Abstract
Ankylosing spondylitis (AS) is an inflammatory systemic disease affecting the spine, sacroiliacal and peripheral joints. Although the aetiology of AS remains unknown, the strong association with the HLA-B27 allele might reflect directly a detrimental effect of the HLA-B27 molecule itself, resulting from its potential capability to present ‘arthritogenic’ peptides to CD8+ T cells. Because some forms of SpA are triggered by enterobacterial infection, such arthritogenic peptides might originate from autologous and/or bacterial proteins triggering cross-reactive CD8+ T cell clones. Intriguingly, two peptides from the second extracellular domain of HLA-B*2705 share sequence homologies with several enterobacterial antigens, exhibit the HLA-B27-binding-motif, and are presented by HLA-B*2705 itself. The objective of this study was to examine the clonal T cell reactivity against these peptides in patients with AS. To this end, we screened peripheral blood lymphocytes (PBL) of 26 patients with AS and 24 healthy donors for TNF-α-producing cells using ELISPOT assays. PBL and synovial fluid-derived lymphocytes (SFL) of peptide-responsive patients were then stimulated and cultured with the relevant peptide and control peptides in vitro. Antigen-specific T cell lines (TCL) were identified by standard chromium release assays. Clonal analysis was performed subsequently applying TCRB-CDR3 spectratyping. Among eight peptides tested, only the HLA-B27 168–176 peptide LRRYLENGK was recognized by PBL from B27+ AS patients but not from B27+ healthy controls (P = 0·001). LRRYLENGK-specific T cell clones used preferentially the TCRBV5S1 and the BV14 segment. These results suggest that an HLA-B27-derived peptide with homology to bacterial peptides may play a role in AS.
Keywords: arthritogenic peptide, spondyloarthropathies, TCRBV-CDR3 spectratyping
INTRODUCTION
Ankylosing spondylitis (AS), acute reactive arthritis (ReA), Reiter's syndrome (RS) and spondyloarthropathy (SpA) in association with chronic inflammatory bowel disease (IBD) constitute a group of inflammatory rheumatic diseases that show a strong association with the human leucocyte antigen (HLA)-B27, but this association and the actual role of HLA-B27 in the disease pathogenesis is not understood. However, a current hypothesis attempting to explain chronicity and autoimmunity in AS and other SpA proposes that peptides derived from the HLA-B27 heavy chain itself could be presented by MHC molecules and become targets for autoreactive CD4, CD8 or γδ T cells [1]. It has been demonstrated that peripheral blood mononuclear cells (PBMC) of patients with uveitis but not from healthy controls reacted against an HLA-B27-derived peptide (B27PD) [2]. In a recent study on rats it was demonstrated that peptides derived from HLA-B27 were cross-recognized with cytokeratin-derived peptides and that immunization with such peptides induced arthritis [3]. In a previous study, we showed that a 13mer peptide from the HLA-B27 heavy chain (B27PA) induced significant proliferative responses in 17 of 55 AS patients, and that γδ T cells were the major population stimulated and expanded after in vitro stimulation with the indicated peptide [4].
Although it is clear that B27 is the predominant predisposing genetic factor for AS, other genetic and environmental factors are probably involved [5]. From analogous aetiopathogenic concepts in enterogenic reactive arthritis it is evident that certain enterobacteria are important in the pathogenesis of this group of SpA, possibly by triggering detrimental T cell responses. A similar scenario might exist in AS, where a defective first line of defence against Klebsiella pneumoniae was reported that resulted eventually in a decreased peripheral T cell response to this microbe [6]. In contrast, T cells with specificity for Klebsiella and other enterobacterial antigens were found to accumulate in the joints of AS patients and could be cloned from synovial fluids of AS patients [6]. Studies of peripheral blood T cell responses to different bacteria, including K. pneumoniae from monocygotic twin pairs concordant or discordant for AS using interferon (IFN)-γ ELISPOT assays, led to similar results [7]. Analysis of the T cell receptor (TCR) β repertoire using CDR3 spectratyping in these twin pairs demonstrated that AS was associated with increased T cell oligoclonality in both CD8+ and CD4+ T cell subsets, indicating a role of conventional T cell antigens in AS pathogenesis [8].
One current hypothesis, the so-called ‘arthritogenic peptide model’ [9] links the spondyloarthropathies to HLA-B27, preceding bacterial infections and CD8+ T cells. It proposes that HLA-B27 serves as restriction molecule for antigenic peptides derived from bacterial proteins and/or structurally related self-peptides that would be presented to and cross-recognized by cytotoxic CD8+ T lymphocytes. Supporting this theory, HLA-B27-restricted CD8+ CTL clones with specificity for bacteria or autoantigens were detected in the synovial fluid and the peripheral blood of patients with ReA or AS [10].
While potentially autoantigenic and arthritogenic peptides are still unknown, nonapeptides from the second extracellular domain of the HLA-B*2705 that share the B27 binding motif and display sequence homology with both enterobacterial antigens and the HLA-B27 heavy chain itself were identified previously by a systematic sequence-database analysis [11]. One of these nonapeptides that was derived from the 3rd hypervariable region of the HLA-B27 molecule (LRRYLENGK, HLA-B27 168–176) was demonstrated to bind to HLA-B*2705 in vitro [12]. Interestingly, it showed sequence homologies to different bacterial antigens derived from Escherichia coli, Pseudomonas aeruginosa and Bacillus megaterium. Boisgérault et al. [13] eluted endogenous peptides from the human C1R cell line transfected with B*2705. One of these peptides (RRYLENGKETL) was also HLA-B27-derived (amino acids 169–179) and overlapped with the LRRYLENGK peptide in eight amino acids, indicating that potentially cross-recognized peptides are in fact presented by HLA-B27.
Because current data on potentially autoantigenic peptides with the HLA-B*2705 binding motif (LRRYLENGK and RRYLENGKETL) are merely theoretically deduced or based on in vitro experiments, the primary aim of this study was to assess whether CD8+ T cells with specificity for such peptides are detectable in patients with AS. To this end, we screened a significant number of patients with regard to precursor frequencies of CD8+ T cells specific for B27-derived peptides ex vivo applying a sensitive ELISPOT assay. Due to the potentially harmful role of cross-reactive T cells, it was of further interest to identify and characterize such cells on the clonal level, eventually to define clonotypic target structures of possible therapeutic value. We therefore generated peptide-specific T cell lines (TCL) from PBMC and synovial fluid mononuclear cells (SFMC) of peptide-responsive AS patients and analysed the clonal complexity of these TCL at different time-points applying T cell receptor-CDR3 size analysis (CDR3 spectratyping).
