Abstract
Aspergillus fumigatus (Afu) is an important fungal pathogen causing allergic and invasive respiratory disorders. A plethora of multi-functional allergens/antigens secreted by Afu have been implicated in pathogenesis. The present study was undertaken to identify and characterize novel Afu allergen/antigen by cDNA library approach. cDNA library of Afu was immunoscreened with pooled sera of allergic bronchopulmonary aspergillosis (ABPA) patients. The cDNA clone, TS1, reacting significantly with specific IgG antibodies, was selected. cDNA was subcloned and expressed in Escherichia coli. Sequencing of the cDNA revealed an open reading frame (ORF) of 1179 bases coding for a protein with an approximate molecular weight of 44 kDa. Immunoreactivity of the recombinant TS1 protein (rTS1) was evaluated by ELISA and Western blot analysis using pooled sera of ABPA patients. The rTS1 exhibited binding to specific IgG and IgE antibodies present in sera of ABPA patients. The deduced amino acid sequence showed homology to 60S ribosomal protein L3 (RpL3) of Aspergillus nidulans, Saccharomyces cerevisiae and Homo sapiens. The RpL3 of S. cerevesiae, tcm1, to which TS1 sequence shows significant homology (72% identity), is known to be responsible for conferring resistance against trichodermin (antibiotic, inhibiting protein synthesis). The present study has led to identification, cloning and expression of a 44-kDa novel allergen/antigen of Afu with sequence homology to L3 ribosomal protein with a probable role in resistance of Afu to antifungal drugs. Sixty-four per cent sequence identity of Afu RpL3 with human RpL3 and common regions in their predicted epitopes suggest a possibility of involvement of Afu RpL3 in autoimmune reactions due to molecular mimicry.
Keywords: ABPA, allergen/antigen, cDNA library, immunoreactivity, pathogenic fungi
INTRODUCTION
Aspergillus fumigatus (Afu), a ubiquitous mould, is an opportunistic pathogen associated with allergic and invasive aspergillosis [1]. This pathogenic fungus secretes several virulence factors which facilitate crossing of epithelial barriers, leading to disease development [2]. A number of multi-functional allergens/antigens secreted by Afu also contribute to pathogenicity [3].
Elevated serum levels of IgG and IgE antibodies against allergens/antigens of Afu are used as an important diagnostic criteria in Afu-induced allergic disorders [4]. A major problem in the diagnosis of Afu-induced allergic disorders arises from the lack of standardized diagnostic reagents. Commercial Afu extracts show variable allergenicity owing to the differences in the strain used, growth conditions, harvesting and extraction procedures [5]. Although the extracts of Afu are known to contain approximately 200 different proteins, glycoproteins and low molecular weight compounds, the current update of Afu allergens by the International Union of Immunological Societies (WHO/IUIS, http://www.allergen.org/List.htm) lists only 19 allergens from Afu [6].
Introduction of the molecular biology approaches such as the cDNA library has allowed cloning, characterization and production of large amounts of single and highly pure Afu allergens in a rapid manner [7,8]. Screening of cDNA libraries with patient sera leads to identification of several previously undescribed allergens in virtually a single experiment. Positive clones are sequenced and compared to known sequences in the electronic databank. Identification and characterization of various allergens/antigens of Afu would contribute substantially to understanding of pathogenesis and biology of the fungus. Such studies would also facilitate development of novel effective therapeutic strategies, in view of the serious limitations of the currently available antifungal therapies, such as toxicity and development of drug-resistant strains.
The present study describes the molecular cloning and expression of a novel 44-kDa immunoreactive protein from the Afu cDNA library. The deduced amino acid sequence of the cDNA shows homology with L3 ribosomal protein (RpL3), a component of 60S ribosomal subunit, from different organisms, including H. sapiens. This protein is an important component of peptidyl transferase centre of the ribosome and has also been involved in conferring resistance to protein synthesis inhibiting drugs such as trichodermin in S. cerevisiae.
