Abstract
Transforming growth factor-β (TGF-β) is an inhibitory cytokine recognized as a key regulator of immunological homeostasis and inflammatory responses. TGF-β is involved in experimental models of oral tolerance and in the pathogenesis of experimental colitis. Patients with inflammatory bowel disease (IBD) have inappropriate T cell responses to antigenic components of their own intestinal microflora, suggesting the presence of a disorder in the normal mucosal immune mechanism that ensures the down-regulation of responses to harmless constituents in the microflora. To evaluate the contribution of TGF-β to this imbalance, we measured TGF-β1 production by lamina propria mononuclear cells (LPMC) and T cells isolated from tissue specimens of patients with Crohn's disease (CD), ulcerative colitis (UC) and controls. Cells were cultured in the presence or absence of anti-CD2 plus anti-CD28 MoAbs and TGF-β1 production in culture supernatants was measured by ELISA. LPMC isolated from CD patients produced significantly less TGF-β1 than controls when stimulated via CD2 plus CD28 pathways (P = 0·001)] conversely, in UC patients increased production of TGF-β1 compared to controls was observed (P = 0·0005). These differences were also observed with purified lamina propria (LP) T cells in both diseases and were associated with the presence of inflammation. Thus, TGF-β1 production shows contrasting secretion in CD and in UC, probably as a consequence of the different Th polarization. The absolute or relative defect in TGF-β1 production observed in CD and UC may contribute to the perpetuation of inflammation.
Keywords: cytokines, human, inflammation, mucosa, tolerance, suppression, anergy
INTRODUCTION
Transforming growth factor βs (TGF-βs) belong to a family of multi-functional polypeptides produced by a wide variety of lymphoid and non-lymphoid cells. They exist in five different isoforms, three of which are expressed in mammals and designated as TGF-β1, TGF-β2 and TGF-β3 [1]. TGF-βs are synthesized as precursors proteins which are cleaved intracellular by the endopeptidase furin prior to secretion. The proteolysis yields two products that assemble into dimers, latency-associated peptide (LAP) and mature TGF-β. The presence of the LAP can facilitate the transport of the TGF-β found on the cell surface and makes the TGF-β biologically inactive. Mature TGF-β associated with LAP is called latent TGF-β. Latent TGF-β is secreted in this inert precursor form and is converted to its biologically active form extracellularly by the release of the LAP fragment or by undergoing a conformational change, which exposes specific TGF-β binding sites [1]. TGF-β1, TGF-β2 and TGF-β3 have an important role in the regulation of immune cells. Targeted disruption in the mouse TGF-β1 gene results in a severe, multi-focal inflammatory response and early death, while mutation of TGF-β2 or TGF-β3 results in 100% embryonic lethality, preventing the determination of their roles in immune regulation in vivo. However, all three TGF-β isoforms seem to have the same effects on immune cells in vitro[2,3].
TGF-β can act in both autocrine and paracrine modes to control the differentiation, proliferation and state of activation of immune cells. In particular, TGF-β1 can inhibit the production of and response to cytokines associated with CD4+ Th1 T cells and CD4+ Th2 T cells [2,3].
TGF-β has also been found to be involved in experimental models of oral tolerance [4,5]. In humans, induction of antigen-specific TGF-β1 secreting Th3 cells by oral administration of myelin in multiple sclerosis patients has been observed [6]. TGF-β production is also relevant in the pathogenesis of experimental colitis. In two different models of Th1-mediated murine experimental colitis, it has been shown that protection from colitis development is strictly associated with the presence of increased numbers and/or up-regulation of TGF-β1 producing cells [7,8]. In humans, it has been reported that mucosal T cell unresponsiveness to luminal antigens is mediated by production of inhibitory cytokines [interleukin-10 (IL-10) and TGF-β] after specific antigen recognition [9]. In normal gut, regulatory mechanisms are involved in maintaining a state of controlled inflammation, which is in turn believed to be induced by enteric flora and food antigens. In patients with inflammatory bowel disease (IBD), this physiological controlled inflammation is overwhelmed by a persistent and damaging inflammation, suggesting the presence of a disorder in the normal mucosal immune mechanism that ensures the down-regulation of responses to harmless constituents in the microflora or the food stream [10,11]. To evaluate the contribution of TGF-β to this imbalance, we measured TGF-β1 production by lamina propria mononuclear cells (LPMC) and T cells isolated from tissue specimens of patients with Crohn's disease (CD) and ulcerative colitis (UC).