MATERIALS AND METHODS
Patients and healthy donors
PB and/or SFs were obtained from 26 patients with AS (25 HLA-B27+, 1 HLA-B27–; 24 males and two females; median age: 45·0 years, range: 19–62 years) and 24 healthy donors (20 HLA-B27+, 4 HLA-B27–; median age: 58·0 years, range: 25–88 years). All AS patients were seen by a rheumatologist (E. M.-H) and met the criteria of clinically active disease, such as inflammatory-type back pain requiring treatment with non-steroidal anti-inflammatory drugs, morning stiffness of ≥45 min duration and/or active peripheral arthritis. None of the patients received steroids or disease-modifying antirheumatic drugs. The healthy controls were slightly older than the patients to reduce the likelihood of developing AS later in life.
Preparation of mononuclear cells
PBMC and/or SFMC were isolated from heparinized peripheral blood or synovial fluids by Ficoll-Hypaque (Seromed Biochrom, Berlin, Germany) density centrifugation according to standard protocols.
Synthetic peptides and in vitro binding assay
Peptides with known binding motif for the HLA-B*2705 molecule were synthesized by the Department of Immunohaematology and Blood Bank, Leiden University Medical Center (the Netherlands). The peptides used in the TNF-α ELISPOT assay were nonapeptides derived from self proteins and peptides derived from bacterial or viral proteins (Table 1). In vitro binding assays were performed on transporter associated with antigen processing (TAP)-deficient T2 cells transfected with HLA-B*2705 according to the method described by Houbiers et al. [14]. Untransfected T2 cells (with low constitutive expression of HLA-A2) served as controls. Aliquots of 5 × 105 T2-B*2705 were incubated overnight at 37°C with 100 µg/ml of synthetic peptides and 10 µg/ml β2-microglobulin in RPMI-1640 supplemented with 1% penicillin/streptomycin and 1% l-glutamine. The HLA-B27 molecules stabilized at the cell surface were assessed by indirect fluorescence. Cells were incubated with 100 µl ME-1 monnoclonal antibody (MoAb) [supernatant 1 : 100 in phosphate buffered saline (PBS)] for 30 min on ice, washed in PBS and then stained with phycoerythrin (PE)-conjugated F(ab′)2 goat antimouse immunoglobulins for 30 min on ice. After washing twice, each sample was analysed on a logarithmic scale using a FACScan (Becton-Dickinson, Mountain View, CA, USA). The results were expressed as fluorescence ratio (FR) calculated by the formula: FR = mean fluorescence experimental sample/mean fluorescence background (cells without peptide stimulation). Cells incubated with an HLA-A3 binding peptide (kind gift from T. Wölfel III. Department of Medicine, Johannes Gutenberg-Universität Mainz, Mainz, Germany) (KIFSEVTPK) [15] served as control. A fluorescence ratio above 1·2 was defined as a cut-off for significant peptide binding. Experimental sample mean fluorescence was higher than background mean fluorescence plus two standard deviations. Synthetic peptide binding was tested in at least three separate assays and was highly reproducible.
Table 1.
Mean fluorescence (MF), standard deviation (s.d.) and fluorescence ratio (FR) of the peptide binding assays
| Peptides and protein source | Sequences | MFa | s.d.a | FRa | MFb | FRb |
|---|---|---|---|---|---|---|
| Influenza NP 383–391 | SRYWAIRTR | 25·88 | 2·04 | 1·52 | 16·45 | 0·97 |
| HIV-gp 120 | RIQRGSGRAFVTIGK | 23·54 | 2·36 | 1·46 | 14·29 | 0·8 |
| Human histon H3·3 | RRYQKSTEL | 22·23 | 1·97 | 1·38 | 14·84 | 0·87 |
| Yers. urease β 153–161 | RRAAERGFK | 23·62 | – | 1·47 | 14·47 | 0·85 |
| Chlamydien Hsp57 | RRKAMFEDI | 22·27 | 2·61 | 1·39 | 15·98 | 0·94 |
| HLA-B27 168–176 | LRRYLENGK | 22·82 | 1·36 | 1·48 | 13·76 | 0·81 |
| HLA-B27 169–179 | RRYLENGKETL | 22·30 | 0·06 | 1·39 | 15·95 | 0·94 |
| Human Hsp60 | RRGVMLAVD | 23·37 | 0·33 | 1·44 | 16·09 | 0·95 |
| HLA-A3 binding control peptide | KIFSEVTPK | 15·63 | 0·63 | 1·0 | 13·69 | 0·80 |
T2-B*2705 cells incubated with peptides
T2 cells incubated with peptides. Mean fluorescence background (T2-B*2705/T2 cells without peptide): 16·07/17·01.
Enzyme-linked immunospot assay (ELISPOT) for TNF-α secreting CD8+ T cells
To obtain pure CD8+ T cell populations, 40–45 × 106 PBMC and/or SFMC from AS patients and healthy blood donors were incubated with anti-CD8 conjugated magnetic MicroBeads (MiniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany) and separated according to the manufacturer's recommendations. Purity was >93% as determined by FACS analysis using an anti-CD8 antibody reagent (Coulter Immunotech, Hamburg, Germany).
Peptide-specific CD8+ T cell responses were quantified by ELISPOT assays as described by Herr et al. [16]. All determinations were performed in triplicate. In brief, 96-well filtration plates (Millipore Corp., Bedford, MA, USA) were coated with antihuman TNF-α capture MoAb (clone 195 Roche, Mannheim, Germany), and 1 × 105 responder cells were seeded into the wells along with 0·5 × 105 irradiated feeder cells (T2 transfected with B*2705) with 20 µg/ml β2M. Cells were incubated for 40 h either in the presence or absence of peptide (200 µg/ml) or PHA (1 µg/ml). After washing with PBS/0·05% Tween20, the plates were incubated for 2 h at 37°C with 100 µl of rabbit polyclonal antihuman TNF-α antibody (Serotec/Biozol Diagnostica, Oxford, UK) diluted at 1 : 250 in PBS/0·5% BSA. After washing, biotinylated antirabbit IgG F(ab′)2 fragments (Roche) were added for 2 h at 37°C. After another washing step spots were visualized using freshly prepared substrate buffer [0·03% (wt/vol) 3-amino-9-ethyl-carbazole, 0·015% (v/v) H2O2 in 0·1 m sodium acetate, pH 5] and were counted by computer-assisted image analysis (Quantimet 600 S, Leica, Wetzlar, Germany).