MATERIALS AND METHODS
Materials
Serum samples of allergic bronchopulmonary aspergillosis (ABPA) patients (n = 30) (following Rosenberg's criteria) and normal subjects (n = 10) were obtained from Vallabhbhai Patel Chest Institute, Delhi as per the guidelines of the institutional human ethics committee [9]. The parameters taken into account for the selection ABPA patients are: asthma, peripheral blood eosinophilia (>1·0 × 109/l), immediate cutaneous reactivity to Afu antigen, precipitating antibodies against Afu antigen, elevated total serum IgE (>1000 ng/ml), chest X-ray infiltrates (or history of), transient or fixed proximal bronchiectasis, elevated serum IgE and IgG antibodies (specific to Afu antigen).
Restriction enzymes and ligase were purchased from New England Biolabs (Beverley, MA, USA). Polymerase chain reaction (PCR) was performed using the thermal cycler from Perkin Elmer Cetus. DNA amplification reagents were purchased from Bangalore Genei (Bangalore, India). GFXTM DNA and gel band purification kit for purification of PCR products was obtained from Amersham Pharmacia Biotech Inc. (Piscataway, NJ, USA). Nitrocellulose membranes were purchased from Schleicher and Schuell (Keene, NH, USA). Peroxidase conjugated antihuman IgG and antihuman IgE antibodies were obtained from Sigma (St Louis, MO, USA).
cDNA library
Afu cDNA library constructed in Uni-ZAP XR lambda vector was obtained from Stratagene (La Jolla, CA, USA). Escherichia coli strains XL-1 Blue MRF′ and SOLR (Stratagene, La Jolla, CA, USA) were used for recombinant DNA manipulations. The cDNA library was amplified using the manufacturer's instructions.
Antibody screening of cDNA library and selection of clone
Immunoscreening of a λZAP cDNA library was performed under standard conditions [10]. Briefly, E. coli XL-1 blue were infected with 6 × 108 phages containing cDNAs and plated onto NZY-agar plates. Expression of fusion protein was induced by overlapping nitrocellulose filters impregnated with 10 mm isopropyl-beta-D-thiogalactopyranoside (IPTG) and followed by incubation of the plates for 4 h at 42°C. The plates were incubated further at 37°C for another 4 h. Filters were washed first with Tris-buffer saline (TBS) (10 mm Tris, pH 8·0, 150 mm NaCl, TBS) with 0·05% v/v Tween 20. They were then blocked with 3% skimmed milk powder in TBS at 37°C for 1 h and were incubated overnight with pooled sera of ABPA patients (n = 10) (preabsorbed against E. coli proteins and diluted 1 : 100 in TBS). Filters were washed in TBS containing 0·05% (v/v) Tween 20 (TBST), and reacted with HRP conjugated antihuman IgG (diluted 1 : 1000 in TBS). Membranes were given a final wash in TBST and were developed with 3,3′ diaminobenzidine tetrahydrochloride and 0·3% v/v hydrogen peroxide. TS1 was one of the positive clones. Selected plaque was rescreened, purified to homogeneity and corresponding pBluescript SK (+/–) phagemid was rescued by in vivo excision using ExAssist helper phage. The E. coli SOLR cells were infected with the phagemid and were plated onto LB-ampicillin (50 µg/ml) agar plates and incubated at 37°C overnight. Colonies appearing on the plate containing the pBluescript double-stranded phagemid having the cloned DNA insert was used for colony PCR using T3 (5′AAT TAA CCC TCA CTA AAG GG-3′) and T7 (5′CGG GAT ATC ACT CAG CAT AAT G-3′) primers. PCR cycling conditions were 94°C/4′ and 28 cycles of 94°C/1′, 58°C/1·5′, 72°C/2′ followed by a terminal extension cycle at 72°C/7′.
Sequence analysis of TS1
The PCR product thus obtained was sequenced using T3, T7 and two internal primers, Int 1 (5′-GAC AGT ATC TTC GAG AAG GAC GA-3′) and Int 2 (5′-CGC AAC CTG TTC GAG AAG CCC ATT-3′). Automated DNA sequencing and oligonucleotide synthesis was performed by sequencing facility at the Institute of Genomics and Integrative Biology, using ABI-3100 DNA sequencer (Applied Biosystems). The deduced amino acid sequence was determined using the Translate Tool software in the Expasy Molecular Biology Server (http://www.expasy.com). The deduced amino acid sequence was submitted to the database of National Center for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (blast) server for nucleotide and amino acid homology searches (http://www.ncbi.nlm.nih.gov/BLAST).
