INTRODUCTION
Autoantibodies which are reactive with intracellular antigens have been studied extensively in autoimmune rheumatic diseases such as systemic lupus erythematosus (lupus), Sjögren's syndrome, scleroderma, dermatomyositis/polymyositis, and other related diseases [1]. Autoantibodies are being recognized with increasing frequency in other illnesses including type I diabetes [2], the paraneoplastic neurological disease syndromes [3,4], liver diseases [5], bullous skin diseases, inflammatory bowel diseases, thyroid and other endocrinopathies, haematopoietic disorders and many others [6]. Autoantibody to p53 was detected in breast cancer [7] but for a time the main interest was in T cell-mediated autoimmunity [8], until it became evident that humoral immunity was also a prominent response in many different types of cancer [9–11].
In this paper, we will review and discuss the special features of spontaneously occurring autoantibodies and in the case of cancer, how some patients’ immune systems appear to be sensing aberrant cellular mechanisms related to tumorigenesis and reporting these events in the form of the contemporaneous appearance of neo-antibodies. There are some similarities between autoantibodies in lupus (and other autoimmune diseases) and autoantibodies in cancer and there are some differences, but important insights into aetiology, pathogenesis and therapeutic strategies could be gained by careful analysis of the deeper implications of autoimmune responses in both lupus and cancer.
AUTOANTIBODIES IN LUPUS
Autoepitopes are highly conserved
SS-B/la is an intranuclear protein which is one of the principal target antigens of autoantibodies in the sera of patients of lupus and Sjögren's syndrome [1,12]. This protein binds to a number of small RNA species including precursors of 5S RNA and tRNAs, but not with their corresponding mature species, and has a functional role in the maturation of these RNAs [13]. Human autoantibodies to SS-B/La were known to be reactive with this cellular protein across a wide range of species, but the uniqueness of this property was appreciated fully when comparing epitopes recognized by human autoantibodies with epitopes recognized by murine monoclonal antibodies obtained by experimental immunization with the purified antigen [14]. Five IgG1 κ monoclonal antibodies all reacted in Western immunoblotting with full-length SS-B/La antigen, but differences in epitope recognition between murine and human antibodies became apparent in reactivities with different fragments of SS-B/La after partial digestion with Staphylococcus aureus V8 enzyme. Perhaps the most informative data came from studies in immunohistochemistry using cell lines from different animal species as tissue substrates. As shown in Table 1, Sjögren's syndrome serum Ze (a reference serum for anti-SS-B/La) reacted positively with the protein in nuclei of cells from human, rabbit, bovine, rat, mouse and other species. Two of the murine monoclonal antibodies were non-reactive and three monoclonals were reactive with human, monkey, rabbit and bovine cells, but not reactive with mouse, hamster, rat and rat kangaroo cells, the latter group coming from species more closely related to each other but more distant to human and monkey species in evolution. Human autoantibody to SS-B/La was recognizing an epitope(s) which was more highly conserved compared to experimentally induced antibodies.
Table 1.
Species-specific reactions of human autoantibody compared to MoAbs as detected by immunofluorescence
| Immunofluorescence* | |||||||
|---|---|---|---|---|---|---|---|
| Murine MoAbs | |||||||
| Species | Cell line origin | A1 | A2 | A3 | A4 | A5 | Human serum Ze‡ |
| Human | HEp-2, larynx | + | + | + | – | – | + |
| Human | HeLa, cervix | + | + | + | – | – | + |
| Human | Raji, Burkitt's lymphoma | + | + | + | – | – | + |
| Monkey | Vero, kidney | + | + | + | – | – | + |
| Rabbit | R9ab, lung | + | + | + | – | – | + |
| Bovine | MDBK, kidney | + | + | + | – | – | + |
| Hamster | BHK-21, kidney | – | – | – | – | – | + |
| Rat | 6m2, kidney | – | – | – | – | – | + |
| Mouse | 3T3, fibroblasts | – | – | – | – | – | + |
| Rat kangaroo | PtK2, kidney | – | – | – | – | – | + |
Cells were grown in Laboratory-Tek tissue culture chambers and fixed in a mixture of acetone and methanol (3 : 1) at −20°C for 2 min.
Ze serum is the CDC reference serum for anti-SS-B/La specificity ([14]).
Autoantibodies react with functional or binding sites which are conformation-dependent
Proliferating cell nuclear antigen (PCNA) was defined with antibody from a lupus patient [15]. It was observed first as an immunoprecipitin reaction between lupus serum and an antigen in thymus extracts. Mature, non-activated lymphocytes contained low or undetectable amounts of PCNA but lymphocytes stimulated to undergo proliferation with the mitogens, phytohaemagglutinin or concanavalin A contained high amounts of the antigen. PCNA was identical to the auxiliary protein of DNA polymerase delta and was an essential component of DNA synthesis in replicating cells [16]. Independently, Celis and coworkers [17] identified a nuclear protein (cyclin) by two-dimensional gel electrophoresis which correlated with the proliferative state of cultured mammalian cells and PCNA and cyclin were shown subsequently to be identical proteins [18].
