INTRODUCTION
Autoimmune diseases arise as a result of a complex interplay of factors, but it is often considered that the failure of the immune system to recognize ‘self’ frequently leads to substantial quantities of autoantibodies that are capable of binding to specific amino acid sequences on a given protein or other molecule. Why a particular protein, or fragment of a protein is targeted for attack by one's own immune system has been speculated upon for many years. The antibody-producing B cells respond to threats by viruses, bacteria and other foreign material entering our bodies, leading to the production of excess antibody that can bind to epitopes of self-antigens. This cross-reactivity has often been attributed to an autoimmune response driven as a consequence of homology between endogenous proteins and pathogen-derived molecules. However, up to a third of autoantibodies can bind to self-antigens [1], many of which bear no relation to microbe-derived proteins. Moreover, autoantibody production itself is often insufficient to cause clinical features of autoimmune disease. Therefore, attention has been focused on patterns of autoantibody production against clusters of protein complexes, in particular protein complexes that are formed when cells are undergoing stress due to a variety of modifications induced by, for example, UV, heat shock, cell cycle changes, hormones, viruses, drugs and ionic conditions. Such complexes are now considered to possess immunological features that under appropriate conditions can modulate the host's immune response.
THE RO/SSA COMPLEX AND SYSTEMIC LUPUS ERYTHEMATOSUS
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease characterized by the production of autoantibodies directed against cellular proteins and nucleic acids in complex with one another. Some of the autoantibodies are thought to have pathogenic consequences [2], and clinical features of SLE can range from a simple skin rash to a rapidly progressive lethal multiorgan disease involving kidneys, brain, blood vessels and cells, lung and heart. These patients also suffer from defective apoptosis mechanisms [3] and many of the dominant autoantigens that are frequent targets are found in the apoptotic blebs that are cleared inefficiently in these patients during programmed cell death [4]. Some of the components that accumulate in the blebs of cell debris are ribonuclearprotein (RNP) polypeptides (Ro/SSA and La/SSB) that associate with small chromosomal RNAs designated hYRNA 1, 3, 4 and 5, situated predominantly in the cytoplasm, but also in the nuclear compartments. The Ro/SSA complex of ribonuclear proteins consists of at least three proteins of 60-, 52-(subtypes α- and β-) and 48 kDa, while the La/SSB protein is 48 kDa. Autoantibodies are often directed against these proteins, with anti-Ro/SSA and anti-La/SSB antibodies detected in 30% and 15% of sera of non-selective patients with SLE, respectively. In patients with hereditary C2, C4 or C1q complement deficiency, anti-Ro/SSA is associated with up to 90% of the lupus individuals [3]. Under normal circumstances these potential ‘self-antigens’ remain non-immunogenic to the immune system, but when in complex with other proteins can become more antigenic in nature. One such candidate is the Ca2-binding protein, calreticulin, which resides normally in the endoplasmic reticulum, and is known to have many cellular functions [5], including regulation of Ca2+ homoeostasis and chaperone activity. Calreticulin is also considered a stress protein induced by heat, food deprivation and chemical stress. For example, overexpression of calreticulin increases the content of ER Ca2+ and affords protection to cells against NO-mediated apoptosis [6]. However it is precisely under specific conditions of stress, such as apoptosis, that calreticulin appears to come into contact with the Ro and La proteins. A combination of in vitro electromobility gel shift analysis and in vivo yeast di-hybrid analysis [7] has confirmed that calreticulin interacts with the Ro52 polypeptide. The subcellular and extracellular localization, together with the subsequent co-localization of calreticulin with the Ro proteins, has been the subject of intense interest [5], not least because the rough ER apoptotic blebs of keratinocytes are known to contain Ro52, Ro60 and the stress proteins Grp78 (BiP) and calreticulin [8], all of which are potentially antigenic, leading to the development of autoantibodies against each protein in either SLE or rheumatoid arthritis patients [9]. Why these carefully packaged components in apoptotic blebs should provoke an autoimmune response leading to B cell hyperactivity and autoantibody production remains unknown. However, in this issue Staikou and coworkers have demonstrated that calreticulin also binds to small polypeptide fragments of the Ro60 protein. Moreover, the interactions of the peptide–calreticulin complexes are favoured by heat treatment, divalent cations and ATP. This is of importance, as calreticulin also undergoes frequent, ion-induced conformation changes which may affect its function and its ability to interact with other proteins [10]. The significance of this is that the autoimmune sera obtained from SLE and primary Sjögren's syndrome (pSS) showed up to a fourfold enhanced recognition of the Ro60 peptides when in association with calreticulin, than with any of the Ro60 peptides or calreticulin alone. This work provides evidence that calreticulin may induce conformation-dependent recognition of the previously linear Ro60 polypeptides, leading to their eventual recognition by anti-Ro60-positive sera from autoimmune patients. The data imply that under changing ionic conditions, calreticulin–peptide interactions have the capacity to enhance the recognition of host proteins by autoantibodies.