Statistical analysis
Three experiments per patient/normal donor were performed, and the mean numbers of these triplicates were indicated as spot-forming cells (SFC) per 1 × 105 cells for each person. Spot counts were compiled on a Microsoft Excel spreadsheet and means and standard deviations were calculated. To calculate the number of antigen-responsive T cells per 1 × 105 CD8+ T lymphocytes, the mean numbers of spots induced by T2-B*2705 alone (background) were subtracted from mean spot numbers induced by antigen-loaded T2-B*2705. For statistical evaluation, the t-test for unpaired samples was used. Values of P < 0·01 were considered significant.
Generation of peptide-specific T cell lines
SFMC and/or PBMC of patients who had been responsive in the ELISPOT assay were used to generate peptide specific T cell lines; 1 × 105 PBMC and/or SFMC were incubated with 50 µg/ml of the indicated peptide in one well of a 96-well U-plate (Greiner, Nürtingen, Germany) in a total volume of 150 µl RPMI/10%HUS supplemented 1% l-glutamine, 1% penicillin/streptomycin. Medium was changed on day 3 to rIL-2 (20 U/ml). The cell lines were then expanded and fed with rIL-2 (20 U/ml) every other day. After 8–12 days the cell lines were specifically restimulated with 1 × 105 irradiated autologous PBMC and 50 µg/ml of the indicated peptide. After a second specific restimulation, the specificity of the cell lines was tested in a standard chromium release assay.
51Chromium (51Cr) release assay
SFMC- and PBMC-derived CTL were characterized functionally in a standard 4 h 51Cr release cytotoxicity assay [10]. Peptide pulsed target cells (T2, T2-B*2705, C1R-B*2705) were labelled with Na251CrO4 (Amersham Pharmacia Biotech, Freiburg, Germany) at 37°C for 1 h. Target cells without peptides served as controls. After washing, 51Cr-labelled target cells were incubated with CTL at different E : T ratios in 96-well round-bottomed plates. After 4 h of incubation at 37°C, supernatants were collected and released radioactivity was measured in a scintillation counter (Wallac, Turku, Finland). Percentage of specific lysis was calculated as: 100 × [(release by CTL – spontaneous release)/(maximal release – spontaneous release)]. Maximal release was determined by the addition of 1% Triton X-100 (Sigma, Deisenhofen, Germany). The spontaneous release, in the absence of CTL, was generally <15% of the maximum release. All samples were prepared and measured in triplicate.
RNA and reverse transcription
Total cellular RNA was extracted from 0·5 to 2·0 × 106 T cells using a protocol from Qiagen (RNeasyTM RNA extraction kit, Qiagen, Hilden, Germany). One µg of RNA from each sample was reverse-transcribed to first-strand cDNA in a volume of 75 µl reaction mixture containing 3 µl of oligo (dT)16 0·5 mg/ml (Sigma), 60 U of M-MLV reverse transcriptase (Gibco BRL, Eggenstein, Germany), 40 µ of RNasin (Promega, Madison, USA) and dATP, dTTP, dGTP, dCTP (Roche) at 125 µm each in 50 mm Tris-HCL pH 8·3, 75 mm KCL, 3 mm MgCl2, 10 mm DTT (Gibco BRL). Samples were diluted in 14 µl double-distilled water and heated at 65°C for 10 min. Next, reverse transcription (RT) reagent mixture was added. RT was carried out at 39°C for 1 h after which samples were heated at 65°C for 5–10 min to terminate reverse transcription.
Polymerase chain reaction (PCR)
To identify selectively rearranged TCRBV families in cDNA from T cells we used a panel of 26 5′-primers specific for 24 different BV families [17,18] together with a 3′-primer specific for the two constant loci TCRBC1 and BC2 [17]. All TCRBV family gene specific primers were tested for productive amplification on cDNA derived from control PBMC. Five µl of normalized cDNA (diluted 1 : 3) was added to each of the 26 reaction tubes containing the PCR mix (45 µl). For CDR3 spectratyping of unstimulated or stimulated T cells (see below), we adopted a multiplex PCR strategy using a non-radioactive method for T cell receptor CDR3 length analysis [19]. PCR reaction mixture consisted of 1 × PCR buffer (Roche), 12·5 pmol each of the 5′-variable and 3′-constant primers, 1·5 mm MgCl2, 0·1 mm each of dATP, dCTP, dGTP and dTTP (Roche) and 1·25 U Taq polymerase (Perkin Elmer/Applied Biosystems, Weiterstadt, Germany). Amplification of cDNA was carried out in a thermal cycler (Hybaid Omni-Gene, Middlesex, UK) at 35–38 sequential cycles at 94°C for 45 s, 60°C for 45 s and 72°C for 45 s. PCR products were then separated on a 2% agarose gel (Biozym, Hameln, Germany) and visualized by ethidium bromide staining.
CDR3 spectratyping
Detection of clonal expansions was performed by CDR3 length analysis, as described elsewhere [20,21]. In brief, amplified BC-BV PCR-products were separated (3h15′ 40 mA) on denaturing sequencing gels (6% polyacrylamid gel) using a modified sequencing protocol from Promega (Silver Sequence TM; Promega).
After fixing with 10% glacial acetic acid and silver staining, gels were developed with sodium carbonate (Na2CO3). After drying overnight, gels were documented using positive contact films (Typopaque TRDO; Promega).
RESULTS
Peptide binding assay
The eight potentially binding peptides as well as one control peptide were tested for actual binding to T2-B*2705. Strong binding to HLA-B*2705 have been shown previously in other binding tests for the influenza NP 383–391 peptide [22] and the HIV-gp 120 peptide [23]. The fluorescence ratio (FR) of each peptide was greater than 1·2 with the exception of the HLA-A3 binding control peptide. None of the peptides bound to the untransfected T2 line (Table 1).