PATIENTS AND METHODS
Patients
The study was approved by the local ethical committee. Fifteen CD patients (age: range 22–75 years), eight UC patients (age: range 20–50 years) and 21 controls (age: range 40–74 years) were studied. In CD patients, the disease involved the ileum as recurrent disease in three patients and as primary disease in eight. In the remaining four patients disease involved the colon as primary disease. At the time of surgery, one CD patient was being treated with corticosteroids, two patients had never received treatment with steroids or immunosuppressive agents, the remaining patients had discontinued corticosteroid treatment approximately 30 days prior to surgery. Indications for surgery in CD consisted of recurrent small bowel obstruction in seven patients, stenosis in three, presence of fistulae in one patient and intractable disease in four patients. In UC patients, the disease involved the entire colon in four patients and the left colon in the remaining four. Seven patients underwent surgery because of intractable disease and one because of toxic megacolon. At the time of surgery three UC patients were being treated with corticosteroids. Control samples in the studies on CD were obtained from macroscopically and microscopically unaffected areas of resected distal ileum of nine patients undergoing bowel resection for right colon neoplasia. Control samples for studies on UC and colonic CD consisted of tissue from macroscopically and microscopically unaffected areas of resected colons from 12 patients with colon cancer.
LPMC isolation
LPMC were isolated form freshly resected mucosa using a previously described DTT-EDTA-collagenase method [12]. In brief, strips of mucosa (6–8 g total weight) were washed in Hanks's balanced salt solution (HBSS) free of calcium and magnesium (HBSS-CMF Hyclone, Europe Ltd, Cramlington, NE, USA). They were then washed in HBSS-CMF containing 1 mmol/l DTT (Sigma Chemical Co., St Louis, MO, USA) and antibiotics (penicillin, 100 U/ml; streptomycin, 100 µg/ml; and fungizone, 0·25 µg/ml) for 15 min at room temperature. After three washes in HBSS-CMF, the mucosal strips were cut into small pieces (approximately 3 × 3 mm) and incubated four to five times in HBSS-CMF containing 0·75 mmol/l EDTA, 10 mmol/l HEPES buffer and antibiotics for 45 min in a humid 5% CO2 atmosphere to remove epithelial cells. After two washes, the mucosal fragments were incubated for 10–13 h at 37°C in a humid 5% CO2 atmosphere in medium [RPMI-1640 plus 10 mm HEPES buffer, 2 mm l-glutamine, 10% heat-inactivated FCS (Hyclone) and antibiotics] containing 25 U/ml collagenase V (Sigma). After incubation, the supernatant was collected and washed twice in HBSS-CMF and LPMC were subjected to centrifugation through a 30–40–60–100% Percoll gradient (Sigma). LPMC were collected at the 40/60% interface and consisted of: 84–91% CD3+, 2–5%CD 14+, 5–11% CD20+. Purified lamina propria (LP) T cells were obtained from LPMC by immunomagnetic negative selection using immunomagnetic beads coated with anti-CD14 and anti-CD19 mouse-antihuman MoAb (Dynal, Oslo, Norway) using the procedure recommended by the manufacturer. The resultant (unbound) T cell population contained greater than 95% CD3+ cells as assessed by flow cytometric analysis.
Cell cultures
Isolated LPMC or LP T cells were resuspended at a final concentration of 1 × 106 cells/ml in complete medium consisting of RPMI-1640 plus 10 mm HEPES buffer, 2 mm l-glutamine and antibiotics supplemented with 1% Nutridoma (Boehringer Mannheim Biochemicals, Mannheim, Germany) and cultured for 72 h in 48-well plates (Costar, Corning Incorporated, Corning, NY, USA) in humid 5% CO2 atmosphere. For evaluation of TGF-β1 production, occurring spontaneously or via activation, cells were cultured either in the absence of a stimulus or the presence of soluble anti-CD28 MoAb plus soluble anti-CD2 MoAbs (T112 and T113). In preliminary experiments cells were also cultured in the presence of anti-CD2 MoAbs alone or in the presence of plate-bound anti-CD3 MoAb (OKT3) alone or with the addition of soluble anti-CD28 MoAb. The anti-CD2 MoAb pair (T112 and T113) was provided by Dr Ellis Reinhertz (Dana Farber Cancer Institute, Boston, MA, USA). The anti-CD28 MoAb (clone 9·3) was obtained from Prof Carl June (Cancer Center, University of Pennsylvania, Philadelphia, PA, USA). The anti-CD2 (T112 and T113) was used at a 1 : 1000 final dilution and the anti-CD28 MoAb was used at a final concentration of 1 µg/ml. In some experiments, in separate cultures anti-IL-12 MoAb (final concentration 2 µg/ml) and anti-IFN-γ MoAb (final concentration 2 µg/ml) (both from R&D Systems, Minneapolis, MN, USA) were added to cells stimulated with anti-CD2 plus anti-CD28 MoAbs in order to block the production and the effect of IFN-γ.
