Abstract
Cancer-related cytokines may interfere with the differentiation and migration of dendritic cells (DCs) and with the associated up-regulation of co-stimulatory molecules in vitro. We determined whether cytokines affected the distribution and activation of DCs in patients with colorectal cancer by measuring the levels of serum cytokines [transforming growth factor (TGF)-β1 and vascular endothelial growth factor (VEGF)], DC numbers and phenotype from peripheral blood and mesenteric lymph nodes draining the cancer, and the infiltration of DCs into colorectal cancer. A significant increase in the serum level of TGF-β1 correlated with a significant reduction in the level of circulating DCs in cancer patients that was associated with an increased infiltration of Langerhans cells into colorectal mucosa. The prevalence but not intensity of co-stimulatory molecule expression in circulating and mesenteric lymph node DCs was reduced in patients with colorectal cancer compared to patients with inflammatory bowel conditions. There was no correlation between co-stimulatory molecule expression and serum TGF-β1. Thus the circulating DC depletion in colorectal cancer could be explained by a TGF-β1-related DC redistribution from the circulation into the colorectal cancer and adjacent mucosa where DC levels were increased. There was an impairment of DC activation within colorectal cancer that was not related to serum level of cytokines.
Keywords: dendritic cells, human colorectal cancer, Langerhans cell, transforming growth factor-β1
INTRODUCTION
Dendritic cells (DCs) capture antigens in the periphery, and then process and present them subsequently for recognition during T helper cell activation within secondary lymphoid organs [1]. Cytotoxic T cells are then recruited to lyse the antigen source [2]. DCs bearing epitopes of the tumour-associated glycoprotein (TAG-72) and colon-associated antigen have been identified in mesenteric lymph nodes draining colorectal cancer [3,4], and increased DC infiltration into the primary tumour is associated with improved colorectal cancer survival [5]. Although only one mature DC is required to stimulate 100–3000 T cells in vitro [6], effective in vivo antigen-presentation requires a larger quantity of mature DCs [7]. Both decrease in DC number and impaired up-regulation of CD40, CD80 and CD86 co-stimulatory molecules may inhibit the DC/T cell interaction, resulting in an attenuated T cell response [8] with tumour escape from immunological control [9].
DCs derive from CD34 positive bone marrow progenitor cells [10] and reduced circulating DCs have been reported in malignancies of the gastrointestinal tract [11], head and neck, breast and lung [12]. In vitro studies suggest that the cytokines transforming growth factor (TGF)-β1 [13,14] and vascular endothelial growth factor (VEGF) [15,16] inhibit DC differentiation and maturation, and up-regulation of CD40, CD80 and CD86 co-stimulatory molecules, and also that TGF-β1 polarizes DC progenitor cell differentiation towards immature Langerhans cells [17,18]. Thus tumour-associated release of these cytokines [19,20] might impair T cell function in colorectal cancer by reducing DC activity.
In the present study in patients with colorectal cancer, we examined the relationship between serum level of TGF-β1 and VEGF with levels of circulating and infiltrating DCs, and also expression of the DC co-stimulatory molecules CD40, CD80 and CD86.
PATIENTS AND METHODS
Patients
One hundred and ninety-four patients were studied (colorectal cancer, 106 patients; large bowel inflammatory conditions, 27; and ‘no-cancer’ controls, 61).
Colorectal cancer
Patients with histologically proven adenocarcinoma of the colon or rectum who had not undergone chemotherapy treatment and who did not have active infections at the time of analysis were included. Blood and tissue samples were collected from patients undergoing primary tumour resection. Blood samples were also collected from patients who had undergone colorectal cancer resection more than 3 months and less than 24 months previously but had liver metastases on hepatic computerized tomographic scan (CT) together with a rising serum carcinoembryonic antigen level.
Seventy-one patients had primary colorectal cancer (M : F, 41 : 30; mean age, 70 years, interquartile range [iqr] 63–77; primary tumour stage Dukes’ A, 10 tumours; B, 24; C, 19; stage unknown, four). Synchronous metastases were identified in 14 of these patients. In addition, 35 patients who had previously undergone primary colorectal cancer resection and had been diagnosed with liver metastases (M : F, 24 : 11; mean age, 58 years, iqr 55–64; median liver metastasis volume 158·9 ml, iqr 54·4–511·5), were also studied.
Large bowel inflammatory conditions
Blood and mesenteric lymph node samples were collected from patients undergoing surgical excision of diverticulitis, ulcerative colitis or Crohn's disease who were not receiving immunosuppressant therapy.
Causes of large bowel inflammation in the 27 patients (M : F, 17 : 10; mean age, 55 years, iqr 36–70) were: diverticulitis 10 patients; Crohn's disease, 11; ulcerative colitis, 6.
Controls
Blood was collected from healthy individuals with no history of cancer, undergoing intermediate surgical procedures such as herniorrhaphy or haemorrhoidectomy. Colorectal mucosa was collected from a smaller subset of these ‘no-cancer’ control subjects undergoing reversal of colostomy procedures or mucosectomy for rectal prolapse. Control subjects were followed-up for a mean of 2 years to determine whether colorectal cancer developed in this follow-up period.
The sex and age distribution of the 61 ‘no cancer’ control patients was: M : F, 31 : 30; mean age, 65 years, iqr 59–76.
Specimen sampling
Blood
Fifteen ml of peripheral venous blood was taken preoperatively and at 6–8 weeks after operation. All preoperative blood samples were taken in the morning, after overnight fasting and before any therapeutic intervention including blood transfusion had been initiated. For measurement of circulating DC and leucocyte levels, 5 ml of blood was collected into an EDTA tube. For cytokine measurements, the remaining 10 ml of blood was then collected into a serum separator tube and allowed to clot for at least 2 h before centrifugation for 20 min at 1000 g. The serum was then removed and stored at ≤−20°C until use.