Analysis of the CD8+ T cell response to synthetic peptides using a TNF-α ELISPOT assay
CD8+ T cells from PBL of 20 HLA-B27+ healthy donors, 25 HLA-B27+ patients with AS, four HLA-B27– healthy controls and one HLA-B27– patient with AS were analysed in a TNF-α ELISPOT assay for reactivity against the peptides with known binding to HLA-B*2705 (Table 1). T2-HLA-B*2705 served as a peptide-presenting cell line. T2-B*2705 cells without peptide loading served as a negative control. Maximum stimulation of T cells was achieved by incubation with PHA. Because none of the donors was HIV positive, HIV-gp 120-peptide was considered to be a negative control regarding the ex vivo immune response. Due to the high prevalence of anti-influenza immune responses in the population, reactivity to the influenza NP 383–391 peptide was considered a positive control.
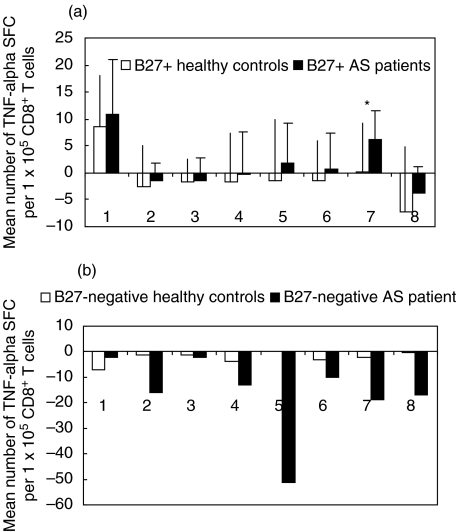
Figure 1a shows the mean number of TNF-α spot-forming cells (SFC) per 1 × 105 CD8+ T cells in response to the different peptides tested in B27+ AS patients and B27+ healthy controls. Both AS patients and healthy controls exhibited remarkable mean precursor frequencies of influenza NP-specific CD8+ T cells (9 per 1 × 105 CD8+ T cells in healthy controls versus 11 per 1 × 105 in AS patients). T cell responses against HIV-gp 120 were not observed in any individual. Although the CD8+ T cell responses against the two bacteria-derived antigens (Yersinia RRAAERGFK and Chlamydia RRKAMFEDI) were higher in AS patients than in controls, there was no statistically significant difference between patients and healthy controls (P = 0·26 and P = 0·35) (Fig. 1a).
Fig. 1.
Frequencies of HLA-B27-restricted CD8+ T cells with specificity for different B*2705-binding synthetic peptides indicate differential reactivity against the HLA-B27 derived nonapeptide (168–176) in HLA-B27+ AS patients and HLA-B27+ healthy controls (*P < 0·01). Mean numbers of spot-forming cells (SFC) in TNF-α ELISPOT assay of (a) 25 HLA-B27+ AS patients and 20 HLA-B27+ healthy controls, and (b) of one HLA-B27– AS patient and four HLA-B27– healthy donors are given. Peptide antigens used: SRYWAIRTR, Inf NP 383–391 (1), RIQRGSGRAFVTIGK, HIV-gp 120 (2), RRYQKSTEL, human histon H3·3 (3), RRGVMLAVD, human Hsp 60 (4), RRAAERGFK, Yers. Urease β 153–161 (5), RRKAMFEDI, Chlam. Hsp 57 (6), LRRYLENGK HLA-B27 168–176 (7), RRYLENGKETL, HLA-B27 169–179 (8). All experiments were performed in triplicate. Mean numbers of these triplicates are given as SFC per 1 × 105 cells for each patient or normal donor. Mean numbers of spots induced by T2-B*2705 alone (background) were subtracted from mean spot numbers induced by antigen-loaded T2-B*2705. ‘Negative’ responses indicate spot numbers lower than background.
We were unable to detect T cells with specificity for any of the B27-binding peptides in any of the five HLA-B27-negative individuals (one AS patient and four healthy controls). In these individuals, mean numbers of TNF-α spot-forming cells were equal to or lower than background (Fig. 1b).
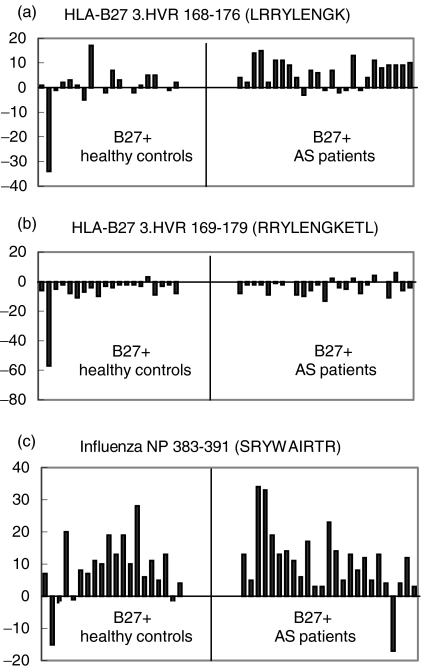
Among all peptides tested, only peptide LRRYLENGK, a peptide derived from the 3rd hypervariable region of some MHC class I molecules, including HLA-B27, was recognized differently in the group of AS patients as compared to the group of HLA-B27+ control donors (a significantly higher number of LRRYLENGK-specific precursor frequencies in AS patients, P = 0·007, Fig. 1a). The frequencies of TNF-α spot-forming lymphocytes in AS patients ranged between −3 and 15 per 1 × 105 CD8+ T cells (Fig. 2a).
Fig. 2.
T cell reactivity against LRRYLENGK but not against RRYLENGKETL exists in patients with AS. Results from TNF-α ELISPOT assays from individual donors (mean numbers of TNF-α spot forming CD8+ T cells) for the nonapeptide LRRYLENGK (a), the 11-mer RRYLENGKETL (b) and the Inf NP 383–391 peptide SRYWAIRTR (c). All experiments were performed in triplicate. Mean numbers of these triplicates are given as SFC per 1 × 105 cells for each patient or normal donor. Mean numbers of spots induced by T2-B*2705 alone (background) were subtracted from mean spot numbers induced by antigen-loaded T2-B*2705. ‘Negative’ responses indicate spot numbers lower than background.
Of note, no response was observed against the naturally processed endogenous B27-derived 11-mer RRYLENGKETL in most of the patients (Fig. 2b). In both patients and controls, the mean precursor frequencies against this peptide were lower than background (‘negative’ responses) (Fig. 1a).