TGF-β1 production measurement
At the end of the 72-h culture period, supernatants were collected and stored at − 80°C until tested for TGF-β1 content. TGF-β1 production was assayed in duplicate, after samples acidification, by ELISA using commercial kits (R&D Systems) following the instructions from the manufacturer.
IFN-γ and IL-5 production measurement
In a subset of supernatants IFN-γ and IL-5 production were also measured, using commercially available kits (Endogen, Woburn, MA, USA distributed by Tema Ricerca, Bologna, Italy).
Statistical analysis
Statistical analysis of the data was performed using non-parametric Mann-Whitney U-test.
RESULTS
LPMC isolated from CD involved tissue show defective TGF-β1 production while LPMC isolated from UC involved tissue show increased TGF-β1 production after stimulation
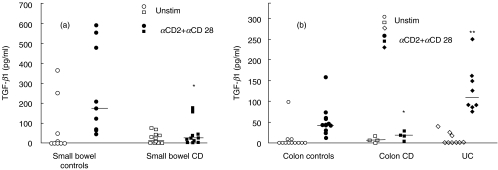
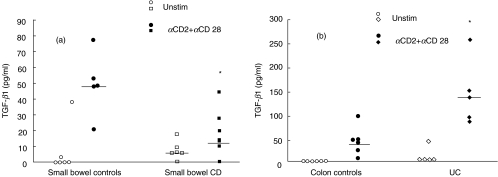
In preliminary experiments we evaluated the ability of different stimulation pathways to induce TGF-β1 production after 72 h of cell culture. In particular we analysed CD3 and CD2 stimulation pathways either alone or in combination with the costimulatory pathway CD28. We found, as described previously in studies on lymphocyte proliferation and production of different cytokines [13], that CD2 + CD28 stimulation was able to induce the highest production of TGF-β1 both in control and IBD samples (data not shown). In this setting, we evaluated TGF-β1 production in unstimulated and stimulated LPMC isolated from involved ileum of CD patients. As shown in Fig. 1a, while there were no differences in TGF-β1 production by unstimulated cells, LPMC isolated from CD patients showed significantly less production of TGF-β1 than controls when stimulated via the CD2 + CD28 pathway (P = 0·001). To verify that the observed reduction of TGF-β1 production was also present when CD was localized in the colon, TGF-β1 production was analysed from LPMC isolated from colonic LP of four CD patients. A significant reduction of TGF-β1 production by isolated LPMC in CD was again observed when compared to LPMC isolated from control colons (Fig. 1b, P = 0·008). This reduction was comparable to levels that were found in CD patients with ileal involvement, although the absolute production of TGF-β1 by LPMC isolated from colonic specimens was lower than that observed in the ileum. Thus, the above data suggest that LPMC isolated from inflamed CD tissue produce lower amounts of TGF-β1 when compared to controls. In contrast, CD2 + CD28 stimulation of LPMC isolated from UC involved tissue showed a higher production of TGF-β1 when compared to colonic controls (P = 0·0005) (Fig. 1b). These observed differences in TGF-β1 production were still appreciable when purified T lymphocytes obtained from a subset of small bowel and colonic controls and CD and UC involved tissues were cultured with antiCD2/CD28 (Fig. 2a,b).
Fig. 1.
TGF-β1 production by isolated LPMC from: (a) small bowel controls, small bowel CD involved tissue (b) colon controls, colon CD involved tissue and UC involved tissue. TGF-β1 production was measured in unstimulated cultures (open symbols) and in αCD2 + αCD28 stimulated cultures (closed symbols). Median is indicated by horizontal bars. (a) *P = 0·001 small bowel CD involved tissue versus small bowel controls] (b) *P = 0·008 colon CD involved tissue versus colon controls, **P = 0·0005 UC involved tissue versus colon controls.