RO/SSA, CALRETICULIN AND EPITOPE SPREADING
The new observations by Staikou et al. [11] take on greater significance when one considers that SLE may be predominantly an autoimmune disease of B cell hyperactivity, with the production of characteristic patterns of autoantibodies [12]. In part, they may explain some of the unusual features of epitope spreading associated with the components of the Ro/SSA complex and the stress proteins Grp78 and calreticulin. The ER blebs of apoptotic cells are an immunological time bomb, because most of the contents of such blebs, including the proteins mentioned above, are potentially autoantigenic to varying degrees. If these blebs are not cleared efficiently, as has been suggested for SLE patients [13], then their contents could eventually be released from the membrane-bound blebs, providing a cluster of proteins to evoke an immune challenge (Fig. 1). If stress proteins such as calreticulin can induce conformational changes in peptides they associate with [14], then this could render cryptic epitopes buried deep within the fold of the associated proteins to become exposed to autoreactive T cells with or without the help of B cells with autospecificity for La or Ro epitopes. Elegant experiments performed by McCluskey and colleagues evaluated this possibility in vivo by experimental immunization of mice with one component of the Ro/SSA complex at a time, to determine if there is a response against any of the other components [15]. Immunization of normal inbred mice with either the 46, 52 or 60 kDa Ro proteins led to a consistent pattern of response, with the generation of high titre antibodies against the initial immunized protein, followed 2 weeks later by autoantibody production of the other components of the apoptotic blebs, included antibodies against calreticulin [16] and Grp78 [17], after mice were inoculated with Ro52 and Ro60, respectively. As calreticulin and Grp78 are not located in the nucleocytoplasmic compartments of non-stressed cells, the association of these ER stress proteins with Ro antigen complexes occurs probably in the apoptotic blebs. Autoreactivty to calreticulin and Grp78 is not restricted to experimental models, as up to 40% of SLE patients possess anticalreticulin antibodies [18] and between 30 and 60% of rheumatoid arthritis patients exhibit anti-Grp78 autoantibodies [9,19]. In a recent issue of this journal [20], Purcell and colleagues demonstrated intermolecular spreading of B cell immunity between Grp78 and the Ro autoantigens providing biochemical evidence of their association and subsequent cascade of responses leading ultimately to enhanced immunogenicty.
Fig. 1.
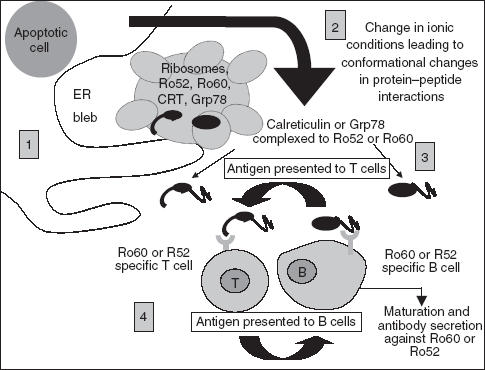
Model showing possible effects of stress protein interaction with Ro polypeptides. (1) Defective clearance of apoptotic blebs containing a combination of stress proteins and ribonuclear proteins could result in their release into the extracellular environment. (2) Changes in the ionic conditions may lead to conformational changes in the both the stress proteins and Ro autoantigens, leading to closer interaction with one another, resulting in structural modification and exposure of cryptic epitopes. (3) Such changes may impair the tolerance-inducing function of the apoptotic process leading to autoimmunity as the protein clusters are taken up by either Ro specific B or T cells. (4) After antigen processing, MHC class II molecules with peptides from the ER cluster of proteins are presented to either naïve Ro, Grp78 or calreticulin Th-cells or directly to B cell specific cells, ultimately leading to B cell maturation and secretion of autoantibodies against the protein clusters.
SIGNIFICANCE OF ENHANCED AUTOANTIBODY PRODUCTION AGAINST STRESS PROTEINS
It is clear that the relationship between the production of autoantibodies and pathology remains controversial and explanations for the tissue destruction observed in multi-system autoimmune diseases remain uncertain. However, with the ever-growing number of extracellular roles being elicited to proteins such as calreticulin the production of autoantibodies could have profound effects on their physiological functions. For example, calreticulin has now been implicated as an important bridging molecule which aids C1q-bound apoptotic debris to be engulfed by macrophages after binding to the α2-macroglubulin receptor (CD91) via calreticulin [21]. Clearly, the presence of autoantibodies to calreticulin could possibly interfere with the attachment of calreticulin to both C1q and CD91 and impair this pathway of clearance of cell debris. In this regard it is noteworthy that clearance of apoptoic debris is indeed impaired in many patients with SLE, but a correlation between anticalreticulin levels and apoptotic dysfunction has not been documented. Grp78 has also been found in the extracellular environment [22]. It is clear from this recent work that interactions of stress proteins with polypeptides encountered in apoptotic bodies can influence the conformational characteristics of the interacting peptides and the chaperones themselves, provoking an increase cascade of immune responses towards the associated proteins, further fuelling the debate as to the precise role of stress proteins in physiological or pathological pathways [23]. Further work in this field will hopefully provide new explanations for the production of autoantibodies against clusters of self-proteins and determine whether the resulting autoantibodies generated are responsible for the tissue damage observed in autoimmune disorders such as SLE and rheumatoid arthritis.