With few exceptions, the influenza NP 383–391 (positive control) was recognized from both AS patients and healthy controls. The mean numbers of SRYWAIRTR specific SFC for AS patients ranged between 17 spots below (‘negative’ response) and 34 spots above background (Fig. 2c).
Peptide-specific T cell lines
We next established antigen-specific HLA-B*2705-restricted TCL from PBMC and/or SFMC of those patients who had shown CD8+ T cells responses against the LRRYLENGK peptide in the ELISPOT assay. PBMC or SFMC were stimulated with the indicated peptide as outlined in Materials and methods. A T2 cell line transfected with HLA-B*2705 (T2-B*2705) and a C1R cell line transfected with HLA-B*2705 (C1R-B*2705) served as target cell lines in a standard 51Cr release assay.
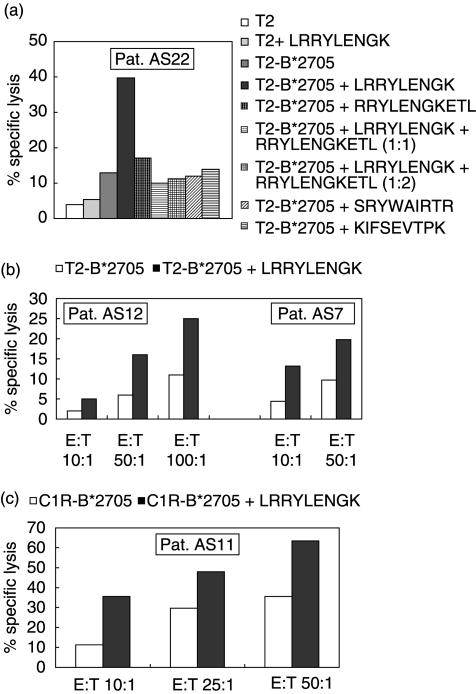
Cytotoxicity of one HLA-B27-restricted peptide-specific CTL line derived from PBMC of patient AS22 is shown in Fig. 3a. Here we tested the reactivity against both the nonamer (LRRYLENGK) and the 11-mer (RRYLENGKETL) peptide of the 3rd hypervariable region of the B27 molecule. Marked specific lysis of LRRYLENGK pulsed T2-B*2705 was observed at an E : T-ratio of 50 : 1. In contrast, T2-B*2705 alone or pulsed with RRYLENGKETL or other control peptides (SRYWAIRTR, KIFSEVTPK), T2 alone or T2 pulsed with LRRYLENGK were not recognized by this CTL line. Notably, LRRYLENGK specific lysis was completely blocked when the LRRYLENGK pulsed targets were co-incubated with the 11-mer RRYLENGKETL at two different ratios (Fig. 3a).
Fig. 3.
HLA-B27-restricted CTL lines with specificity for the HLA-B*2705 derived nonapeptide LRRYLENGK can be obtained from AS patients. (a) Specific lysis of T2-B*2705 target cells pulsed with the peptide LRRYLENGK, RRYLENGKETL and two control peptides by an HLA-B27-restricted peptide specific CTL line generated from PBMC of patient AS22. T2-B*2705 cells pulsed with control peptides (influenza NP 383–391 (SRYWAIRTR) and an HLA-A3 binding peptide (KIFSEVTPK)) were not killed. T2 target cells pulsed with LRRYLENGK, T2 alone and T2-B*2705 alone served as controls. (b) Specific lysis of T2-B*2705 target cells with and without peptides by two HLA-B27 restricted peptide specific CTL lines generated from PBMC of patient AS12 and SFMC of patient AS7. (c) Specific lysis of C1R-B*2705 target cells with and without peptides by an HLA-B27 restricted CTL line generated from PBMC of patient AS11. All experiments were performed in triplicate.
Two other patients, AS12 and AS7, showed moderate cytotoxicity against T2-B*2705 pulsed with peptide LRRYLENGK (Fig. 3b). Specific lysis of CTL line of patient AS12 ranged from 10% to 14% over background at an E : T ratio of 50 : 1 and 100 : 1. Ten per cent specific lysis was observed with a CTL line of patient AS7 at each E : T ratio.
Cytotoxicity of an HLA-B27-restricted peptide specific CTL line derived from PBMC of patient AS11 is shown in Fig. 3c. In this case C1R-B*2705 served as the target cell line. C1R-B*2705 cells pulsed with peptide LRRYLENGK were lysed effectively by this CTL line at each E : T ratio tested.
TCRB-CDR3 size spectratyping
To analyse further the peptide-specific T cell response on a clonal level, we performed TCRB-CDR3 size analyses on the respective TCL.
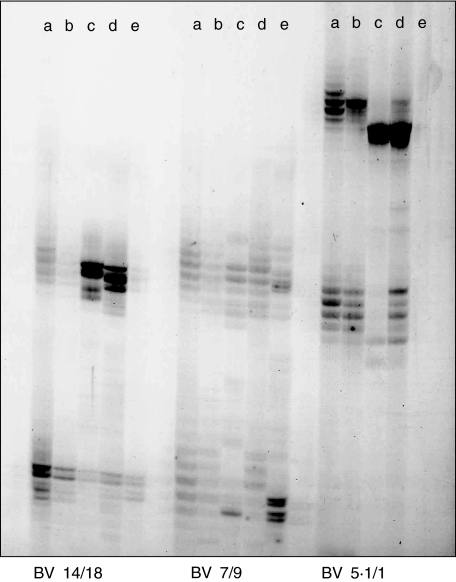
From patient AS7 we analysed freshly isolated SFMC, SFMC stimulated with PHA, SFMC stimulated with LRRYLENGK + rIL2, SFMC after two rounds of stimulation with LRRYLENGK + rIL2 and SFMC cultured with rIL-2 alone (Fig. 4, lanes a–e). CDR3 spectratyping revealed band intensities from freshly isolated cells that were distributed normally and typical for polyclonal T cell populations. These patterns remained largely unaltered under stimulation with the polyclonal T cell activator PHA. In contrast, remarkable alterations of CDR3 band patterns that generally indicate oligo- or monoclonal T cell expansions were found in LRRYLENGK-stimulated TCL. In this patient, T cell expansions were confined to the BV5S1 and BV14. rIL-2 alone had surprisingly little effect, and in no case expanded CDR3 bands identical to those that were induced by the peptide (Fig. 4, lane e). This finding suggested that clonal T cell expansions were elicited directly by the peptide and were not reflecting bystander activation of IL-2 responsive T cells.