Subcloning, expression and purification of rTS1
The cDNA insert was amplified using the modified forward primer (5′-AGC GAC AAC AGG AAT TCG ATG AGT CAC CGG AAG TAC-3′, carrying an EcoRI site) and T7 as reverse primer. The amplified product was digested with EcoRI and XhoI, and the resulting fragment was inserted into pGEX-5X-3 expression vector, which was digested previously with the same restriction enzymes. The resulting plasmid was designated as pGEX-TS1. E. coli strain BL21 was transformed with plasmid pGEX-TS1 following the standard method [11]. The transformed cells were grown in 2 YT medium containing 100 µg/ml ampicillin at 37°C until the OD600 reached 0·5. IPTG was then added to a final concentration of 0·5 mm, and the culture was grown for an additional 5 h at 37°C with shaking. Cells were harvested by centrifugation at 5000 g for 15 min and resuspended in 20 ml sonication buffer (50 mm Tris/HCl pH 7·4, 150 mm NaCl, 1 mm EDTA, 10% (v/v) glycerol, 1 mm PMSF and 10 µg/ml aprotinin). The cells were sonicated on ice for 2 min, and sonicate was supplemented with Triton X-100 to a final concentration of 1% before centrifugation at 30 000 g for 30 min at 4°C. The supernatant was incubated overnight at 4°C with glutathione–Sepharose 4B matrix (Pharmacia Biotech). The resin bound to protein was packed into a column and washed with five bed volumes of phosphate-buffered saline (PBS) (1·44 g disodium hydrogen phosphate, 0·24 g potassium dihydrogen orthophosphate, 0·2 g potassium chloride, 8·0 g sodium chloride in 1 l distilled water, pH 7·2, PBS). Protein was eluted with 50 mm Tris/HCl pH 8·0 containing 1 mm 1,4-dithiothreitol (DTT), 5 mm MgCl2 and 15 mm glutathione. Fractions containing purified rTS1 protein were pooled and the purity of the recombinant protein was confirmed on 10% SDS-PAGE.
Immunoblotting of recombinant protein with pooled sera of ABPA patients
For immunoblot analysis, rTS1 was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane at constant current of 100 mA for 4 h in Bio-Rad mini Trans-blot cell. The nitrocellulose membrane was blocked with 3% non-fat milk in PBS at 37°C for 1 h followed by washing. The membrane strips were incubated at 4°C overnight with 1 : 100 v/v dilution (for IgG) or 1 : 50 dilution v/v (for IgE) of pooled sera of ABPA patients and pooled sera of controls. After overnight incubation, the strips were washed and treated with peroxidase-conjugated antihuman IgG (1 : 1000 v/v) and antihuman IgE (1 : 1000 v/v) antibodies for 1 h at 37°C. The membrane was washed further and incubated with substrate, 3,3′ diaminobenzidine tetrahydrochloride and 0·3% v/v hydrogen peroxide. The enzyme reaction was stopped by rinsing with deionized water.
Specific IgG and IgE binding of TS1 fusion protein by indirect ELISA
An indirect ELISA [12] was used to screen the pooled sera of ABPA patients and pooled sera of controls (1 : 100 v/v for IgG and 1 : 50 v/v for IgE) for the presence of serum antibodies specific to rTS1.
Immunodot-blot
The allergenicity of rTS1 was determined in vitro by dot-blot immunoassay using a panel of sera from 30 ABPA patients following standard protocol [13]. Briefly, 1 µg of rTS1 fusion protein was applied onto Hybond C nitrocellulose membrane (Amersham Life sciences, UK). Five µg of Afu 3-week culture filtrate (3 wcf) was used as positive control while 1 µg of purified glutathione-S-transferase (GST) was used as a negative control (because culture filtrate is a mixture of number of allergens/antigens, five times more quantity was used than the purified proteins). Dot blots were blocked with 5% non-fat milk powder in PBST (PBS with 0·05% Tween 20). The blots were incubated separately with 1 : 50 (v/v) dilution of sera of 30 ABPA patients for 3 h at 37°C. After three washes with PBST, the blots were incubated with peroxidase conjugated antihuman IgE (1 : 1000 v/v) for 1 h at 37°C. A similar washing procedure was followed and the blots were developed with substrate, 3,3′ diaminobenzidine tetrahydrochloride and 0·3% v/v hydrogen peroxide. All reactions with visible dots (compared to positive control dots) were scored as positive. The enzyme reaction was stopped by rinsing with deionized water.