REFERENCES
- 1.Isenberg D, Morrow J. Friendly fire. Oxford: Oxford University Press; 1995. [Google Scholar]
- 2.Paul E, Manheimer-Lory A, Livneh A, et al. Pathogenic anti-DNA antibodies in SLE: idiotypic families and genetic origins. Int Rev Immunol. 1990;5:295–313. doi: 10.3109/08830189009056736. [DOI] [PubMed] [Google Scholar]
- 3.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 4.Huggins ML, Todd I, Cavers MA, Pavuluri SR, Tighe PJ, Powell RJ. Antibodies from systemic lupus erythematosus (SLE) sera define differential release of autoantigens from cell lines undergoing apoptosis. Clin Exp Immunol. 1999;118:322–8. doi: 10.1046/j.1365-2249.1999.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11:122–9. doi: 10.1016/s0962-8924(01)01926-2. [DOI] [PubMed] [Google Scholar]
- 6.Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001;98:10845–50. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng ST, Nguyen TQ, Yang YS, Capra JD, Sontheimer RD. Calreticulin binds hYRNA and the 52-kDa polypeptide component of the Ro/SS-A ribonucleoprotein autoantigen. J Immunol. 1996;156:4484–91. [PubMed] [Google Scholar]
- 8.Eggleton P, Llewellyn DH. Autoimmune disease and calcium binding proteins. In: Pochet R, Donato R, Haiech J, Heizmann C, Volker G, editors. Calcium: the molecular basis of calcium action in biology and medicine. Amsterdam: Kluwer Academic Publishers; 2000. pp. 317–31. [Google Scholar]
- 9.Blass S, Union A, Raymackers J, et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 2001;44:761–71. doi: 10.1002/1529-0131(200104)44:4<761::AID-ANR132>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Corbett EF, Michalak KM, Oikawa K, et al. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J Biol Chem. 2000;275:27177–85. doi: 10.1074/jbc.M002049200. [DOI] [PubMed] [Google Scholar]
- 11.Staikou EV, Routsias JG, Makri AA, et al. Calreticulin binds preferentially with B cell linear epitopes of Ro60 kD autoantigen, enhancing recognition by anti-Ro60 kD autoantibodies. Clin Exp Immunol. 2003;134:6–8. doi: 10.1046/j.1365-2249.2003.02246.x. Also published online, doi:10.1046/j.1365-2249.2003.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsky PE. Systemic lupus erythematosus. an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson SJ, Hakannson KO. Biochemical and molecular properites of calreticulin. In: Eggleton P, Michalak M, editors. Calreticulin. 2. Georgetown: Landes Bioscience; 2003. pp. 9–18. [Google Scholar]
- 15.Keech CL, Gordon TP, McCluskey J. The immune response to 52-kDa Ro and 60-kDa Ro is linked in experimental autoimmunity. J Immunol. 1996;157:3694–9. [PubMed] [Google Scholar]
- 16.Kinoshita G, Keech CL, Sontheimer RD, Purcell A, McCluskey J, Gordon TP. Spreading of the immune response from 52 kDaRo and 60 kDaRo to calreticulin in experimental autoimmunity. Lupus. 1998;7:7–11. doi: 10.1191/096120398678919606. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita G, Purcell AW, Keech CL, Farris AD, McCluskey J, Gordon TP. Molecular chaperones are targets of autoimmunity in Ro (SS-A) immune mice. Clin Exp Immunol. 1999;115:268–74. doi: 10.1046/j.1365-2249.1999.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishore U, Sontheimer RD, Sastry KN, et al. The systemic lupus erythematosus (SLE) disease autoantigen-calreticulin can inhibit C1q association with immune complexes. Clin Exp Immunol. 1997;108:181–90. doi: 10.1046/j.1365-2249.1997.3761273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigall VM, Bodman-Smith MD, Fife MS, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–8. doi: 10.4049/jimmunol.166.3.1492. [DOI] [PubMed] [Google Scholar]
- 20.Purcell AW, Todd A, Kinoshita G, et al. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin Exp Immunol. 2003;132:193–200. doi: 10.1046/j.1365-2249.2003.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delpino A, Castelli M. The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci Rep. 2002;22:407–20. doi: 10.1023/a:1020966008615. [DOI] [PubMed] [Google Scholar]
- 23.Eggleton P, Llewellyn DH. Pathophysiological roles of calreticulin in autoimmune disease. Scand J Immunol. 1999;49:466–73. doi: 10.1046/j.1365-3083.1999.00542.x. [DOI] [PubMed] [Google Scholar]