Fig. 4.
TCRB-CDR3 size analysis indicates oligoclonal T cell expansions induced by the HLA-B27-derived nonamer peptide LRRYLENGK in SFMC from patient AS7. Lanes (a) freshly isolated SFMC, (b) SFMC + PHA, (c) SFMC + peptide + rIL-2, (d) SFMC + peptide + rIL-2 after restimulation with peptide + rIL-2 and (e) SFMC + rIL-2. BV families are indicated. Where two BV gene-specific PCR reactions were run in one tube (multiplex PCR), the BV number before the slash indicates the longer PCR product. Peptide-induced T cell expansions are confined to BV14 and BV5·1. Other BV families analysed were not or only moderately altered (additional polyclonal BV families are not shown).
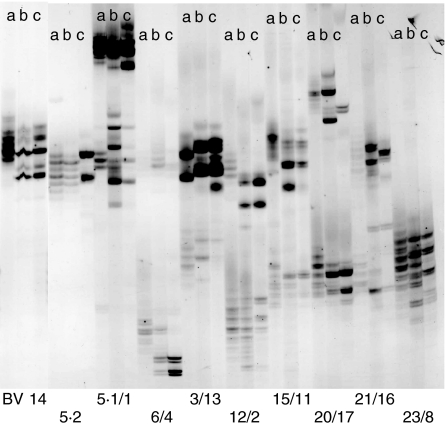
From a second patient (AS12) we analysed freshly isolated PBL, PBL stimulated with peptide LRRYLENGK + rIL-2, and PBL after two rounds of stimulation with the same peptide + rIL-2 (Fig. 5, lanes a–c). In this case, the T cell expanding effect of the peptide was even greater than in the TCL from patient AS7 as peptide-induced band-expansion appeared in significantly more BV families. Notably, as in patient AS7, BV5S1 and BV14 were also affected. Other altered BV-families were BV5S2, 1, 4, 12, 15, 20 and 17. Some T cell expansions associated with peptide stimulation were stable over time, i.e. they were detected after both rounds of stimulation (BV 14 and 17). Other strong expansions were present only transiently after the first stimulation, for instance in BV20, whereas expansions belonging to a third group were only weakly present after the first stimulation, but were enhanced strongly after the second round of stimulation (e.g. in BV4 and 12).
Fig. 5.
TCRB-CDR3 size analysis indicates strong oligoclonal T cell expansions induced by the HLA-B27-derived nonamer peptide LRRYLENGK in PBMC from patient AS12. Lanes (a) freshly isolated PBMC, (b) PBMC stimulated with 50 µg/ml LRRYLENGK + rIL-2 and (c) PBMC restimulated with LRRYLENGK and irradiated autologous PBMC + rIL-2.
TCL from seven other AS patients were tested in the same way. Oligoclonal expansions were observed predominately in the BV families 5S1 (n = 7) and 14 (n = 6) of the stimulated TLC. Only three patients showed expansions in the BV5S1 family of the unstimulated T cell population (data not shown).
DISCUSSION
HLA-B27 may bind peptides that are derived from degraded HLA-B27 itself. The fact that such peptides share structural similarities to enterobacterial peptides offers a tempting hypothesis for the understanding of the spondyloarthropathies. As a variation of the molecular mimicry hypothesis, bacteria-derived peptides might be presented to and cross-recognized by CD8+ T cells which would then fuel an ongoing HLA-B27-dependent autoimmunity, thus explaining the strikingly strong association of the SpA with both HLA-B27 and enterobacterial infection.
To address this problem directly, we ask here whether patients with AS, a form of SpA, where no bacterial trigger is yet known, harbour HLA-B27-restricted T cell clones with specificity for such HLA-B27-derived peptides.
We used TNF-α ELISPOT assays to characterize the CD8+ T cell response against HLA-B27-binding peptides derived from different bacterial proteins, and from the HLA-B*2705 molecule itself, comparing B27+ healthy donors and patients with AS. Peptide antigen from influenza NP that is known to bind efficiently to B27 served as a positive control and was recognized by most individuals from both groups, whereas a HIV-derived peptide with similar B27-binding capacity was not recognized by any (HIV-negative) individual tested. Among all other peptides tested only a nonapeptide from the 3rd hypervariable region of the HLA-B*2705 molecule (peptide LRRYLENGK) was recognized uniquely by CTL from AS patients, i.e. significantly higher LRRYLENGK specific precursor frequencies were measured in AS patients compared to HLA-B27+ control donors, P < 0·01 (Fig. 1a).
The mean number of peptide-responsive CD8+ T cells (e.g. for LRRYLENGK: range −3–15, mean 6·3 per 1 × 105 = 0·0063%) was low compared to a recently published study where the HLA-B27 restricted T cell response to a self-peptide from vasoactive intestinal peptide receptor 1 (VIP1R400-408) was analysed in patients with AS and B*2705-positive healthy controls [24]. Applying IFN-γ ELISPOT assays, the frequencies of peptide-responsive T cells in that study ranged between 100 and more than 4000 in 1 × 106 PBMC (approximately 0·01–0·4%). This difference may be explained by the fact that, in that study [24], T cell responses were evaluated after 10 days of culture including two rounds of in vitro stimulation with the peptide. Hence, T cell reactivity of in vitro-generated T cell lines was measured rather than precursor frequencies ex vivo. In contrast, the data presented here were yielded ex vivo and may represent appropriately the number of peptide-reactive CD8+ T cells in AS patients [16].
A high mean number of background spots was detected in both AS patients and healthy controls (data not shown). This is a frequently observed phenomenon in ELISPOT assays [25]. Nevertheless, LRRYLENGK-specific SFC in AS patients were 16·7% above background (versus 0·2% in B27+ healthy donors).