Fig. 2.
TGF-β1 production by LP purified T lymphocytes. (a) Small bowel controls, CD involved tissue] (b) colon controls, UC involved tissue. Bars represent median. TGF-β1 production was measured in unstimulated cultures (open symbols) and in αCD2+ αCD28 stimulated cultures (closed symbols). (a) *P = 0·009 small bowel CD versus small bowel controls (b) *P = 0·009 UC versus colon control. LPMC TGF-β1 production in parallel cultures stimulated with αCD2+ αCD28 MoAbs were: small bowel controls: range: 48–124 pg/ml, median: 62·5, small bowel CD: range: 2·18–175 pg/ml, median: 16·8. colon controls: range: 23·1–73 median 47·5 pg/ml, UC: range 90–249 pg/ml median 160.
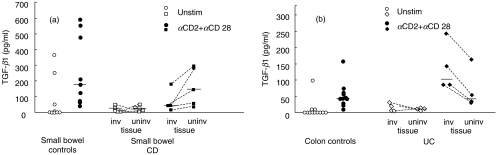
In five of 11 CD patients, and in four UC patients in whom the disease was localized to the left colon alone, it was possible to evaluate TGF-β1 production both in macroscopically involved and uninvolved tissue from the same surgery specimen. As shown in Fig. 3, stimulation of LPMC isolated from uninvolved tissue induces comparable amount of TGF-β1 as controls both in CD (Fig. 3a, P = 0·232) and in UC (Fig. 3b, P = 0·428). Thus, the differences in TGF-β1 production observed both in CD and UC are associated with the presence of inflammation, as demonstrated by the finding that LPMC isolated from CD small bowel uninvolved tissue and UC uninvolved tissue showed TGF-β1 production which was comparable to small bowel and colonic controls, respectively.
Fig. 3.
TGF-β1 production by isolated LPMC from: (a) small bowel controls, small bowel CD involved tissue, small bowel CD uninvolved tissue] (b) colon controls, UC involved tissue, UC uninvolved tissue. Bars represent median. TGF-β1 production was measured in unstimulated cultures (open symbols) and in αCD2 + αCD28 stimulated cultures (closed symbols).
The differential production of TGF-β1 in CD and UC is associated with different Th polarization
Studies in the murine system have shown that TGF-β producing cells are negatively and positively affected in their differentiation by the presence of IFN-γ and IL-4, respectively [14]. We and others have demonstrated previously that LP T cells from the involved area of CD patients produce higher amounts of IFN-γ than comparable T cells of control tissues, while LP T cells isolated from UC patients produce higher amount of IL-5 compared to T cells from control tissues [13,15–15]. Therefore, in the present study in a subset of samples we measured IFN-γ and IL-5 production in addition to TGF-β1 to ascertain that a similar pattern of cytokine production was indeed present in the patients enrolled in the present study. As shown in Table 1 we found an increased production of IFN-γ in CD patients when compared to controls while IL-5 secretion was higher in UC patients, demonstrating that the differential levels of TGF-β1 production observed in CD and UC were, in fact, associated with different Th cells polarization. It is known that once cells become committed to produce TGF-β in priming cultures, they are only marginally sensitive to modulation by IFN-γ and completely resistant to IL-4 modulation [14,19]. However, to investigate the possibility that the observed low TGF-β1 production by CD LPMC was the result of an in vitro inhibitory effect of IFN-γ, we added anti-IL-12 and (neutralizing) anti-IFN-γ antibodies to cultures of cells from both CD and control samples stimulated with anti-CD2+ anti-CD28. As shown in Table 2, we found that whereas cells in αIL-12 and αIFN-γ-treated cultures produced about twofold more TGF-β1 than cells in untreated cultures, the effect of the antibodies was the same in cultures of both CD and control cells. These results indicate that the differences between patients and controls in in vitro TGF-β1 production were not due to differences in the amount of IFN-γ produced during the culture period.
Table 1.