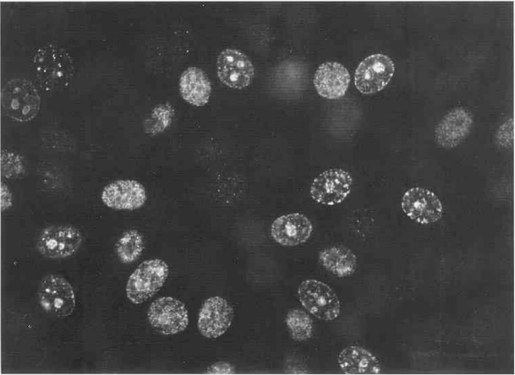
An interesting feature of most human autoantibodies to PCNA is their reactivity with the antigen when the latter is associated with the replication complex and engaged in DNA synthesis in the cell cycle. This was observed first as a variable pattern of nuclear staining in nonsynchronized cells, with early G1 cells staining poorly and late G1 and S phase cells showing nucleolar or uniform nucleoplasmic staining [Fig. 1]. In cells synchronized by starvation or by double thymidine block, nucleolar localization of PCNA was associated with the late G1 and early S phase of DNA synthesis, while the more uniform nucleoplasmic localization was observed at mid- and late S phase [19].
Fig. 1.
PCNA localization on nonsynchronized HEp-2 cells using human lupus autoantibody. Nuclei of cells in different phases of the cell cycle showed different patterns of staining, some with nucleolar staining associated with speckled staining at the nuclear periphery while others showed uniform fine-speckled nucleoplasmic staining. Nucleolar localization of PCNA was associated with early S phase of DNA synthesis while uniform nucleoplasmic localization was associated with mid and late S phase.
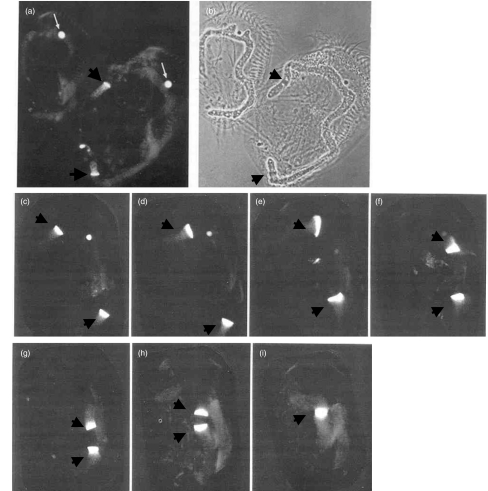
In contrast to the multiple foci of DNA synthesis in mammalian cell nuclei, the ciliated protozoan Euplotes eurystomus exhibits only two sites of DNA synthesis, the germline micronucleus and a macronucleus with DNA synthesis taking place at the replication band (RB). At the beginning of the macronucleus S phase, an RB forms at each tip of a macronucleus and with progression of S phase, the RBs migrate towards each other, fusing at the termination of S phase. Lupus anti-PCNA sera recognize the RBs of E. eurystomus[20] and demonstrate dramatically the movement of RBs through the S phase of macronuclear DNA synthesis [Fig. 2]. This study supports not only the association of PCNA with DNA synthesis but also the highly conserved nature of PCNA epitopes recognized by human autoantibodies. It has also been shown that PCNA co-localized with labelled nucleotide uptake at sites of DNA synthesis in mammalian tissue culture cells [21,22]. A critical proof that an autoantigen is related to or an important part of a cellular pathway or function would be the demonstration that the autoantibody would block or neutralize this function. This was shown for PCNA in DNA polymerase δ-dependent DNA synthesis [23], and this ability to neutralize function appears to be a common feature of autoantibodies found in lupus and many other autoimmune conditions. These include neutralizing activities of autoantibodies to histidyl-, threonyl- and other tRNA synthetases [24,25], to small nuclear ribonucleoprotein antigens of the Sm family involved in premRNA splicing [26,27], to DNA topoisomerase I [28] and to RNA polymerase I [29], among others.
Fig. 2.
Immunofluorescent localization of PCNA in the protozoan Euplotes eurystomus. Cells were permeabilized before fixation and arranged with the anterior end pointing up. (a) and (b) are immunofluorescent and phase contrast images of the same field showing micronuclei (thin arrows) and the RBs of macronuclei (thick arrows). The macronuclei are in the form of inverted Cs and the cell on the right shows RB at both ends of the macronucleus at the beginning of the S phase. Frames (c–i) show immunofluorescent images of RBs advancing towards each other until fusion at the termination of S phase (from [20]).
