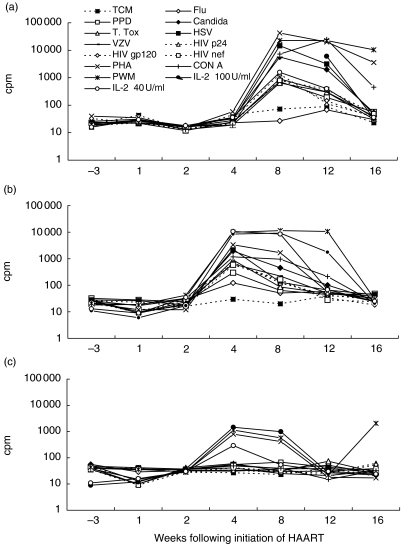
Detailed kinetics of lymphocyte proliferation, IL-2 production and IL-4 production for a representative of the baseline non-responder subgroup
The LPR, IL-2 production and IL-4 production of patient 1 are described in detail (Fig. 2) as a representative of the majority of ‘baseline non-responsive’ patients. CPM are presented in order to illustrate the unmanipulated data and background levels (cells alone in TCM). This individual showed a complete lack of responsiveness to in vitro stimuli before HAART (Fig. 2a). From weeks 8–12 responses to Herpes simplex virus (HSV) and candida became prominent. This feature is characteristic of the majority of ‘baseline non-responsive’ patients. These responses appeared to diminish by week 16. Such loss of responses by week 16 was evident in 11 of the 36 patients (31%) (data not shown). Patient 1's responses to mitogens rose and persisted from week 8 to week 16.
Fig. 2.
(a) Lymphocyte proliferative responses, (b) soluble bioavailable IL-2 production (c) and soluble bioavailable IL-4 production are shown for patient 1 in cpm, as a representative of the ‘baseline non-responsive’ patients, following initiation of HAART.
IL-2 production, detected in the supernatants of these proliferation assays by antigen, mitogen or IL-2 stimulation first became apparent at week 4 for patient 1 (Fig. 2b), before proliferative responses which arose at week 8 (Fig. 2a). By week 12, IL-2 was detected only in the supernatants of PBMCs stimulated with PWM; at week 16 there was no IL-2 detectable in supernatants, while LPR to all three mitogens were robust at this time-point.
Detection of IL-4 from proliferation assay supernatants remained low at all time-points. Where IL-4 production was detected, it was weak, as is shown for patient 1 in response to IL-2 or mitogens at week 4 and week 8 (Fig. 2c).
Improvement in lymphocyte proliferative responses following initiation of HAART is most apparent in a ‘new responder’ subgroup
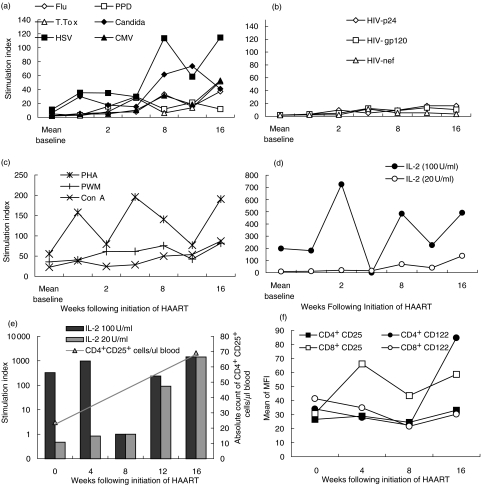
LPR for the ‘new responder’ subset of patients are summarized as mean SI, for recall and HIV antigenic, mitogenic and IL-2 stimuli in Fig. 3. The patients we defined as ‘new responders’ were 18 individuals who were non-responsive to recall and HIV-1 antigen at all three baseline time-points, and a further five patients who demonstrated positive LPR at baseline, but whose responses increased following initiation of HAART. As described for patient 1, LPR to HSV and candida were prominent following initiation of HAART in the new responders (Fig. 3a). The mean SI to HSV at week 16 increased significantly to 120 from 6 at baseline (P = 0·001). For candida the mean SI at week 16 was 43, compared with 6 at baseline (P = 0·001). The mean SI response to CMV (Cytomegalovirus) at week 16 was 51, compared with a baseline of 3·4 (P = 0·003). In contrast, SI responses to purified protein derivative (PPD), influenza and tetanus toxoid did not rise above 39, which was for influenza, compared with 2·1 at baseline (P = 0·059). Responses to these three antigens remained persistently weak for the majority of these patients.