Identification of epitopic regions
The dnastar module of the Lasergene software package was used for computational analysis of deduced amino acid sequence. Various algorithmic programs were used, such as the Chou–Fasman algorithm (prediction of secondary structure), the Hopp–Woods method (protein antigenic determinants), the Kyte and Doolittle method (regional hydropathy of protein) and Jameson Wolf's method for antigen index and identification of probable epitopic regions [14].
RESULTS
PCR and sequence analysis of the cDNA clone, TS1
Sequencing of the cDNA insert using T3, T7, Int1 and Int2 revealed an ORF of 1179 base pairs coding for 392 amino acids. The cDNA has a complete 3′ untranslated region with a poly A tail. A polyadenylation signal, AATAAA, was present 18 base pairs upstream of the poly A tail. The sequence of TS1 cDNA was submitted to the NCBI GenBank (Accession number: BankIt439468, AF464911).
blastx homology search results showed that the deduced amino acid sequence shares significant homology with L3 ribosomal proteins of different organisms (Fig. 1). It showed 91% identity with Emericella nidulans (A. nidulans), 72% with S. cerevisiae, 71% with Schizosaccharomyces pombe and 64% with H. sapiens.
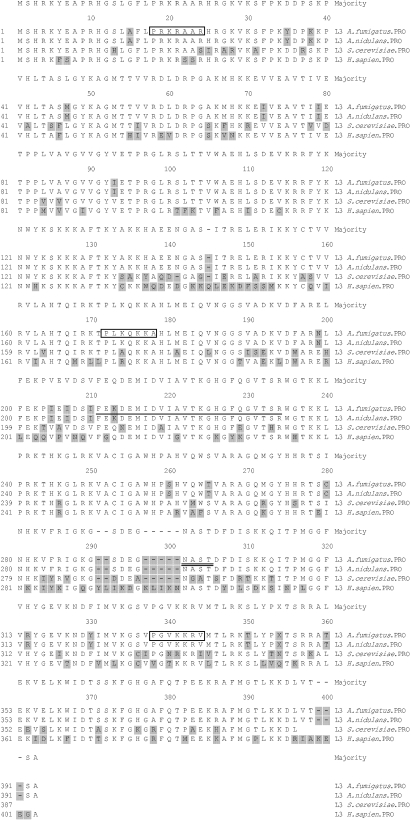
Fig. 1.
Multiple sequence alignment of the amino acid sequences of L3 ribosomal protein from Aspergillus fumigatus, A. nidulans, Saccharomyces cerevisiae and Homo sapiens. The sequences have been aligned with clustal w (dnastar). The amino acid residues that differ from the consensus are shaded. The result indicates a high degree of homology among the various L3 ribosomal proteins from different organisms. The L3 signature is underlined. The boxed amino acid sequences represent the nuclear localization signals. The potential N-glycosylation site is underlined with a double line.
The sequence analysis of TS1 revealed that a ribosomal protein L3 signature ([FL]·{6}[DN]·{[2} [AGS]·[ST]· G[KRH]G·{2}G·{3{R) was present at residue 210–233. Four nuclear localization signals were present in the sequence (PRKR at position 18, PRKRAAR at 18, PLKQKKA at 171, PGVKKRV at 330). In addition to this, one potential glycosylation site at position 294–297 (N[^P][ST][^P] motif) and four potential myristoylation sites at positions 142, 186, 289 and 327 (G[^EDRKHPFYW]·{2}[STAGCN][^P] motif) were also present (Fig. 1).