Defining the amino acid sequence and the conformational structure of antigenic determinants or epitopes recognized by autoantibodies is important because these autoepitopes reflect how the patient's immune system has reacted to the antigen. In a study of autoepitopes on PCNA recognized by lupus sera, recombinant DNA constructs encoding different N- and C-terminal deletions were analysed by immunoprecipitation of in vitro transcription–translation products and by Western blotting of expressed fusion proteins [30]. In addition, overlapping 15-mer synthetic peptides covering the full-length protein were tested. The differences between two experimentally induced antibodies to PCNA (a rabbit antipeptide antiserum and a murine monoclonal antibody) and lupus sera were striking. None of 14 lupus sera reacted with the synthetic linear sequence peptides in contrast to the experimental antibodies which reacted with some of these linear sequences. All 14 lupus sera reacted positively in immunoprecipitation of labelled full-length transcription–translation products, but very few reacted with truncated products. Reaction in Western blotting with fusion proteins was variable, with only five of the 14 sera reacting with full-length or truncated proteins. These and other data suggested that epitopes of PCNA recognized by lupus sera comprised higher ordered conformational structures, such as might be seen with protein folding resulting in approximation of discontinuous sequences [31]. It was found that a compound peptide joining a sequence of 7 aa residues (159–165) from the mid-region with a sequence of 7 aa residues (255–261) at the extreme C-terminus simulated the characteristics of lupus antibody. Immunization with the compound peptide produced antibody that showed S-phase-related cell-cycle staining, but the antipeptide antibody had much lower avidity than lupus antibodies. Extensive studies by others have demonstrated that the majority of B cell epitopes are discontinuous and highly conformational [32]. Antibodies against discontinuous regions of a picornavirus protein have been demonstrated in foot and mouth disease of cattle [33]. In studies of human choriogonadotrophin, a region of the α subunit (residues 41–60) was joined to a region of the β subunit (residues 101–121) and antibodies to the compound peptide inhibited the binding of human choriogonadotrophin to its receptor [34]. Autoreactive epitopes defined by type 1 diabetes-associated human monoclonal antibodies have been mapped to the middle and C-terminal domains of GAD65 [35]. Further studies have shown that these autoantibodies target conformation-dependent chimeric peptides [36]. In the use of antigenic peptides for immunotherapy, increased attention should be given to use of constructs which simulate what the immune system sees in vivo.
AUTOANTIBODIES IN CANCER
In recent years there has been a steadily increasing number of studies describing and characterizing autoantibodies in cancer. Beginning with the observation of antibodies to p53 in patients with breast cancer [7], the expanding list of autoantibodies to tumour-associated antigens (TAAs) include HER-2/neu [37], ras proteins [38], cell-cycle associated proteins such as cyclin B1 [39], cancer/testis antigens [40], antigens associated with paraneoplastic neurological disease syndromes [3,4,41], carbohydrate antigens such as MUC1 and gangliosides [42] and others (see [10] for review). A technique which has been very productive in identifying TAAs uses antibody-containing sera to screen cDNA expression libraries, a technique which was used first to isolate a cDNA clone encoding the human lupus SS-B/La antigen [43], other autoimmune antigens [44,45] and subsequently used in the cloning of TAAs [46,47]. It is widely acknowledged, however, that many of the antigens identified by these methods are not necessarily tumour-associated and that follow-up studies should be conducted to distinguish between antigens which are also associated with other conditions and those which are more tumour-related. A paradigm for defining what is a TAA has not been established but one approach would be stringent demonstration that the antigen is significantly less or non-reactive with sera from a wide variety of non-malignant conditions.
TAAs providing insights into immunogenicity of intracellular proteins
Unlike in lupus and other systemic autoimmune diseases, where knowledge of factors which might initiate immunogenicity of autoantigens is not available, in cancer important insights into this phenomenon have been reported. An informative study of p53 mutations in lung cancer showed that point mutations leading to missense protein products were correlated highly with presence of autoantibodies whereas stop, splice/stop, splice and frameshift mutations were not [48]. The point mutations occurred in the hot spot regions of p53 in exons 4–9 and resulted in the expression of abnormal proteins which have altered functions and increased stability compared to rapidly degraded wild-type protein. No antip53 antibodies were detected in patients whose tumours had stop, splice and frameshift mutations and there were some patients with missense p53 mutations who did not develop antip53 antibodies. The latter could be related to MHC and other antigen-processing immune response regulators in the individual patient. It might have been expected that missense p53 proteins had acquired abnormal antigenic determinants and that autoantibodies to p53 were targeted against these neoepitopes. However, antip53 autoantibodies reacted equally well with wild-type or mutated p53 proteins and the autoepitopes were at the amino- and carboxy-terminal regions, away from the central region where the hot-spot mutations were identified [49,50]. These findings in cancer support the findings in autoimmune diseases where autoantibodies can be used in immunofluorescent microscopy to track the localization and movement of native proteins such as centromeres/kinetochores in normally occurring chromosome condensation and segregation during mitosis [51]. It may not be the abnormal structure of the mutated protein itself that is immunogenic but factors such as the increased antigenic load of the aberrant protein or its ectopic localization which might be responsible for the immunogenic stimulus.