Fig. 3.
Mean lymphocyte proliferative responses to recall antigens (a), HIV-1 antigens (b), mitogens (c) and high (100 U/ml) and low (20 U/ml) concentration IL-2 (d) are shown as SI for the ‘new-responder’ patient subset following initiation of HAART (not including patients 11 and 30 at week 16). Responsiveness to high concentration (100 U/ml) and low concentration (20 U/ml) IL-2 are shown (e) as SI together with the absolute number of CD4+CD25+ T cells/µl whole blood for patient 19 as a representative of patients who generate an equivalent response to low and high concentration IL-2 following initiation of HAART. The mean CD25 and CD122 mean fluorescence intensity (MFI) on CD4+ and CD8+ T cells are shown (f) for a subset of 11 of the 18 patients who developed equivalent responses to low and high concentration IL-2 by week 16.
LPR to HIV-1 gag-p24, env-gp120 and nef (Fig. 3b) also remained persistently weak for the new responders, as for baseline responders. The mean gag-p24 SI at week 16 for the new responders was 17. However, this was an eightfold increase over the mean gag-p24 response at baseline and was statistically significant (P = 0·002). Gp120 and nef responses were not significant.
Responses to mitogenic stimuli (Fig. 3c) were stronger for the majority of patients. PHA induced LPR rose to a peak at week 4, fell back to baseline values at week 12 and rose at week 16. This was not statistically significant. There was a less aggressive and non-significant increase in the mean Con A and mean PWM responses over the 16 weeks.
High concentration IL-2 induced robust LPR in nearly all individuals following initiation of HAART (Fig. 3d). As many as 25 patients made baseline responses to high concentration IL-2. Because these responses were ubiquitous they were not included as a definition of baseline ‘non-responsiveness’. Thus the subset of 18 baseline non-responders, plus five baseline partial responders, included patients who responded to high concentration IL-2. For this patient subset there was a substantial increase in mean SI from 208 (median SI = 78) at baseline to 510 at week 16 (median = 98). These responses peaked at week 2, mean SI = 725 (median = 159) (Fig. 3d), but were not significant.
Responses to low concentration IL-2 also increased, although to a lesser extent. The baseline mean SI of 9·4 (median SI = 0·8) increased to 144 (median SI = 4·7) at week 16. All 23 ‘new-responders’ demonstrated increased LPR to low concentration IL-2 by week 16 (SI range 7·6–1432). However, 18 patients (50% of the whole group) developed an equivalent response to high and low concentration IL-2 subsequent to initiation of HAART, none of which did so at baseline. These responses were often transient and were not significant. Cryo-preserved PBMCs from a subset of these patients (n = 11) who had stored cells available were analysed subsequently by flow cytometry for expression of CD25 (IL-2 receptor α chain) and CD122 (IL-2 receptor β chain) on CD4+ and CD8+ T lymphocytes. The absolute numbers of CD4+CD25+ T cells/µl whole blood were calculated from the percentage cells stained as a function of absolute CD3 T cell count. Lymphocyte proliferative responsiveness to high and low concentration IL-2 are shown together with the absolute count of CD4+CD25+ T cells at weeks 0 and 16 for patient 19 as a representative of these patients (Fig. 3e). In some cases the numbers of CD4+CD25+ T cells increased, but counts remained very low. The mean absolute number of CD4+CD25+ T cells at week 0 for these patients was 15 cells/µl and at week 16 was 24 cells/µl. No change was seen for the absolute count of CD4+CD122+ T cells. However, in many cases the mean fluorescence intensity of both CD25 and CD122 expression changed substantially, suggesting up-regulation of expression of these components of the IL-2 receptor on CD4+ T cells, and in the case of CD8+ T cells decreased expression of the β chain (Fig. 3f). This subset of patient's mean CD25 MFI on CD4+ T cells increased by 24·4% from 26·6% to 33·1% between weeks 0 and 16. The mean CD122 MFI on CD4+ T cells increased by 149% from 34% to 84·7% between weeks 0 and 16. On CD8+ T cells the mean CD25 MFI increased by 91·3% from 30·7% to 58·7% between weeks 0 and 16. However, the mean expression of CD122 on CD8+ T cells for this subset of patients decreased by 26·6% from 41·4% to 30·4%. While the numbers here are not sufficient for statistical analysis, the increase in intensity of CD25 expression coupled with CD122 on CD4+ T cells may explain the increased IL-2 responsiveness observed in proliferation assays.