Characterization of purified rTS1
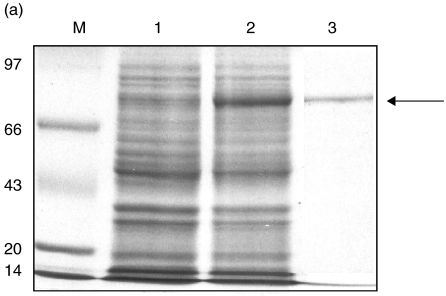
The purified rTS1, expressed as GST fusion protein, resulted in a single band on denaturing 10% SDS-PAGE (Fig. 2a). The predicted size of GST-TS1 was 73 kDa (44 kDa for TS1 protein and 29 kDa for the attached GST protein).
Fig. 2.
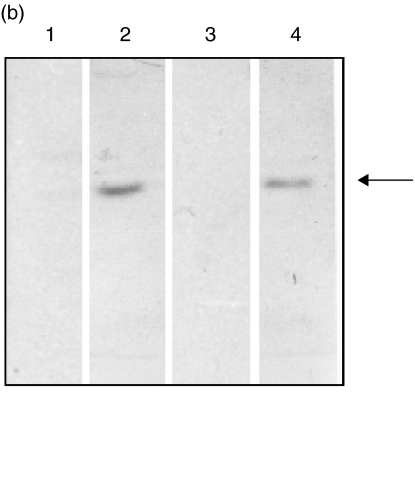
(a) 10% SDS-PAGE stained with Coomassie blue R-250 showing expression and purification of rTS1 fusion protein from a cDNA clone. Lane M, molecular weight marker in kDa; lane 1, uninduced E. coli cell lysate; lane 2, induced Escherichia coli cell lysate (IPTG-induced expression of rTS1 fusion protein from E. coli cells); lane 3, purified rTS1 fusion protein (∼73 kDa). (b) Specific IgG and specific IgE binding of rTS1. The recombinant protein was resolved on 10% SDS-PAGE and electroblotted onto nitrocellulose membrane at 50 mA overnight 4°C. The blots were probed with pooled sera of ABPA patients and pooled sera of controls. Lane 1, IgG/sera of controls (1 : 100); lane 2, IgG/sera of ABPA patients (1 : 100); lane 3, IgE/sera of controls (1 : 50); lane 4, IgE/sera of ABPA patients (1 : 50).
Immunoreactivity of rTS1
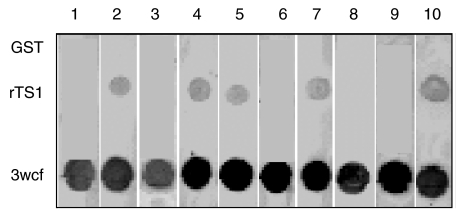
rTS1 showed reactivity with IgE and IgG antibodies in the pooled sera of ABPA patients on Western blot (Fig. 2b). The ELISA absorbance value of rTS1-specific IgG antibodies in pooled sera of ABPA patients was 5 fold higher than the controls (Table 1). The ELISA absorbance value of rTS1-specific IgE antibodies in pooled sera of ABPA patients was 6·4-fold higher than the controls (Table 1). In the dot-blot analysis, seven of 30 ABPA patients (26·7%) showed presence of IgE antibodies against rTS1 (Fig. 3). All the 30 sera showed presence of specific IgE antibodies against 3 wcf of Afu. Because rTS1 was expressed as a fusion protein with GST, purified GST alone was used as a negative control to rule out non-specific reaction of ABPA sera with GST. None of the 30 sera reacted with GST.
Table 1.
The indirect ELISA optical density values of rTS1-specific IgG and IgE antibodies
| Absorbance values | ||
|---|---|---|
| Serum samples used | IgG | IgE |
| Pooled ABPA patients sera | 0·624 ± 0·050 | 0·256 ± 0·021 |
| Normal human sera | 0·124 ± 0·054 | 0·040 ± 0·034 |
All the readings are mean values representing three readings for each sample.
Fig. 3.
Allergenicity of rTS1 by dot-blot immunoassay. Ten representative panels of the IgE dot-blot immunoassay are shown. Purified rTS1 and GST were used at 3 µg/dot and 3 wcf was used at 6 µg/dot.