Production of TGF-β, IFN-γ and IL-5 by LPMC isolated from CD and UC involved tissue and small bowel and colonic controls. Cells were cultured for 72 h in medium containing 1% Nutridoma
| TGF-β1 (ng/ml) | IFN-γ (ng/ml) | IL-5 (ng/ml) | ||||
|---|---|---|---|---|---|---|
| Unstim median (range) | αCD2+ αCD28 median (range) | Unstim median (range) | αCD2+ αCD28 median (range) | Unstim median (range) | αCD2+ αCD28 median (range) | |
| Small bowel controls | 3·65 | 211 | ND | 1651 | ND | 1·36 |
| (n = 4) | (ND-7) | (58–554) | (429–4562) | (ND-2) | ||
| Small bowel CD | 3 | 32 | ND | 5000 | ND | ND |
| (n = 3) | (ND-15) | (27–33) | (3513–10 220) | (ND-28) | ||
| Colon controls | 3·33 | 38 | ND | 3105 | ND | 0·8 |
| (n = 5) | (ND-13) | (14–61) | (2467–3515) | (ND-23) | ||
| UC | ND | 95 | 8·9 | 2404 | ND | 179·6 |
| (n = 4) | (50–249) | (ND-29) | (1604–10 400) | (104–389) | ||
ND = not detectable.
Table 2.
TGF-β1 production (pg/ml) in cultures of LPMC isolated from small bowel controls, small bowel CD involved tissue, colon controls and UC involved tissue stimulated with αCD2 + αCD28MoAb in the presence or in the absence of aIL-12 and aIFN-γ blocking antibodies
| αCD2 + αCD28 median (range) | αCD2 + αCD28 + αIL-12+αIFN-γ median (range) | |
|---|---|---|
| Small bowel controls | 69·4 | 123 |
| (n = 5) | (48-–209) | (62–602) |
| Crohn's disease | 23 | 37 |
| (n = 5) | (2·18–33) | (30–43) |
| Colon controls | 21 | 33·5 |
| (n = 4) | (ND-44·6) | (24–58) |
| Ulcerative colitis | 68 | 71 |
| (n = 3) | (38–125) | (38–160) |
ND = not detectable.
DISCUSSION
In the present study, we observed that isolated LPMC stimulated via the CD2/CD28 pathway are able to produce measurable amounts of TGF-β1 and that the secretion levels obtained allow meaningful comparisons between IBD patients and controls. We found that, whereas LPMC isolated from inflamed CD tissue produced significantly decreased amounts of TGF-β1 than control LPMC after stimulation, LPMC isolated from UC tissue produced significantly increased amounts of TGF-β1 after stimulation. These studies are the first to show that the changes in TGF-β1 production in IBD is different in Crohn's disease and ulcerative colitis and, as such, provide a base of information with which to study the role of this regulatory cytokine in the pathogenesis of IBD.
Using stimulation of CD2 and CD28 activation pathways, we observed opposite changes in TGF-β1 production in CD and in UC. These differences are not related to different anatomical sites of inflammation (small bowel versus colon), as these differences are still present when CD localized to the colon, UC and colonic controls were compared. However, in both UC and CD the variation of TGF-β1 production appears to be related to the presence of inflammation, since TGF-β1 produced from lymphocytes isolated from uninvolved mucosa is comparable to the production from controls. Finally, these differences in TGF-β1 secretion are still observable in both diseases when T lymphocytes were purified, suggesting that the contribution to the production of TGF-β1 by non-T lymphocytes and accessory cells does not affect the observed difference.