DISCUSSION
Absence of LPR to recall and HIV-1 antigens and to mitogens before initiation of HAART is consistent with data published by other groups [1,2]. Recovery of these responses after initiation of HAART has also been described [27,29–31]. We find that where LPR to recall antigens do not exist prior to HAART there is a consistent rise in these responses following administration of therapy. While increases in response to HIV-1 antigens are seen, their weakness, in an environment of increasing functional immunocompetence, may result from lack of HIV-1 antigenic stimulus, due to HAART.
Recall antigen responses that become positive are largely directed to HSV, candida and CMV, but not to influenza, PPD and tetanus antigens, which induced weaker responses. It is important to note that these antigens are derived from organisms that present clinically in immunocompromised patients. In contrast, antigens that induced weak responses in the majority of patients are derived from organisms to which exposure is likely to be short-lived, as at time of vaccination (PPD and tetanus), or infrequently (influenza). Furthermore, HSV and CMV are both permanent infections, while Candida albicans persists in immunocompromised patients. There have been hints at this disparity between responsiveness to ‘persistent’ and ‘transient’ or ‘neo’ antigens in other studies [30]. Ongoing antigenic presentation to T lymphocytes from persistent organisms may explain the magnitude of the LPR we observed for these antigens compared to those for antigens of transient organisms (Table 2). HIV-1 is also a persistent organism. Table 2 demonstrates the disparity between LPR to HIV-1 and recall antigens in the ‘persistent antigen’ group, both before and after initiation of HAART, even though there is some improvement in responses to HIV-1 over time. The absence of renewed memory responses to ‘transient’ antigens, such as tetanus and influenza and to HIV-1, may suggest either T cell anergy in some clonal populations or simply very low clone numbers, as suggested by perturbations in the T cell repertoire described by Connors et al. [34]. The profile of cytokine secretion and responsiveness by T cells in these assays favours the former explanation, or at least a combination of the two.
Table 2.
Comparison of proliferative responses to different categories of recall antigens before and after initiation of HAART. Each patient was assessed for proliferative responses following initiation of HAART at the single timepoint at which their responses peaked. Ten patients peaked at week 16. Six patients peaked at week 12. Six patients peaked at week 8. Seven patients peaked at week 4. No patients peaked at week 2 and 1 patient peaked at week 1. Four patients failed to demonstrate any positive responses to antigenic stimulus following initiation of HAART
| Number of patients responding | ||
|---|---|---|
| At baseline (%) | Following initiation of HAART (%) | |
| Transient antigens | ||
| PPD | 8 (22) | 17 (47) |
| FLU | 7 (19) | 17 (47) |
| Tetanus | 11 (31) | 15 (42) |
| Persistent antigens | ||
| HSV | 17 (47) | 27 (75) |
| CMV | 10 (28)* | 21 (60)* |
| Candida | 11 (31) | 24 (67) |
| HIV-1 p24 | 2 (6) | 10 (27) |
| HIV-1 gp120 | 2 (6) | 11 (31) |
| HIV-1 nef | 2 (6) | 19 (7) |
Patient 1 was not assessed for CMV responses, thus the sample size for CMV was 35 patients.
Measurements of IL-2 and IL-4 production demonstrate near parallel kinetics of the production of bioavailable IL-2 with LPR. Detection of IL-2 production following initiation of HAART complements previous work by our group utilizing a subset of these patients, where a shift in production of cytokines from an IL-4/IL-10 secreting phenotype to an IL-2/interferon (IFN)-γ secreting phenotype is demonstrated by reverse transcriptase-PCR [35].