Mesenteric lymph node
Mesenteric lymph node samples were collected from the excised colonic or rectal specimen immediately after resection and transported in cold (4°C) Hanks's balanced salt solution (HBSS) (Sigma, Poole, UK). After removal of surrounding fat, half of the lymph node was disaggregated manually through a 50-µm mesh filter (Becton Dickinson, Oxford, UK). No enzymatic digestion was performed to avoid the loss of cell surface co-stimulatory molecules. The resulting cellular suspension was layered onto Histopaque®-1077 (Sigma, Poole, UK) and centrifuged for 20 min (1000 g) at room temperature. Mononuclear cells were collected from the interface and washed twice in phosphate buffered saline (PBS). Cellular viability was assessed by trypan blue (0·4%, Sigma, Poole, UK) exclusion, and was greater than 90% in all cases. Cellular density was adjusted to 2–3 million cells/ml before staining for flow cytometric analysis. The remaining half of the lymph node was snap-frozen in liquid nitrogen and stored at −70°C until use.
Colorectal carcinoma and adjacent mucosa, and control colorectal mucosa
At the time of lymph node harvesting, 5 mm3 samples from the intraluminal surface of the colorectal cancer, and from macroscopically normal colorectal mucosa situated at 10–15 cm away from the tumour edge, were removed. Normal colorectal mucosa from ‘no-cancer’ control subjects undergoing reversal of colostomy or mucosectomy for rectal prolapse was also collected. All samples were snap-frozen in liquid nitrogen and stored at −70°C until use.
Dendritic cell assessment
Blood samples
Whole blood (100 µl) was stained directly with FITC-conjugated antibodies (‘lineage cocktail 1’; 10 µl) reactive against CD3 (clone SK7), CD14 (clone MφP9), CD16 (clone 3G8), CD19 (clone SJ25CI), CD20 (clone L27) and CD56 (clone NCAM16·2) [21] and PerCP-conjugated anti-HLA-DR antibodies (clone L243, 10 µl) per tube. PE-conjugated anti-CD86 antibodies [clone 2331 (FUN-1), 10 µl] or the isotype-matched antibody to control for non-specific binding were added to each tube. All antibodies were obtained from Becton Dickinson, Oxford, UK.
Circulating DC population (lineage 1–/HLA-DR+ events) and CD86+ DCs were identified and analysed by an observer (PA) blinded to the clinical details using flow cytometry as described previously [22].
Mesenteric lymph node samples
100 µl of mononuclear cellular suspensions obtained from lymph node disaggregation were stained in the same way as for whole blood, except that lysis of erythrocytes was not required because the latter had been removed during gradient centrifugation. In addition to staining with lineage cocktail 1–FITC (10 µl) and HLA-DR-PerCP (10 µl) antibodies, cells were also stained with CD86 [clone 2331 (FUN-1), 10 µl], CD80 (clone BB1, 10 µl) or CD40 (clone 5C3, 20 µl) PE-conjugated antibodies. All the latter antibodies and isotype controls were obtained from BD PharMingen, Oxford, UK. Analyses of the DC population and the expression of co-stimulatory molecules were performed as above.
Blood leucocyte count measurement
Absolute numbers of lymphocytes, monocytes and neutrophils were measured, in the same EDTA-blood sample used for DC measurement, by an automated flow cytometric cell analyser (Coulter®STKS and Coulter®GEN·STM systems, Beckman Coulter, High Wycombe, UK). The lymphocyte, monocyte and neutrophil populations were differentiated according to cellular volume, conductivity and laser light scatter, and the absolute count of each cellular population was measured with reference to a standard sample.
Serum TGF-β1 and VEGF measurement
Serum cytokines were measured using the quantitative sandwich ELISA technique according to the manufacturer's instructions (Quantikine®; R&D systems, Abingdon, UK). Before assay, serum for TGF-β1 measurement was activated (according to the manufacturer's instructions) with 2·5 N acetic acid/10 m urea and neutralized with 2·7 N sodium hydroxide/1 m HEPES, to release the active cytokine from the latent complex. The intra-assay variation was 3·7–7·3% and the interassay reproducibility was 9·8–12·8%. The minimum detectable TGF-β1 level was 7 pg/ml. The VEGF immunoassay detected the VEGF165 isoform, and the minimum detectable VEGF level was 9 pg/ml.