Prediction of antigenic index and epitopic analysis
The deduced amino acid sequence of TS1 was analysed for the prediction of potential antigenic determinants by using the protean module of lasergene. This analysis predicted several potential antigenic sites and 11 regions were observed to have a high antigenic index (Table 2).
Table 2.
The amino acid sequences of epitope regions of rTS1
| Predicted epitopic regions | ||
|---|---|---|
| Number | Amino acid positions | Amino acid sequence |
| 1 | 1–14 | MSHRKYEAPRHGSL |
| 2 | 17–43 | LPRKRAARHRGKVKSFPKYDPKKPVHL |
| 3 | 56–75 | VVRDLDRPGAKMHKKEIVEA |
| 4 | 94–103 | ETPRGLRSLT |
| 5 | 109–130 | HLSDEVKRRFYKNWYKSKKKAF |
| 6 | 133–158 | YAKKHAEENGASITRELERIKKYCTV |
| 7 | 166–180 | QIRKTPLKQKKAHLM |
| 8 | 231–250 | TSRWGTKKLPRKTHKGLRKV |
| 9 | 274–285 | HHRTSCNHKVFR |
| 10 | 286–309 | IGKGSDEGNASTDFDISKKQITPM |
| 11 | 343–370 | LYPQTSRRATEKVELKWIDTSSKFGHGA |
DISCUSSION
The current study, involving immunoscreening of the cDNA library of Afu, led to identification and expression of a 44-kDa protein with sequence homology to L3 ribosomal proteins. Significantly higher levels of specific IgG and IgE antibodies to the purified protein observed in sera of ABPA patients than controls suggested that it may be a novel allergen/antigen of Afu. Although other ribosomal proteins such as P2, L7 and L12 have been found to be allergenic/antigenic in Afu, Cladosporium herbarum and Brucella melitensis, none of the RpL3s has been proposed as an allergen/antigen until now [15–17]. The presence of IgE antibodies to rTS1 in 26·7% of the ABPA patients indicated that it may be a minor allergen of Afu according to the allergen nomenclature guidelines given by the IUIS (IUIS, allergen nomenclature subcommittee, http://www.allergen.org/Editoral.htm).
Multiple sequence alignment using the sequences of RpL3 from Afu, A. nidulans, S. cerevisiae and H. sapiens showed that this protein belongs to the category of highly conserved proteins. RpL3 is vital for the function of the ribosome and has been shown to participate in or initiate the early steps of the ribosomal assembly, where it binds with high affinity to the 23S rRNA [18,19]. It is involved in the formation of the peptidyltransferase centre and is essential for its catalytic activity [20–23]. The L3-binding site has been localized to a long double helix, containing a large internal loop and a sarcin/ricin loop in domain VI of 23S rRNA [24,25].
The deduced amino acid sequence showed significant homology to tcm1, which encodes RpL3 of S. cerevisiae. It is interesting to note that tcm1 has been implicated in resistance of S. cerevisiae to trichodermin (an inhibitor of ribosome peptidyl transferase activity) [26,27]. Although many strains of Afu have been shown to be resistant to antibiotics, only limited information is yet available regarding the genes conferring antibiotic resistance to Afu. However, recently Afu ribosomal stalk proteins such as P0 phosphoprotein has been implicated in resistance to sordarin antifungals [28]. On the basis of significant sequence homology to tcm1, the RpL3 of Afu could be implicated in antibiotic resistance of Afu, although this requires experimental confirmation.
Sixty-four per cent sequence identity of Afu RpL3 with human RpL3 and common regions in their predicted epitopes suggests the possibility of involvement of Afu RpL3 in autoimmune reactions. There are reports of the involvement of phylogenetically conserved proteins of Afu in autoimmune reactions in patients of aspergillosis, such as Asp f6 (manganese superoxide dismutase), Asp f8 (P2 acidic ribosomal protein) and Asp f11 (cyclophilin) (sequence identity of >50% with the corresponding human protein) [15,29,30]. In a previous study, significantly high levels of specific IgG and IgE antibodies to human fibronectin and collagen type IV were observed in sera of ABPA patients, suggesting the role of autoimmune reactions in immunopathogenesis of aspergillosis [3]. Tissue damage due to the inflammatory process observed in patients of aspergillosis might result in release of autoantigens [31]. Exposure to autoantigens containing cross-reactive determinants (molecular mimicry) can result in an autoimmune response by recruiting the memory T cell repertoire at the site of inflammation.