In one healthy individual (HC2), spot numbers after stimulation with peptide LRRYLENGK were markedly lower than background (40%) (Fig. 2a). The potentially skewing effect of that individual prompted us to recalculate the statistical analyses after its removal from the analysis. The resulting difference of the frequency of LRRYLENGK-responder T cells in AS patients and B27+ healthy controls remained statistically highly significant (P = 0·006, t-test for unpaired samples). Of note, in the present study the endogenously processed 11-mer derived from HLA-B*2705 (169–179) was not recognized by HLA-B27+ patients or controls (Figs 1a and 2b), although it binds efficiently to B*2705 and overlaps with the well-recognized nonamer HLA-B*2705 (168–176) in eight of nine residues. The number of TNF-α spot-forming CD8+ T cells after stimulation with the 11-mer was even lower than background (‘negative’ response) (Fig. 1a). The reason for this observation is not clear. One explanation might be that background reactivity of CD8+ T cells against T2-B*2705 cells reflects a response to free B*2705 heavy chain (HC) on the surface of T2-B*2705 cells. Abundance of this naturally processed 11-mer might stabilize correctly folded B27/β2M on the cell surface and therefore reduce the anti-HC response. In addition, this peptide might have a competitive or antagonistic effect on the LRRYLENGK peptide that is antigenic in AS patients. The finding that the LRRYLENGK-dependent T cell response can in vitro be inhibited by coincubation with RRYLENGKETL supports this notion (Fig. 3a).
Although direct evidence of arthritogenic bacterial or self peptide is still lacking, the presence of CTL specific for a nonamer peptide derived from the 3rd hypervariable region of the HLA-B27 molecule (LRRYLENGK) from PBMC or SFMC of patients with AS suggests that these CTL may be important in disease pathogenesis.
Recently, in addition to the previously reported 11-mer peptide (RRYLENGKETL, HLA-B27 169–179 [13]), a 13-mer B27-derived peptide (RRYLENGKETLQR, HLA-B27 169–181) was shown to be a natural ligand of B*2705 [26]. However, the LRRYLENGK nonamer or its N-terminal precursors were not generated by the 20S proteasome in vitro, as there was no cleavage after the Lys-176 residue. Accordingly, LRRYLENGK was not found as a natural ligand of HLA-B27. Notably, however, these analyses were performed in a cell line (C1R) transfected with the HLA-B*2705 molecule or other B27-subtypes but not with cells derived from patients or healthy controls, which besides the HLA-B27 are characterized by a genetically defined MHC haplotype.
It is important to note that this peptide (LRRYLENGK) is not unique to HLA-B27, but also exists in numerous other HLA-A and HLA-B alleles that are not associated with SpA. However, this does not necessarily speak against a role of this peptide in SpA but suggests rather that additional preconditions are to be met to induce the disease. Such might be (i) a MHC haplotype that allows presentation of this peptide to T cells; and (ii) according to the ‘arthritogenic peptide model’, this haplotype must also allow presentation of a microbial peptide that can be cross-recognized with the LRRYLENGK peptide. Failure to meet one or both of these prerequisites would consequently rescue these individuals from SpA.
CTL lines with specificity for the peptide LRRYLENGK could be established from PBMC and SFMC of four AS patients (Fig. 3) and were of great use to characterize further the nonamer-specific T cell response. CDR3 spectratyping of these lines demonstrated that this nonamer is a potent inducer of clonal T cell expansions and that such expanded T cell clones use repeatedly a limited set of TCRBV families (Figs 4 and 5).
Interestingly, a recent compiling analysis of all available HLA-B27- or SpA-related TCRB-CDR3 sequences argued strongly for a role of HLA-B27-restricted CD8+ T cells with specificity for an autologous determinant present in the synovium in early reactive arthritis [27]. The properties of the TCR involved were conserved strikingly between different ReA-patients and are therefore of potential diagnostic or therapeutic value. In vitro expanded B27-nonamer-specific T cells in this study did not use the same BV segments than the ReA-derived clones (BV1, BV23), and the number of TCRBV families used by those T cell clones varied between CTL lines from different patients. This finding is not unexpected, because the reported TCR motifs were highly specific for ReA, i.e. not present in patients with AS.
Interestingly, however, a small selection of BV families (BV5S1, 14 and 17) were used repeatedly by nonamer-expanded T cell clones in all CTL lines examined. These BV families match previous TCR studies from our group showing a preferential TCR BV14 and 17 usage in HLA-B27 restricted autoreactive or Yersinia-specific CD8+ T cells [28]. These data may therefore aid identification of pathogenetically relevant T cell clones and their corresponding antigens and may lay the basis to define more specific targets for future therapeutic interventions in AS. However, it should be noted that there were no obviously dominating bands detected in PBMC and/or SFMC ex vivo that corresponded to the peptide-induced bands. This suggests that, in vivo, LRRYLENGK-specific T cell clones are not, or only very subtly, expanded.
In summary, the data presented here provide evidence that CD8+ T cells with specificity for the B27-binding nonapeptide LRRYLENGK that is derived from the HLA-B27 heavy chain itself are present in the peripheral blood of AS patients, but not in healthy B27-positive individuals. As this autoantigenic peptide shows homology with peptides from P. aeruginosa, E. coli and Bacillus megaterium in five consecutive amino acids, such an antiself immune response might be initiated or perpetuated by an infection with enterobacteria. In consistency with the ‘arthritogenic peptide model’, our results therefore favour a role of autoreactive HLA-B27-restricted/HLA-B27-specific T cells in ankylosing spondylitis.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), SFB 548 (B4) to Elisabeth Märker-Hermann. The authors wish to thank Mrs Jutta Weber for technical assistance and Dr Ariane Kemkes-Grottenthaler (Institute of Anthropology, Johannes Gutenberg-University of Mainz) for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Baum H, Davies H, Peakman M. Molecular mimicry in the MHC:. hidden clues to autoimmunity? Immunol Today. 1996;17:64–70. doi: 10.1016/0167-5699(96)80581-0. [DOI] [PubMed] [Google Scholar]
- 2.Wildner G, Thurau SR. Cross-reactivity between an HLA-B27 derived peptide and a retinal autoantigen peptide: a clue to major histocompatibility complex association with autoimmune disease. Eur J Immunol. 1994;24:2579–85. doi: 10.1002/eji.1830241103. [DOI] [PubMed] [Google Scholar]