The easiest explanation for these findings is inherent to the different type of T helper polarization in the two diseases observed in previous studies and confirmed in the present study. It has been demonstrated that CD is a Th-1 orientated inflammation in which cells overproduce cytokines such as IL-12 and IFN-γ[13,15–18], while in UC an increase in IL-5 (a Th-2 cytokine) production has been reported with normal levels of IFN-γ[13]. Studies in the murine system have shown that differentiation of TGF-β producing cells is negatively and positively affected by the presence of IFN-γ and IL-4, respectively [14]. Therefore, it is quite possible that the presence of an established Th-1 response as in CD may prevent the in vivo expansion of TGF-β1 producing cells. On the other hand, the presence of Th-2 type cytokines, as in UC, may favour the emergence of TGF-β1 producing cells. In several ways, Th-1 type cytokines can affect TGF-β production as well as its biological effects. In particular, IL-12 is able to directly down-modulate TGF-β1 gene activity [20], while IFN-γ is able to inhibit TGF-β1 signalling by a Jak1/STAT-1-mediated activation of the synthesis of the inhibitory Smad 7 protein, which in turn prevents the interaction of Smad3 with the TGF-β type I receptor [21,22]. The inhibitory effect of IFN-γ on the TGF-β signalling not only blocks the immunosuppressive effects of TGF-β on the responding cells, but also negatively affects the induction and expansion of TGF-β producing cells themselves. It is also known from animal studies that TGF-β positively affects the development of TGF-β producing cells [14]. This effect is probably mediated by the ability of TGF-β to increase gene expression of its own converting enzyme, furin [23]. Interestingly, it has been demonstrated that Smads can also regulate TGF-β-induced furin transcription, Smad7 being inhibitory of such transcription [24], and it has been observed that Smad7 protein expression is increased in involved tissue of patients with CD compared with normal controls [25]. It is possible that the increase in TGF-β1 production after in vitro Th-1 type cytokines neutralization observed in the present study might be due to partial inhibition of Smad7, which is increased by IFN-γ, TNF-α, IL-1 and LPS [26]. At the same time the observation that, in CD cells, in vitro Th-1 type cytokines neutralization is unable to restore TGF-β production suggests that in vivo priming conditions (presence of high level of IL-12 and IFN-γ) influenced the expansion of TGF-β1 producing cells [27]. This hypothesis is supported by the observation that systemic administration of antibodies to IL-12 in a murine model of oral tolerance to ovalbumin enhances substantially TGF-β production by spleen and PP cells [28]. However, the hypothesis that additional factors affecting TGF-β1 secretion might be present and contribute to TGF-β production has also to be taken into account.
In the present study TGF-β production in in vitro unstimulated cells did not show any statistically significant difference among the various groups. This finding is in contrast with previous reports that have shown in both CD and in UC an increased amount of mRNA for TGF-β evaluated by Northern blot analysis, and an increased number of TGF-β1 positive cells detected by in situ hybridization and immunohistochemistry [29–31]. However, these techniques do not allow the evaluation of the amount of TGF-β secreted, but only the evaluation of intracellular TGF-β RNA content or preformed intracellular TGF-β protein. TGF-β proteins undergo a number of intracellular processing steps prior to their secretion by the cell. An important processing step appears to be the proteolytic digestion of the precursor by the endopeptidase furin [32], transcription of which is influenced negatively by Smad7 [24]. Taken together, this information suggests that the intracellular presence of TGF-β may not necessarily match TGF-β secretion, in particular when cells are exposed to an inflammatory microenvironment [26]. In addition, extracting RNA from tissue specimens or biopsies does not allow discrimination among the different cell sources of TGF-β mRNA (fibroblasts, epithelial cells, lymphocytes). This reason might also explain the increased TGF-β bioactivity detected in sonicated IBD colonic biopsies when compared to control biopsies observed in one of the above-mentioned studies [29].
A somewhat different scenario is present in UC where IFN-γ production is comparable to controls and there is an increased production of IL-5 [13]. While differentiation of naive CD4+ cells into TGF-β producing cells is favoured under priming conditions promoting induction of Th 2 responses, TGF-β can control Th2 differentiation through the inhibition of the transcription factor GATA-3 expression [33,34]. GATA 3 controls Th-2-specific expression of the IL-5 gene [35], suggesting that TGF-β in UC may influence the production of IL-5. In oxazolone colitis, a Th-2 mediated murine experimental colitis [36]] we observed an increased production of TGF-β in the affected tissue. In this colitis, neutralization of TGF-β is associated with the extension of inflammatory lesions from the distal colon to the entire colon. It is therefore possible that, as in UC, TGF-β production may contribute to limit the extension of inflammation. However, although increased, TGF-β is unable to inhibit inflammation. In the report by Monteleone et al. [25], a defect in TGF-β signalling due to increased Smad7 expression has also been described in UC. However, putative factors involved in producing a relative defect of TGF-β production or signalling in UC are not easily recognizable. In conclusion, although the data reported in the present study do allow to ascribe a direct role of TGF-β in IBD, they do indicate that any hypothesis for a TGF-β defect in CD and UC must be quite different, as baseline levels in the two diseases are different. In addition, they suggest that the absolute defect in TGF-β production observed in CD and the inability of TGF-β, in spite of its increased levels to counteract inflammation in UC, may contribute in both diseases to the perpetuation of inflammation.