In conclusion, the present study has led to identification of a novel allergen/antigen of Afu with sequence homology to L3 ribosomal protein, which may be involved in autoimmune reactions in patients of aspergillosis and in conferring drug resistance to Afu.
Acknowledgments
We are grateful to the Institute of Genomics and Integrative Biology for providing the facilities for carrying out the study. We acknowledge the funding for the present study by the Institute of Genomics and Integrative Biology and the Council of Scientific and Industrial Research. We also acknowledge the Vallabhbhai Patel Chest Institute for providing sera samples of ABPA patients. We thank Dr Yogendra Singh and Mr Puneet Chopra for assistance in cloning and protein purification. Ms Shweta Saxena is the recipient of a Senior Research Fellowship from the Council of Scientific and Industrial Research.
REFERENCES
- 1.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurokawa CS, Sugizaki MF, Peracoli MT. Virulence factors in fungi of systemic mycoses. Rev Inst Med Trop Sao Paulo. 1998;40:125–35. doi: 10.1590/s0036-46651998000300001. [DOI] [PubMed] [Google Scholar]
- 3.Purkayastha S, Madan T, Shah A, et al. Multifunctional antigens of A. fumigatus and specific antibodies. Appl Biochem Biotechnol. 2000;83:271–83. doi: 10.1385/abab:83:1-3:271. [DOI] [PubMed] [Google Scholar]
- 4.Greenberger PA. Clinical aspects of allergic bronchopulmonary aspergillosis. Front Biosci. 2003;8:S119–27. doi: 10.2741/943. [DOI] [PubMed] [Google Scholar]
- 5.Crameri R. Recombinant Aspergillus fumigatus allergens: from the nucleotide sequences to clinical applications. Int Arch Allergy Immunol. 1998;115:99–114. doi: 10.1159/000023889. [DOI] [PubMed] [Google Scholar]
- 6.Borga A. 1990. Allergens of Aspergillus fumigatus. PhD thesis, Karolinska Institute, Stockholm. [Google Scholar]
- 7.Kumar A, Reddy LV, Sochanik A, et al. Isolation and characterization of a recombinant heat shock protein of Aspergillus fumigatus. J Allergy Clin Immunol. 1993;91:1024–30. doi: 10.1016/0091-6749(93)90215-2. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee B, Kurup VP, Phadnis S, et al. Molecular cloning and expression of a recombinant Aspergillus fumigatus protein Asp fII with significant IgE reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–62. doi: 10.1016/s0022-2143(96)90093-1. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg M, Patterson R, Roberts M, et al. The assessment of immunologic and clinical stages occuring during corticosteroid therapy for ABPA. Am J Med. 1978;64:599–607. doi: 10.1016/0002-9343(78)90579-x. [DOI] [PubMed] [Google Scholar]
- 10.Nigam S, Sarma PVGK, Ghosh PC, et al. Characterization of Aspergillus fumigatus protein disulfide isomerase family gene. Gene. 2001;281:143–50. doi: 10.1016/s0378-1119(01)00794-6. [DOI] [PubMed] [Google Scholar]
- 11.Mandel M, Higa A. Calcium-dependent bacteriophage DNA infection 1970. Biotechnology. 1992;24:198–201. [PubMed] [Google Scholar]
- 12.Banerjee B, Madan T, Sharma GL, et al. Characterisation of 45 kd glycoprotein antigen of A. fumigatus. Serodiagnosis and immunotherapy. Infect Dis. 1995;7:147–52. [Google Scholar]
- 13.Ramos JD, Cheong N, Lee BW, et al. cDNA cloning and expression of Blo t11, the Blomia tropicalis allergen homologous to paramyosin. Int Arch Allergy Immunol. 2001;126:286–93. doi: 10.1159/000049525. [DOI] [PubMed] [Google Scholar]
- 14.Van Regenmortel MH. Protein antigenicity. Mol Biol Rep. 1992;16:133–8. doi: 10.1007/BF00464700. [DOI] [PubMed] [Google Scholar]