Immunohistochemical staining and quantification
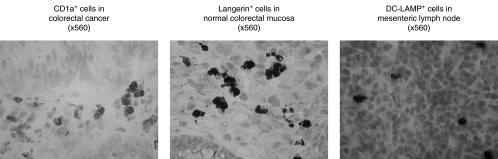
Frozen tissues were mounted onto embedding medium, and 4–6 µm tissue sections were then cut by cryostat. After air-drying overnight and fixation in absolute acetone, the slides were stored at −20°C until immunohistochemical staining. Endogenous peroxidase activity was quenched for 1 h at room temperature with 70% methanol containing 0·3% hydrogen peroxide and non-specific binding was blocked for 1 h at room temperature with rabbit serum [10% solution in Tris-buffered saline (TBS)]. Staining for 60 min at room temperature was performed with murine primary monoclonal antibodies recognizing the following molecules: CD1a (clone O10, IgG1; neat; Serotec, Oxford, UK), Langerin (clone DCGM4, IgG1; 1/40 dilution; Immunotech, High Wycombe, UK) and DC-LAMP (clone 104.G4, IgG1; 1/10 dilution; Immunotech, High Wycombe, UK). Langerhans cells express specifically CD1a (Fig. 1) [23] and Langerin (CD207, Fig. 1) [24,25], whereas mature DCs express DC-LAMP (CD208, Fig. 1) [26]. For negative control sections, the primary antibody was replaced with non-immune mouse serum. Normal skin, which is abundant in Langerhans and dendritic cells, was used for positive control sections. Secondary staining with the rabbit antimouse horseradish peroxidase-conjugated antibody (IgG; 1/50 dilution; Serotec, Oxford, UK) was performed for 60 min at room temperature. Freshly prepared 0·1% (w/v) 3,3′-diaminobenzidine tetrahydrochloride (Fast DAB, Sigma, Poole, UK) solution containing 5 µl of 30% hydrogen peroxide was used as a chromogen substrate, and was applied to slides at room temperature for 10 min. Counterstaining with Mayer's haematoxylin (Sigma, Poole, UK) was performed for 30 s.
Fig. 1.
Langerhans cells express CD1a and Langerin, whereas mature DCs express DC-LAMP.
Positively stained cells were identified as an intensely brown end-product, using light microscopy (Vickers Instruments, London, UK) at a magnification of 400 (×10 ocular lens, ×40 objective lens) surveying an area of 0·196 mm2 per high-power field (hpf). Twenty high-power fields were counted per section to reduce sampling error and the density of positively stained cells within each -power field was scored independently by two observers (AH and NI), who were unaware of clinical details. However, infiltrating cells were distributed heterogeneously with some fields containing many stained cells, whereas others contained none. To account for this heterogeneity, the average density of positively stained cells in the 20 hpf was scored as: 0 (0 cells/hpf), 1 (1–5 cells/hpf), 2 (6–10 cells/hpf) and 3 (more than 10 cells/hpf). There was a >90% concordance in scoring between the two observers.
Liver metastasis volume
In liver metastasis patients, liver metastasis volume was measured within 3 weeks of blood sampling, by single-contrast enhanced liver CT scan [27]. In brief, the area of liver metastasis was measured on each CT slice using a Kontron Elektronik KS100 Version 2·0 image analyser (Kontron Elektronik GmbH, Germany), and the volume for each slice was then calculated by multiplying the area by the CT slice thickness. The volumes for all slices were then summed to obtain a total liver metastasis volume for each patient, as described previously [28].
Statistical analysis
When comparing differences in circulating DC levels, a pilot study of 40 patients suggested a mean DC level of 0·44% for control patients and 0·34% for cancer patients. A power calculation indicated that a sample size of 70 in each group would have a 90% power of detecting a 0·1% mean DC level difference, assuming a common standard deviation of 0·2 and using a two group t-test with 0·05 two-sided significance level. Intergroup differences were analysed by Mann–Whitney U-test, and correlation was measured using Spearman's correlation coefficient test. Pre- and postoperative levels were compared using the Wilcoxon's signed rank test. Assessment for linear trend in the relation between circulating DCs or serum cytokine, and CD1a+ and Langerin+ cells was by anova linear contrast test [29]. P-values of <0·05 were considered statistically significant.
Ethical approval and consent
The study was approved by the Chelsea and Westminster Hospital Ethics Committee, and all patients gave informed consent before participation.
RESULTS
Circulating dendritic cell levels
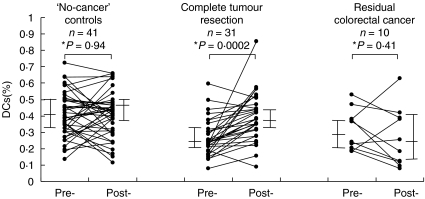
Circulating DC levels were significantly lower in colorectal cancer patients than in ‘no-cancer’ control subjects or patients with inflammatory bowel conditions (Table 1). Circulating DC levels increased significantly by 6–8 weeks from primary tumour resection to reach similar levels to those found in ‘no-cancer’ controls, in patients undergoing complete colorectal cancer resection but not in patients known to have residual colorectal cancer (Fig. 2). There was no significant difference (Mann–Whitney U-test, P = 0·84) in circulating DC levels between colorectal cancer patients with a primary tumour but without liver metastases (n = 53, median 0·25%, iqr 0·20–0·38), and those with liver metastases in whom the primary tumour had been excised (n = 7, median 0·25%, iqr 0·24–0·33). There was a significant lymphopenia and no significant change in the absolute numbers of monocytes or neutrophils in cancer patients compared with control subjects (Table 1).
Table 1.