We observe that in many untreated patients where LPR are impaired, proliferation can be induced with high concentration IL-2 (Table 1). Because IL-2 has been shown to reverse the anergic phenotype [36] we suggest that lack of recall or HIV-1 responses is not a result of low numbers of specific T cell clones but of functional impairment. Induction of renewed HIV-1 specific responses can even be achieved in late stage HIV-1 disease by cytokine therapy alone [32]. Additionally peripheral CD4+ T cells are capable of producing IFN-γ and TNF-α in response to HIV-1 antigen stimulation, but are incapable of proliferating [6].
Prior to HAART, lack of IL-2 secretion is associated with lack of proliferation to low concentration IL-2, suggesting a relationship between inability to produce IL-2 and dysfunctionality of the IL-2R. The appearance of LPR to recall antigens within weeks of initiation of HAART, observed in the majority of these patients, is likely to be an effect of reversed T cell anergy and at least partial restoration of normal IL-2 receptor expression and/or function. Renewed responses appear to diminish in 11 (31%) of these patients, as shown in Fig. 2. Pontesilli et al. report similar loses of renewed responses in those receiving HAART, although over a longer time period [30]. Observations of increased LPR to tuberculin, a transient antigen, and CMV after 1 year of therapy by Li et al. [29], suggests permanent reconstitution of ‘central memory’ T cell cones. We believe that renewed responses specific to persistent antigens within the first 16 weeks of HAART, represent activated effector recall responses rather than resting ‘central memory’ recall responses. The subsequent loss of responses observed in 31% of our patients may reflect clearance of effector responses following resolution of clinical or subclinical activity of these persistent organisms. Such effector cell populations are short-lived, with a half-life of days [37–39].
In summary, we suggest that following initiation of HAART activated effector HTL are released from lymphoid organs due to loss of viral trapping mechanisms. These cells are at least partially functional and able to secrete pro-proliferative cytokines, such as IL-2, in an increasingly immunocompetent environment. Control of clinical or subclinical herpetic and/or fungal manifestations as a result of increasing immunocompetence within days or weeks of HAART down-regulates antigen driven expansion of activated HTL, resulting in their clearance and loss of these responses. De novo HTL regeneration enables later recovery of ‘central memory’ recall responses, detectable after 1 year of treatment as described previously [29].
These results support the consideration that HAART is insufficient to enable complete immunological reconstitution, particularly of HIV-1 specific responses. Lack of evidence of HIV-1 specific HTL in the periphery after initiation of HAART suggests ongoing anergy of these clones. Strategies for boosting these important responses may increase potency and efficacy of HAART.
Acknowledgments
The authors wish to thank the Wellcome Trust (grant numbers 050020 and 058700) and St Stephen's AIDS Trust for funding. HIV-1 recombinant proteins were provided by the EU Programme EVA/MRC Centralized Facility for AIDS Reagents, NIBSC, United Kingdom (grants QLK-CT 1999–00609 and GP828102). In addition we thank the routine clinical laboratory staff at Chelsea and Westminster Hospital, Department of Immunology. Finally, we wish to thank nursing staff and most importantly the patients at the St Stephen's Clinic.