- 15.Mayer C, Appenzeller U, Seelbach H, et al. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. 1999;189:1507–12. doi: 10.1084/jem.189.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachrach G, Banai M, Fishman Y, et al. Delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infect Immun. 1997;65:267–71. doi: 10.1128/iai.65.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achatz G, Oberkofler H, Lechenauer E, et al. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. Mol Immunol. 1995;32:213–27. doi: 10.1016/0161-5890(94)00108-d. [DOI] [PubMed] [Google Scholar]
- 18.Nowotny V, Nierhaus KH. Initiator proteins for the assembly of the 50S subunit from Escherichia coli ribosomes. Proc Natl Acad Sci USA. 1982;79:7238–42. doi: 10.1073/pnas.79.23.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speirer P, Zimmermann RA. RNA–protein interactions in the ribosome. Binding of proteins L1, L3, L6, L13 and L23 to specific fragments of the 23S RNA. FEBS Lett. 1976;68:71–5. doi: 10.1016/0014-5793(76)80407-3. [DOI] [PubMed] [Google Scholar]
- 20.Hampl H, Schulze H, Nierhaus KH. Ribosomal components from Escherichia coli 50S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981;256:284–8. [PubMed] [Google Scholar]
- 21.Franceschi FJ, Nierhaus KH. Ribosomal proteins L15 and L16 are mere late assembly proteins of the large ribosomal subunit. Analysis of an Escherichia coli mutant lacking L15. J Biol Chem. 1990;265:16676–82. [PubMed] [Google Scholar]
- 22.Green R, Noller HF. Ribosomes and translation. Annu Rev Biochem. 1997;66:679–716. doi: 10.1146/annurev.biochem.66.1.679. [DOI] [PubMed] [Google Scholar]
- 23.Khaitovich P, Mankin AS, Green R, et al. Characterization of functionally active subribosomal particles from Thermus aquaticus. Proc Natl Acad Sci USA. 1999;96:85–90. doi: 10.1073/pnas.96.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leffers H, Egebjerg J, Andersen A, et al. Domain VI of Escherichia coli 23S ribosomal RNA. Structure, assembly and function. J Mol Biol. 1988;204:507–22. doi: 10.1016/0022-2836(88)90351-8. [DOI] [PubMed] [Google Scholar]
- 25.Uchiumi T, Sato N, Wada A, et al. Interaction of the sarcin/ricin domain of 23S ribosomal RNA with proteins L3 and L6. J Biol Chem. 1999;274:681–6. doi: 10.1074/jbc.274.2.681. [DOI] [PubMed] [Google Scholar]
- 26.Fried HM, Warner JR. Cloning of yeast gene for trichodermin resistance and ribosomal protein L3. Proc Natl Acad Sci USA. 1981;78:238–42. doi: 10.1073/pnas.78.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz LD, Friesen JD. Nucleotide sequence of the tcmI gene (ribosomal protein L3) of Saccharomyces cerevisiae. J Bacteriol. 1983;155:8–14. doi: 10.1128/jb.155.1.8-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos C, Ballesta JP. Role of the ribosomal stalk components in the resistance of Aspergillus fumigatus to the sordarin antifungals. Mol Microbiol. 2002;43:227–37. doi: 10.1046/j.1365-2958.2002.02736.x. [DOI] [PubMed] [Google Scholar]
- 29.Mayer CS, Hemmann A, Faith K, et al. Cloning, production, characterization and IgE cross reactivity of different manganese superoxide dismutases in individuals sensitized to Aspergillus fumigatus. Int Arch Allergy Immunol. 1997;113:213–5. doi: 10.1159/000237550. [DOI] [PubMed] [Google Scholar]
- 30.Appenzeller U, Meyer C, Menz G, et al. IgE-mediated reactions to autoantigens in allergic diseases. Int Arch Allergy Immunol. 1999;118:193–6. doi: 10.1159/000024064. [DOI] [PubMed] [Google Scholar]
- 31.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–7. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]