Circulating dendritic cells (DCs) in colorectal cancer patients, ‘no-cancer’ control subjects and patients with inflammatory bowel conditions
| Intergroup differences: Mann–Whitney U-test (P-value) | ||||||
|---|---|---|---|---|---|---|
| Patients with colorectal cancer (n = 78)(group a) | ‘No-cancer’ control subjects (n = 61)(group b) | Patients with inflammatory bowel conditions (n = 27)(group c) | a versus b | a versus c | b versus c | |
| Circulating DCs (%) | 0·25 (0·21–0·38) | 0·41 (0·33–0·51) | 0·36 (0·24–0·59) | 0·0001 | 0·01 | 0·30 |
| Monocytes (×109/l) | 0·5 (0·4–0·7) | 0·5 (0·4–0·6) | 0·7 (0·5–0·8) | 0·52 | 0·005 | 0·0004 |
| Lymphocytes (×109/l) | 1·3 (0·9–1·6) | 1·8 (1·5–2·5) | 1·3 (0·9–2·7) | 0·0001 | 0·09 | 0·06 |
| Neutrophils (×109/l) | 4·5 (3·6–5·9) | 3·7 (3·2–5·1) | 6·1 (4·9–9·3) | 0·08 | 0·004 | 0·0002 |
| CD86+ DCs prevalence (%) | 37 (25–46) | 39 (22–48) | 57 (34–68) | 0·85 | 0·0008 | 0·001 |
| CD86+ DCs immunofluorescence (mean fluorescence intensity) | 192·4 (146·0–282·0) | 173·1 (137·1–219·8) | 173·8 (140·4–347·2) | 0·06 | 0·85 | 0·23 |
Circulating DCs[lineage 1 negative (CD3¯, CD14¯, CD16¯, CD19¯, CD20¯, CD56¯) and HLA-DR positive cells] in peripheral venous blood were identified by flow cytometry, and expressed as a percentage of circulating leucocytes. Absolute numbers of lymphocytes, monocytes and neutrophils were measured, in the same blood sample used for DC measurement, by an automated flow cytometric cell analyser with reference to a standard sample. CD86 positive DCs (prevalence and intensity of immunofluorescence) were defined from the DC population using the appropriate isotype control. All values shown are medians and interquartile ranges, and all analyses were performed using the Mann–Whitney U-test. P <0·05 was considered significant.
Fig. 2.
The level of circulating DCs increased at 6–8 weeks after primary tumour resection in colorectal patients without residual disease (Wilcoxon's signed rank test, n = 31, P = 0·0002) compared with those with residual disease (n = 10, P = 0·41) and with ‘no-cancer’ controls (n = 41, P = 0·94). Pre = preoperatively; Post = 6–8 weeks postoperatively; bars represent median and interquartile range. *Wilcoxon's signed rank test.
Dendritic cells within the primary tumour, adjacent colorectal mucosa and locally draining lymph nodes in colorectal cancer
Langerhans cellular density within the primary tumour and adjacent colorectal mucosa was significantly higher than in colorectal mucosa from ‘no-cancer’ controls (Table 2). Mature DC-LAMP+ cellular infiltration within the primary tumour was significantly higher than in adjacent colorectal mucosa, and in colorectal mucosa from ‘no-cancer’ controls (Table 2).
Table 2.
Infiltration of Langerhans (CD1a+ and Langerin+) cells and mature (DC-LAMP+) dendritic cells in colorectal cancer, adjacent non-cancerous colorectal mucosa and normal colorectal mucosa from ‘no-cancer’ control subjects
| Intergroup differences: Mann–Whitney U-test (P-value) | ||||||
|---|---|---|---|---|---|---|
| Patients with colorectal cancer (group a) | Adjacent noncancerous mucosa from colorectal cancer patients (group b) | Normal colorectal mucosa from ‘no-cancer’ controls (group c) | a versus b | a versus c | b versus c | |
| Number of patients | 43 | 44 | 8 | |||
| CD1a+ cells score | 1 (1–3) | 1 (0–2) | 0 (0–1) | 0·27 | 0·003 | 0·03 |
| Langerin+ cells score | 2 (1–3) | 1 (0–2) | 0 (0) | 0·14 | 0·0002 | 0·005 |
| DC-LAMP+ cells score | 2 (1–3) | 0 (0) | 0 (0–0·5) | < 0·0001 | 0·0001 | 1 |
Langerhans cells and DCs in frozen tissue sections were identified by indirect single immunoperoxidase staining using primary antibodies recognizing CD1a, Langerin and DC-LAMP. The overall density of infiltration of stained cells in the sections was scored as: 0 (0 cell/high-power field), 1 (1–5 cells/high-power field), 2 (6–10 cells/high-power field) and 3 (>10 cells/high-power field). All values shown are medians and interquartile ranges, and all analyses were performed using the Mann–Whitney U-test. P < 0·05 was considered significant.
Very few Langerhans cells were found within the lymph nodes draining primary colorectal cancer [CD1a+ cells: n = 41, median density 0 (iqr 0); Langerin+ cells: n = 41, median density 0 (iqr 0)], but greater numbers of mature DC-LAMP+ cells were identified [n = 41, median density 2 (iqr 1–3)]. There was no difference between the number of mature DC-LAMP+ cells within lymph nodes with metastases and those without (Mann–Whitney U-test >0·5). CD1a+, Langerin+ and DC-LAMP+ cellular densities within the primary colorectal cancer did not correlate (anova test for linear contrast, P > 0·05) with their densities within adjacent colorectal mucosa or the locally draining lymph node.
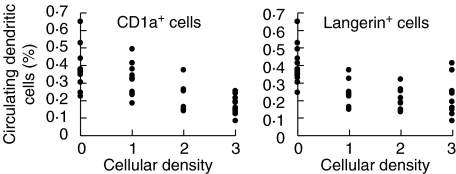
There was a significant inverse relationship (anova test for linear contrast, P < 0·0001) between circulating DC levels and Langerhans (CD1a+ and Langerin+) cellular densities within colorectal mucosa adjacent to colorectal cancer (Fig. 3), but not within the cancer itself (CD1a+ cells: P = 0·94, Langerin+ cells: P = 0·15, n = 43 patients). There was no association between circulating DC level and DC-LAMP+ cellular density in the cancer (P = 0·85, n = 43 patients), in adjacent colorectal mucosa (P = 0·49, n = 44 patients) or in locally draining lymph nodes (P = 0·36, n = 41 patients).
Fig. 3.