REFERENCES
- 1.Ballett JJ, Couderc L-J, Rabian-Herzog C, et al. Impaired T lymphocyte dependent immune responses to microbial antigens in patients with HIV-1 associated persistent generalised lymphadenopathy. AIDS. 1988;2:291–7. doi: 10.1097/00002030-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV, Fahey JL, Smith DC, et al. Early effects of HIV on CD4+ lymphocytes in vivo. J Immunol. 1987;138:3725–30. [PubMed] [Google Scholar]
- 3.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viraemia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 4.Schnittman SM, Lane CH, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T cell functional defects observed in infected individuals. Proc Natl Acad Sci. 1990;87:6058–62. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:483–4. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 6.Wilson JD, Imami N, Watkins A, et al. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J Infect Dis. 2000;182:792–8. doi: 10.1086/315764. [DOI] [PubMed] [Google Scholar]
- 7.Meyaard L, Schuitemaker H, Miedema F. T cell dysfunction in HIV infection: anergy due to defective antigen-presenting cell function? Immunol Today. 1993;14:161–4. doi: 10.1016/0167-5699(93)90279-T. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 9.Romagnami S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 10.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T cell responses during chronic viral infections. J Virol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T cell immunity to influenza. N Engl J Med. 1983;309:13. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 12.Klein MR, van Baalen CA, Holwerda AM, et al. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long term asymptomatics. J Exp Med. 1995;8:903–7. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland Jones SL, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 14.Gallimore A, Cranage M, Cook N, et al. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;11:1167–73. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 15.Kent S, Hu S-L, Corey L, Morton W, Greenburg P. Detection of simian immunodeficiency virus (SIV)-specific CD8+ T cells in macaques protected from SIV challenge by prior SIV subunit vaccination. J Virol. 1996;70:4941–7. doi: 10.1128/jvi.70.8.4941-4947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor D, Friedrich T, Hughes A, Allen TM, Watkins D. Understanding cytotoxic T lymphocyte escape during simian immunodeficiency virus infection. Immunol Rev. 2001;183:115–26. doi: 10.1034/j.1600-065x.2001.1830110.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8:493–9. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 18.Clerici M, Shearer GM. A Th1 to Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–10. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 19.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type-1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different disease stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein–Barr virus in late disease. J Exp Med. 1993;177:249. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotch F, Hardy G, Imami N. Therapeutic vaccines in HIV-1 infection. Immunol Rev. 1999;170:173–82. doi: 10.1111/j.1600-065x.1999.tb01337.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg ES, Walker BD. HIV type-1 specific helper-T cells: a critical host defence. AIDS Res Hum Ret. 1998;14:S143–7. [PubMed] [Google Scholar]
- 23.Buchbinder SP, Katz MH, Hessol NA, O'Malley PM, Holmberg SD. Long-term HIV infection without immunological progression. AIDS. 1994;8:1123–8. doi: 10.1097/00002030-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Easterbrook PJ, Schrager LK. Long-term non progression in HIV infection. Methodological issues and scientific priorities. AIDS Res Hum Ret. 1998;14:1211–28. doi: 10.1089/aid.1998.14.1211. Workshop summary. [DOI] [PubMed] [Google Scholar]
- 25.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 26.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 27.Kelleher AD, Carr A, Zaunders J, Cooper DA. Alterations in the immune response of HIV infected subjects treated with an HIV-specific protease inhibitor, Ritonavir. J Infect Dis. 1996;9:321–9. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 28.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV infection: a composite of redistribution and proliferation. Nature Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 29.Li TS, Tubiana R, Katlama C, et al. Long lasting recovery in CD4 cell function and viral load reduction after highly active anti-retroviral therapy in advance HIV-1 disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 30.Pontesilli O, Kerhof-Garde S, Notermans DW, et al. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 31.Autran B, Carcelain G, Li TS, Blanc C, et al. Positive effects of combined anti-retroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 32.Imami N, Hardy GAD, Nelson MR. Induction of HIV-1-specific T cell responses by administration of cytokines in late-stage patients receiving highly active antiretroviral therapy. Clin Exp Immunol. 1999;118:78–86. doi: 10.1046/j.1365-2249.1999.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imami N, Pires A, Hardy G, Wilson J, Gazzard B, Gotch F. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J Virol. 2002;76:9011–23. doi: 10.1128/JVI.76.18.9011-9023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connors M, Kovacs JA, Krevat S, et al. HIV infection induces changes in the CD4+ T cell phenotype and depletions within the CD4+ T cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–40. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 35.Imami N, Antonopoulos C, Hardy GAD, Gazzard B, Gotch FM. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: Impact of highly active antiretroviral therapy. AIDS Res Hum Ret. 1999;15:1499–508. doi: 10.1089/088922299309784. [DOI] [PubMed] [Google Scholar]
- 36.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–71. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 37.Marrack P, Mitchell T, Bender J, Hildeman D, Kedi R, Teague K, Kappler J. T cell survival. Immunol Rev. 1998;165:279–85. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 38.Akbar AN, Borthwick N, Salmon M, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;178:427–38. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbar AN, Savill J, Gombert W, et al. The specific recognition by macrophages of CD8+, CD45RO+ T cells undergoing apoptosis: a mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994;180:1943–7. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]