- 3.Wildner G, Diedrichs-Mohring M, Thurau SR. Induction of arthritis and uveitis in Lewis rats by antigenic mimicry of peptides from HLA-B27 and cytokeratin. Eur J Immunol. 2002;32:299–306. doi: 10.1002/1521-4141(200201)32:1<299::AID-IMMU299>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Märker-Hermann E, Meyer zum Büschenfelde KH, Wildner G. HLA-B27 derived peptides as autoantigens for T lymphocytes in ankylosing spondylitis. Arthritis Rheum. 1997;40:2047–54. doi: 10.1002/art.1780401118. [DOI] [PubMed] [Google Scholar]
- 5.Brown MA, Kennedy LG, MacGregor AJ, et al. Susceptibility to ankylosing spondylitis in twins. The role of genes, HLA, and the environment. Arthritis Rheum. 1997;40:1823–8. doi: 10.1002/art.1780401015. [DOI] [PubMed] [Google Scholar]
- 6.Hermann E, Sucké B, Droste U, Meyer zum Büschenfelde KH. Klebsiella pneumoniae reactive T cells in blood and synovial fluids of patients with ankylosing spondylitis: a comparison with HLA-B27+ healthy control subjects in a limiting dilution study and determination of the specifity of synovial fluid T cell clones. Arthritis Rheum. 1995;38:1277–82. doi: 10.1002/art.1780380916. [DOI] [PubMed] [Google Scholar]
- 7.Höhler T, Hug R, Schneider PM, et al. Ankylosing spondylitis in monocygotic twins: studies on immunological parameters. Ann Rheum Dis. 1999;58:435–40. doi: 10.1136/ard.58.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchmann R, Lambert C, May E, Höhler T, Märker-Hermann E. CD4+ and CD8+ clonal T cell expansions indicate a role of antigens in ankylosing spondylitis; a study in HLA-B27+ monozygotic twins. Clin Exp Immunol. 2001;123:315–22. doi: 10.1046/j.1365-2249.2001.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin R, Parham P. Guilt by association: HLA × B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–42. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 10.Hermann EYuDT, Meyer zum Büschenfelde KH, Fleischer B. HLA-B27 restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–50. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 11.Scofield RH, Warren WL, Koelsch G, Harley JB. A hypothesis for the HLA-B27 immune dysregulation in spondyloarthropathy: Contributions from enteric organisms, B27 structure, peptides bound by B27, and convergent evolution. Proc Natl Acad Sci USA. 1993;90:9330–4. doi: 10.1073/pnas.90.20.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scofield RH, Kurien B, Gross T, Warren WL, Harley JB. HLA-B27 binding of peptides from its own sequence and similar peptides from bacteria: implications for spondyloarthropathies. Lancet. 1995;345:1542–4. doi: 10.1016/s0140-6736(95)91089-1. [DOI] [PubMed] [Google Scholar]
- 13.Boisgérault F, Tieng V, Stolzenberg MC, et al. Differences in endogenous peptides presented by HLA-B*2705 and B*2703 allelic variants. Implications for susceptibility to spondyloarthropathies. J Clin Invest. 1996;98:2764–70. doi: 10.1172/JCI119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houbiers JGA, Nijman HW, van der Burh SH, et al. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993;23:2072–7. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- 15.Herr W, Protzer U, Lohse AW, Gerken G, Meyer zum Büschenfelde KH, Wölfel T. Quantification of CD8+ T lymphocytes responsive to human immunodeficiency virus (HIV) peptide antigens in HIV-infected patients and seronegative persons at high risk for recent HIV exposure. J Infect Dis. 1998;178:260–5. doi: 10.1086/517449. [DOI] [PubMed] [Google Scholar]
- 16.Herr W, Linn B, Leister N, Wandel E, Meyer zum Büschenfelde KH, Wölfel T. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor alpha spots in response to peptide antigens. J Immunol Meth. 1997;203:141–52. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin ‘superantigens’ with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–5. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genevee C, Diu A, Nierat J, et al. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–9. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 19.May E, Märker-Hermann E, Wittig BM, Zeitz M, Meyer zum Büschenfelde KH, Duchmann R. Identical T-cell expansions in the colon mucosa and the synovium of a patient with enterogenic spondyloarthropathy. Gastroenterology. 2000;119:1745–55. doi: 10.1053/gast.2000.20173. [DOI] [PubMed] [Google Scholar]
- 20.Gorski J, Yassai M, Zhu X, Kissela B, Keever C, Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–19. [PubMed] [Google Scholar]
- 21.Maslanka K, Piatek T, Gorski J, Yassai M, Gorski J. Molecular Analysis of T cell repertoires. Spectratypes generated by multiplex polymerase chain reaction and evaluated by radioactivity or fluorescence. Hum Immunol. 1995;44:28–34. doi: 10.1016/0198-8859(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 22.Colbert RA, Rowland-Jones SL, McMichael AJ, Frelinger JA. Differences in peptide presentation between B27 subtypes. the importance of the P1 side chain in maintaining high affinity peptide binding to B*2703. Immunity. 1994;1:121–30. doi: 10.1016/1074-7613(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 23.Villadangos JA, Galocha B, Garcia F, Albar JP, López de Castro JA. Modulation of peptide binding by HLA-B27 polymorphism in pockets A and B, and peptide specifity of B*2703. Eur J Immunol. 1995;25:2370–7. doi: 10.1002/eji.1830250837. [DOI] [PubMed] [Google Scholar]
- 24.Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8+ T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britten CM, Meyer RG, Kreer T, Drexler I, Wölfel T, Herr W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8+ T lymphocytes in IFN-γ ELISPOT assays. J Immunol Meth. 2002;259:95–110. doi: 10.1016/s0022-1759(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez I, Sesma L, Marcilla M, et al. Identification of novel HLA-B27 ligands derived from polymorphic regions of its own or other class I molecules based on direct generation by 20 S proteasome. J Biol Chem. 2001;276:32729–37. doi: 10.1074/jbc.M104663200. [DOI] [PubMed] [Google Scholar]
- 27.May E, Dulphy N, Frauendorf E, et al. Highly conserved TCR β chain usage in reactive arthritis, evidence for selection by a putative HLA-B27-associated autoantigen. Tissue Antigens. 2002;60:299–308. doi: 10.1034/j.1399-0039.2002.600404.x. [DOI] [PubMed] [Google Scholar]
- 28.Duchmann R, May E, Ackermann B, Goergen B, Meyer zum Büschenfelde KH, Märker-Hermann E. HLA-B27-restricted cytotoxic T lymphocyte responses to arthritogenic enterobacteria of self-antigens are dominated by closely related TCRBV gene segments: a study in patients with reactive arthritis. Scand J Immunol. 1996;43:101–8. doi: 10.1046/j.1365-3083.1996.d01-16.x. [DOI] [PubMed] [Google Scholar]