There was a correlation between reduced circulating dendritic cells and an increased density of Langerhans cell infiltration (0 = 0 cells/high-power field, 1 = 1–5 cells/high-power field, 2 = 6–10 cells/high-power field and 3 = more than 10 cells/high-power field) of CD1a+ (anova test for linear contrast, P <0·0001, n = 44) and Langerin+ (P < 0·0001, n = 44) within the non-cancerous mucosa adjacent to colorectal cancer. One explanation for this is that a cancer-related increase in local levels of TGF-β1 resulted in DC redistribution from the circulation into the peri-tumoural tissues.
Serum TGF-β1
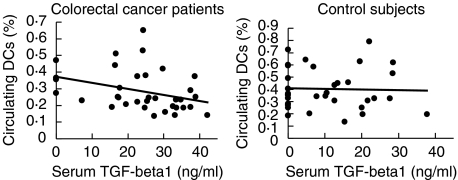
Serum TGF-β1 levels were increased significantly (Mann–Whitney U-test, P <0·0001) in colorectal cancer patients (n = 64, median 30·05 ng/ml, iqr 24·29–36·98) compared with controls (n = 33, median 6·92 ng/ml, iqr 0–16·29). Increased serum TGF-β1 correlated significantly with reduced circulating DCs in colorectal cancer patients, but not in ‘no-cancer’ control subjects (Fig. 4).
Fig. 4.
Serum TGF-β1 correlated significantly with the level of circulating DCs in colorectal cancer patients (Spearman test, n = 33, r =−0·36, P = 0·04, 95% CI = −0·62 to –0·02) but not in control subjects (n = 33, r = 0·07, P = 0·70, 95% CI = −0·28–0·40).
Liver metastasis patients had significantly higher (Mann–Whitney U-test, P = 0·001) serum TGF-β1 levels (n = 40, median 33·34 ng/ml, iqr 25·30–38·13) than colorectal cancer patients without detectable metastases (n = 24, median 24·67 ng/ml, iqr 16·91–33·03), and there was a significant correlation between colorectal liver metastasis volume and serum TGF-β1 (Spearman test, n = 32 patients, r = 0·36, P = 0·04, 95% CI = 0·02–0·63).
Increased serum TGF-β1 was associated significantly with the appearance of CD1a+ (Mann–Whitney U-test, P = 0·003, n = 21 patients) and Langerin+ (Mann–Whitney U-test, P = 0·0006, n = 21 patients) cells within colorectal mucosa adjacent to colorectal cancer, but not with increased CD1a+ or Langerin+ cell density (anova test for linear contrast, CD1a+ cells, P = 0·29; Langerin+ cells, P = 0·07).
Serum TGF-β1 levels did not correlate significantly with CD1a+, Langerin+ and DC-LAMP+ cell identification (n = 21 patients) or density (n = 21 patients) within the primary colorectal cancer. Serum TGF-β1 level also did not correlate with DC-LAMP+ cell identification (n = 21 patients) or density (n = 21 patients) within mesenteric lymph nodes draining colorectal cancer.
Serum VEGF
Serum VEGF levels were increased significantly (Mann–Whitney U-test, P = 0·03) in colorectal cancer patients (n = 35, median 388·08 pg/ml, iqr 181·98–662·37) compared with controls (n = 34, median 205·13 pg/ml, iqr 104·39–469·78). No significant correlation was found between serum VEGF and circulating DCs in colorectal cancer patients (Spearman's test, n = 35, r =−0·13, P = 0·47, 95% CI = −0·44–0·21) or in control subjects (n = 34, r =−0·30, P = 0·09, 95% CI = −0·58–0·05). Similarly, the serum level of VEGF did not correlate with CD1a+, Langerin+ and DC-LAMP+ cellular identification or density within the primary colorectal cancer, adjacent colorectal mucosa or mesenteric lymph nodes.
Dendritic cell co-stimulatory molecule expression, and serum cytokines
The prevalence and immunofluorescent intensity of CD86+ circulating DCs were not significantly different between colorectal cancer patients and ‘no-cancer’ control subjects. Circulating CD86+ DCs were identified significantly more frequently in patients with bowel inflammation than in colorectal cancer or control patients (Table 1).
The prevalence of CD40+, CD80+ or CD86+ DCs within local lymph nodes draining primary colorectal cancer was significantly lower than within nodes draining inflammatory bowel conditions (Table 3). However, CD40+, CD80+ and CD86+ DC immunofluorescent intensities were significantly higher in nodes draining colorectal cancer than inflammation (Table 3).
Table 3.