Acknowledgments
This work was supported partially by the Research Project ‘Malattie infiammatorie croniche intestinali ed autoimmuni – componenti immunoregolatorie della mucosa nella patogenesi e prevenzione’ art 12 del D.L. gs 502/1992.
REFERENCES
- 1.Khalil Nasreen TGF-β: from latent to active. Microbes Infect. 1999;1:1255–63. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 2.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 3.Gorelik L, Flavell RA. Transforming growth factor-β in T cell biology. Nature Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 4.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 5.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998;81:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 6.Fukaura H, Kent SC, Pietrusewicz MJ, Koury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neurath M, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β-mediated oral tolerance. J Exp Med. 1996;183:2605–16. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo UY, Proctor IE, Macpherson AJS. CD4+ T cell down-regulation in human intestinal mucosa. J Immunol. 1997;158:3626–34. [PubMed] [Google Scholar]
- 10.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Bushenfelde KH. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–8. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 14.Seder RA, Marth T, Sieve MC, et al. Factors involved in the differentiation of TGF-β-producing cells from naive CD4+ T cells: IL-4 and IFN-γ have opposing effects, while TGF-β positively regulates its own production. J Immunol. 1998;160:5719–28. [PubMed] [Google Scholar]
- 15.Fais S, Capobianchi M, Pallone F, et al. Spontaneous release of interferon γ by intestinal lamina propria lymphocytes in Crohn's disease: kinetics of in vitro response to interferon γ inducers. Gut. 1991;32:403–7. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breese E, Braegger CP, Corrigan CJ, Walker-Smith JA, MacDonald TT. Interleukin-2 and interferon-γ secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78:127–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Monteleone G, Biancone L, Marasco R, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–78. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 18.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and IL-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Kohyama M, Sugahara D, Hosokawa H, Kubo M, Hozumi N. IL-4 mediated development of TGF-β1-producing cells from naïve CD4+ T cells through a STAT6-independent mechanism. Eur J Immunol. 2001;31:3659–66. doi: 10.1002/1521-4141(200112)31:12<3659::aid-immu3659>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson KA, Aung H, Toossi Z. Modulation of transforming growth factor β-1 gene expression by IL-12. Scand J Immunol. 2000;52:271–7. doi: 10.1046/j.1365-3083.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi H, S.Abdollah Y, Qiu J, et al. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–73. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 22.Ulloa L, Doody J, Massaguè J. Inhibition of transforming growth factor-β/SMAD signaling by the interferon-γ/STAT pathway. Nature. 1999;397:710–3. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 23.Blanchette F, Day R, Dong W, Laprise MH, Dubois CM. TGFβ1 regulates gene expression of its own converting enzyme furin. J Clin Invest. 1997;99:1974–83. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchette F, Rudd P, Grondin F, Attisano L, Dubois CM. Involvement of Smads in TGF-β1-induced furin (fur) transcription. J Cell Physiol. 2001;188:264–73. doi: 10.1002/jcp.1116. [DOI] [PubMed] [Google Scholar]
- 25.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–9. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitzer M, von Gersdoff G, Liang D, et al. A mechanism of suppression of TGF-β/SMAD signaling by NF-kB/RelA. Genes Dev. 2000;14:187–97. [PMC free article] [PubMed] [Google Scholar]
- 27.Strober W, Kelsall B, Fuss I, et al. Reciprocal IFNγ and TGFβ responses regulate the occurrence of mucosa inflammation. Immunol Today. 1997;18:61–4. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 28.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-β secretion and T cell apoptosis. J Immunol. 1996;157:2348–57. [PubMed] [Google Scholar]
- 29.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors α and β in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–84. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 30.di Mola FF, Friess H, Schreuren A, et al. Transforming growth factor-βs and their signaling receptors are coexpressed in Crohn's disease. Ann Surg. 1999;229:67–75. doi: 10.1097/00000658-199901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xian CJ, Xu X, Mardell CE, et al. Site-specific changes in transforming growth factor-α and -β1 expression in colonic mucosa of adolescents with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:591–600. doi: 10.1080/003655299750026056. [DOI] [PubMed] [Google Scholar]
- 32.Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor β1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–24. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- 33.Gorelik L, Fields PE, Flavell RA. Cutting edge. TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–7. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 34.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 35.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 36.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis. a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–39. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]