Expression of co-stimulatory molecules in dendritic cells (DCs) from mesenteric lymph nodes in colorectal cancer patients, ‘no-cancer’ control subjects and patients with inflammatory bowel conditions
| Intergroup differences: Mann–Whitney U-test (P-value) | ||||||
|---|---|---|---|---|---|---|
| Patients with colorectal cancer (group a) | ‘No-cancer’ control subjects (group b) | Patients with inflammatory bowel conditions (group c) | a versus b | a versus c | b versus c | |
| Number of patients | 33 | 3 | 12 | |||
| CD40+ DCs prevalence (%) | 6·0 | 7·1 | 22·0 | 0·84 | 0·0001 | 0·02 |
| (4·0–10·0) | (4·1–9·5) | (16·0–25·0) | ||||
| CD40+ DCs immunofluorescence | 274·0 | 342·2 | 140·9 | 0·47 | 0·007 | 0·10 |
| (mean fluorescence intensity) | (191·3–339·1) | (220·4–350·3) | (94·8–236·0) | |||
| CD80+ DCs prevalence (%) | 3·0 | 1·4 | 13·7 | 0·06 | 0·0001 | 0·009 |
| (2·0–4·5) | (0–1·6) | (7·5–21·0) | ||||
| CD80+ DCs immunofluorescence | 165·7 | 183·9 | 83·4 | 0·75 | 0·004 | 0·77 |
| (mean fluorescence intensity) | (130·6–215·6) | (0–185·5) | (53·9–148·9) | |||
| CD86+ DCs prevalence (%) | 15·5 | 4·0 | 40·0 | 0·05 | 0·0001 | 0·009 |
| (10·0–21·0) | (4·0–12·0) | (34·5–54·0) | ||||
| CD86+ DCs immunofluorescence | 255·0 | 241·8 | 153·5 | 0·74 | 0·04 | 0·15 |
| (mean fluorescence intensity) | (190·2–342·0) | (207·9–421·7) | (142·6–278·1) | |||
DCs[lineage 1 negative (CD3¯, CD14¯, CD16¯, CD19¯, CD20¯, CD56¯) and HLA-DR positive cells] in mononuclear lymph node suspensions were identified by flow cytometry, and the prevalence and intensity of immunofluorescence of CD40, CD80, CD86 positive DCs were defined from the DC population using the appropriate isotype control. All values shown are medians and interquartile ranges, and all analyses were performed using the Mann–Whitney U-test. P < 0·05 was considered significant.
There was no significant correlation (Spearman's test, P > 0·05) between the prevalence and immunofluorescent intensities of circulating or lymph node CD40+, CD80+ and CD86+ DCs, and the serum level of TGF-β1 and VEGF from patients with colorectal cancer.
DISCUSSION
Circulating DC levels in the presence of colorectal cancer were reduced to about 60% of control levels, as reported in patients with other solid malignancies [11,30], and complete colorectal cancer removal restored circulating DC levels to the normal range. As one mature DC stimulates 100–3000 T cells [6], this reduction could impair in vivo antigen-presentation [7]. Although control subjects were not investigated to exclude occult cancer, the younger mean age and 2-year cancer-free follow-up suggested that control patients were unlikely to have harboured an occult cancer at the time of phlebotomy.
Circulating DC depletion was observed both in primary colorectal cancer patients without liver metastases and also in patients with colorectal liver metastases in whom the primary tumour had been excised. This suggested that the circulating DC depletion was independent of the site of the colorectal cancer. Although measuring the frequency of DCs as a percentage of all leucocytes may underestimate the true level, the absence of a significant increase in monocytes and neutrophils and the lymphopenia in cancer patients compared with control subjects was more consistent with an absolute rather than a relative circulating DC reduction. This circulating DC reduction was associated with an increase in Langerhans (CD1a+ and Langerin+) cells within mucosa adjacent to the primary colorectal cancer. Although there is no direct evidence that infiltrating Langerhans cells are derived from circulating DCs, the high level of MHC class II expression in both [23] and their capacity for maturation suggest that they are both immature DCs.
As reported reviously, serum TGF-β1 [19,20] and VEGF [31,32] levels were increased significantly in colorectal cancer patients. The correlation of serum TGF-β1 levels with liver metastasis volume in the present study and the reported association between plasma TGF-β1 level and tumour mRNA expression [20] are consistent with the tumour being a source of TGF-β1. TGF-β1 suppresses mature DC differentiation in murine marrow culture [14] and prevents Langerhans cell maturation [13]. In the present study, increased serum TGF-β1 but not VEGF levels correlated with reduced circulating DC levels in colorectal cancer patients and not in healthy controls.
TGF-β1 also promotes in vitro Langerhans cell differentiation [18,33,34] from CD34-positive progenitors by preventing precursor cell apoptosis [35]. In the present study, there was an increase in Langerhans and mature DCs in both primary colorectal cancer and adjacent mucosa, compared with colorectal mucosa from ‘no-cancer’ control patients. Increased serum TGF-β1 was associated with the appearance of Langerhans cells in mucosa adjacent to colorectal cancer. One explanation for these findings is that a cancer-related increase in local levels of TGF-β1 resulted in DC redistribution from the circulation into the tumour and adjacent mucosa. Measurements of local TGF-β1 levels would be required to demonstrate this association. Other cancer-related effects on mucosa adjacent to colorectal cancer, for example in mucin structure [36], have been identified previously. Studies of the influence of distance from the primary tumour on mucosal Langerhans cell density and of colorectal mucosal Langerhans cell density in colorectal liver metastasis patients in whom the primary tumour has been resected would help to determine whether this was a local tumour-related effect on adjacent mucosa. The findings were consistent with a TGF-β1-induced DC redistribution from the circulation into the primary colorectal cancer and adjacent mucosa, by polarization of DC progenitor cells towards differentiating into immature Langerhans cells [17,18]. Further examination of the relationship between serum TGF-β1 and CD1a+/Langerin+ cellular infiltration in skin specimens from colorectal cancer patients would determine whether this was a tumour-specific phenomenon.
It has been reported that serum TGF-β1 levels in a heterogeneous group of cancer patients did not correlate with the number of immature DCs derived from culture of peripheral blood mononuclear cells from the same patients [12]. However, serum TGF-β1 was not elevated in most of the advanced cancer patients in this study, in contrast to the present study where 85% of colorectal cancer patients had elevated serum TGF-β1 levels − with a greater than fourfold increase in mean TGF-β1 level − compared with control patients. Thus the effect of TGF-β1 on DCs may be observed at elevated levels only, accounting for the lack of relationship between serum TGF-β1 level and circulating DCs in ‘no-cancer’ control subjects.
It has also been suggested that increased serum VEGF is associated with an increase in culture-derived immature DCs [12]. Because stress from culture may activate DCs directly [37], analysis of unmanipulated DCs − as in the present study − may be more representative of the in vivo DC population. Increased serum VEGF levels have also been associated with reduced circulating DCs in patients with various solid malignancies [38]. However, the mean serum VEGF level in these patients was more than fivefold higher [38] than in the healthy control patients in the present and other studies [31,32]. Consistent with the present study, previous studies [38] have not demonstrated an association between serum VEGF and circulating DCs at the serum VEGF levels (less than double those of healthy controls) that have been reported [31,32] in association with advanced colorectal cancer.
Clot formation during blood sampling induces platelet and neutrophil activation resulting in the non-uniform release of VEGF [39]. As platelets and neutrophils contain high VEGF levels, it has been suggested that tissue VEGF levels are more accurately reflected by plasma than serum levels [40]. Thus the absence of a correlation between serum VEGF and circulating DCs in the present study may not exclude a relationship between circulating VEGF and circulating DCs. However, nonuniform VEGF release is reduced [41] by allowing whole blood samples to clot for between 2 and 6 h before serum is collected, as performed in the present study. In addition, preoperative serum VEGF has been shown to be a stronger predictor of colorectal cancer survival than plasma VEGF [42], suggesting some relationship between serum levels and tumour behaviour. Studies of plasma VEGF levels are required to determine whether the lack of association between circulating DCs and serum VEGF indicates that circulating DC levels are independent of circulating VEGF.
The prevalences of DCs expressing CD40, CD80 and CD86 within the blood and lymph nodes of colorectal cancer patients were similar to ‘no-cancer’ control patients, and lower than those in patients with inflammatory bowel conditions. A limitation of this study is that it was possible to obtain only three ‘no-cancer’ control lymph nodes for examination. Truly noncancerous and non-inflammatory mesenteric lymph nodes may be impossible to obtain in large numbers from healthy volunteers. A further limitation is that there may be differences within the heterogeneous group of inflammatory colonic conditions that we included within the ‘inflammatory’ group within this study. A larger, separate study would be required to further evaluate this for different varieties of inflammation.
Nevertheless, the apparent failure of DC up-regulation in cancer patients appeared to be incomplete, since immunofluorescent intensity was higher in blood and lymph node DCs from patients with colorectal cancer than patients with colonic inflammation. This could explain the previous contradictory findings where DCs expressing co-stimulatory molecules were identified in human colorectal cancer [43], while an earlier study did not identify up-regulated DCs [44]. The difference may be explained by a lower prevalence rather than absence of activated DCs in colorectal cancer. Although an increased presence of DC-LAMP+ cells was present within the tumour tissue, DC-LAMP expression is associated with antigenic uptake by DCs and not necessarily the activation of co-stimulatory molecules [26].
It has been suggested that in vitro TGF-β1 [13,45] and VEGF [15,46] interfere with co-stimulatory molecule up-regulation. We did not find a correlation between serum level of these cytokines and levels of CD40+, CD80+ or CD86+ DCs in blood or mesenteric lymph nodes. Although serum cytokine levels may not reflect accurately their activity in the tumour microenvironment [47], the present findings are consistent with factors other than serum level of cytokines influencing in vivo expression of DC co-stimulatory molecules. Although DCs penetrate the gut epithelium to sample luminal antigens [48], failure of cancer antigens to reach DCs is unlikely to be a factor because DCs bearing such antigens are present in the draining lymph nodes [3,4]. Tumour-related inhibition of DC co-stimulatory molecule up-regulation may be due to the lack of appropriate activation signals [49].
Thus the circulating DC depletion in colorectal cancer could be explained by a TGF-β1-related DC redistribution from the circulation into the colorectal cancer and adjacent mucosa where DC levels are consequently increased. There appeared to be an impairment of DC activation within colorectal cancer that was not related to serum levels of TGF-β1 or VEGF. One explanation could be that continuous antigen delivery by DCs draining from the tumour to lymph nodes resulted in peripheral T cell tolerance [50], with attenuation of DC/T cell interaction and T cell dysfunction [8].
Acknowledgments
AH was supported by Colon Cancer Concern, London, UK, and by the Research Trustees of Chelsea and Westminster Hospital, London, UK. NI was funded by the Wellcome Trust UK (grant number 058700). We thank Clare Glover MA CStat for statistical advice.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 3.Mariani-Costantini R, Muraro R, Ficari F, et al. Immunohistochemical evidence of immune responses to tumor-associated antigens in lymph nodes of colon carcinoma patients. Cancer. 1991;67:2880–6. doi: 10.1002/1097-0142(19910601)67:11<2880::aid-cncr2820671129>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Bonnotte B, Favre N, Moutet M, et al. Role of tumor cell apoptosis in tumor antigen migration to the draining lymph nodes. J Immunol. 2000;164:1995–2000. doi: 10.4049/jimmunol.164.4.1995. [DOI] [PubMed] [Google Scholar]
- 5.Ambe K, Mori M, Enjoji M. S-100 protein-positive dendritic cells in colorectal adenocarcinomas. Distribution and relation to the clinical prognosis. Cancer. 1989;63:496–503. doi: 10.1002/1097-0142(19890201)63:3<496::aid-cncr2820630318>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Stockwin LH, McGonagle D, Martin IG, Blair GE. Dendritic cells. immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91–102. doi: 10.1046/j.1440-1711.2000.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–92. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 8.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 9.Shu S, Plautz GE, Krauss JC, Chang AE. Tumor immunology. JAMA. 1997;278:1972–81. [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Lissoni P, Brivio F, Ferrante R, et al. Circulating immature and mature dendritic cells in relation to lymphocyte subsets in patients with gastrointestinal tract cancer. Int J Biol Markers. 2000;15:22–5. doi: 10.1177/172460080001500104. [DOI] [PubMed] [Google Scholar]
- 12.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 13.Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–53. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 16.Ohm JE, Shurin MR, Esche C, Lotze MT, Carbone DP, Gabrilovich DI. Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol. 1999;163:3260–8. [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang YY, Ogata M, et al. Transforming growth factor-beta1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208–20. [PubMed] [Google Scholar]
- 18.Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J Immunol. 1999;163:4869–77. [PubMed] [Google Scholar]
- 19.Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-beta1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–61. doi: 10.1002/(sici)1097-0142(19990201)85:3<554::aid-cncr6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Tsushima H, Kawata S, Tamura S, et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–82. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 21.Savary CA, Grazziutti ML, Melichar B, et al. Multidimensional flow-cytometric analysis of dendritic cells in peripheral blood of normal donors and cancer patients. Cancer Immunol Immunother. 1998;45:234–40. doi: 10.1007/s002620050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones GJ, Watera C, Patterson S, et al. Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients. AIDS. 2001;15:1657–63. doi: 10.1097/00002030-200109070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valladeau J, Duvert-Frances V, Pin JJ, et al. The monoclonal antibody DCGM4 recognizes Langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur J Immunol. 1999;29:2695–704. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 26.de Saint-Vis B, Vincent J, Vandenabeele S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–36. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin MJ, Burke D, Earlam S, Fordy C, Allen-Mersh TG. Measurement of response to treatment in colorectal liver metastases. Br J Cancer. 1995;71:873–6. doi: 10.1038/bjc.1995.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breiman RS, Beck JW, Korobkin M, et al. Volume determinations using computed tomography. Am J Roentgenol. 1982;138:329–33. doi: 10.2214/ajr.138.2.329. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Comparing several groups using analysis of variance. Br Med J. 1996;312:1472–3. doi: 10.1136/bmj.312.7044.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissoni P, Vigore L, Ferranti R, et al. Circulating dendritic cells in early and advanced cancer patients: diminished percent in the metastatic disease. J Biol Regul Homeost Agents. 1999;13:216–9. [PubMed] [Google Scholar]
- 31.Davies MM, Jonas SK, Kaur S, Allen-Mersh TG. Plasma vascular endothelial but not fibroblast growth factor levels correlate with colorectal liver mestastasis vascularity and volume. Br J Cancer. 2000;82:1004–8. doi: 10.1054/bjoc.1999.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werther K, Christensen IJ, Brunner N, Nielsen HJ, Danish RCC. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. Eur J Surg Oncol. 2000;26:657–62. doi: 10.1053/ejso.2000.0977. [DOI] [PubMed] [Google Scholar]
- 33.Caux C, Massacrier C, Dubois B, et al. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J Leukoc Biol. 1999;66:781–91. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- 34.Strobl H, Riedl E, Scheinecker C, et al. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J Immunol. 1996;157:1499–507. [PubMed] [Google Scholar]
- 35.Riedl E, Strobl H, Majdic O, Knapp W. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158:1591–7. [PubMed] [Google Scholar]
- 36.Dawson PM, Habib NA, Fane S, Rees HC, Wood CB, Allen-Mersh TG. Association between extent of colonic mucosal sialomucin change and subsequent local recurrence after curative excision of primary colorectal cancer. Br J Surg. 1990;77:1279–83. doi: 10.1002/bjs.1800771127. [DOI] [PubMed] [Google Scholar]
- 37.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 38.Lissoni P, Malugani F, Bonfanti A, et al. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J Biol Regul Homeost Agents. 2001;15:140–4. [PubMed] [Google Scholar]
- 39.Minagawa N, Akahane K, Nagashima N, Nagata N, Itoh H. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res. 2002;22:2437–42. [PubMed] [Google Scholar]
- 40.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–23. [PubMed] [Google Scholar]
- 41.Werther K, Christensen IJ, Nielsen HJ. Determination of vascular endothelial growth factor (VEGF) in circulating blood. significance of VEGF in various leucocytes and platelets. Scand J Clin Lab Invest. 2002;62:343–50. doi: 10.1080/00365510260296492. [DOI] [PubMed] [Google Scholar]
- 42.Werther K, Christensen IJ, Nielsen HJ. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer. 2002;86:417–23. doi: 10.1038/sj.bjc.6600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwaab T, Weiss JE, Schned AR, Barth RJ., Jr Dendritic cell infiltration in colon cancer. J Immunother. 2001;24:130–7. [PubMed] [Google Scholar]
- 44.Chaux P, Moutet M, Faivre J, Martin F, Martin M. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 co-stimulatory molecules of the T-cell activation. Lab Invest. 1996;74:975–83. [PubMed] [Google Scholar]
- 45.Strobl H, Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–90. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 46.Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol. 1998;161:4842–51. [PubMed] [Google Scholar]
- 47.Melichar B, Savary C, Kudelka AP, et al. Lineage-negative human leukocyte antigen-DR+ cells with the phenotype of undifferentiated dendritic cells in patients with carcinoma of the abdomen and pelvis. Clin Cancer Res. 1998;4:799–809. [PubMed] [Google Scholar]
- 48.Rescigno M, Borrow P. The host–pathogen interaction: new themes from dendritic cell biology. Cell. 2001;106:267–70. doi: 10.1016/s0092-8674(01)00454-8. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–80. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 50.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–80. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]