Another pathway by which tumour proteins might be rendered immunogenic came from studies of RNA binding proteins. In a rhabdomyosarcoma cell line, three proteins were identified which bound to the 5′ untranslated region of insulin-like growth factor II (IGF II) leader 3 mRNA [52]. IGF-II is a growth factor which has been implicated in tumorigenesis [53,54]. Homologues of these proteins, called IMPs (IGF II mRNA binding proteins), have been found in chicken (zip code binding protein [55]), in Xenopus oocyte [56,57] and in the mouse [58]. The mouse homologue of IMP-1, called CRD-BP, binds to the coding region of c-myc mRNA and shields c-myc mRNA from nucleolytic degradation. IMP-1/CRD-BP was detected in 73% of malignant mesenchymal and 40% of benign mesenchymal tumours and high expression was found in all 14 Ewing's sarcoma [59]. Gene amplification of CRD-BP has been found in breast cancer [60]. IMP-3 also called Koc [61] was found to be overexpressed first in human pancreatic cancer and in other cancers. Using autoantibodies from patients with hepatocellular carcinoma (HCC), a cDNA encoding a splice variant of IMP-2 called p62 was isolated [62]. When recombinant protein from the p62 cDNA clone was used as antigen, 21% of a cohort of HCC patients were found to have autoantibodies. It had been shown in the mouse that this small family of IGF-II mRNA binding proteins were regulated developmentally and transcripts were expressed highly in mouse embryo until the 12th to 13th day, but was essentially turned off after that and remained down-regulated in adult tissues [63]. IMP2/p62 transcripts were also demonstrated to be present in human fetal livers from 18 to 24 weeks of age but were not detectable in adult livers by Northern blotting [64]. One-third (9/27) of HCC liver specimens were found to express p62/IMP2 protein in the cancer cells of HCC nodules, whereas adjacent normal liver cells in the same specimens and normal adult liver were devoid of detectable protein by immunohistochemistry [64]. These characteristics of p62 are compatible with those of oncofetal proteins.
The IMP family of IGF-II mRNA binding proteins are distinguished by two different RNA-binding motifs, one set of consensus sequence RNA-binding domain (CS-RBD) at the N-terminus and four repeats of hnRNP K homology (KH) domains in spaced intervals from the mid-region to the C-terminus. There are other groups of RNA-binding proteins where aberrant regulation is related to the paraneoplastic neurological disorder (PND) syndromes. Some neurological symptoms, such as opsoclonus myoclonus ataxia, cerebellar degeneration and limbic and brain stem encephalitis, have strong associations with tumours of the lung, breast, ovary and testes [3,4]. PND patients make antibodies to RNA-binding proteins that are normally neurone-specific but become expressed abnormally in these non-neural tumours. Two classes of these proteins have been identified. The Hu proteins expressed aberrantly in tumours associated with sensory neuroneopathy [65] are highly homologous to the Drosophila protein ELAV (embryonic lethal abnormal vision) and contain three sets of CS-RBP. The ELAV/Hu proteins bind to the AU-rich elements in the 3′-UTR of proto-oncogene and cytokine mRNAs including c-myc, c-fos, c-myb and GM-CSF. The role of the Hu proteins in tumorigenesis appears to parallel the IGF-II mRNA binding proteins. The AU-rich elements promote degradation of mRNAs but binding of the protein to these elements confers resistance to degradation leading to greater production of translation products [66]. A second group of RNA-binding proteins called Nova are involved as aberrantly expressed antigens in PND syndromes and these proteins contain KH-related binding motifs [4]. All small cell lung cancers express the ELAV/Hu protein but not all produce antibodies perhaps for reasons described earlier with antip53 responses.
Autoantibodies in diagnostic and predictive oncology
In lupus, Sjögren's syndrome, scleroderma and other systemic rheumatic diseases, autoantibodies have been useful as diagnostic markers to differentiate one disease from another when a disease is in its early stage or when there are overlapping symptoms with other diseases [1]. Antibodies to double-strand DNA and to Sm small nuclear ribonucleoprotein (Sm–snRNP) antigens are diagnostic of lupus as they rarely occur in other illnesses and there are several other examples of such diagnostic antibodies. Other autoantibodies cross over into more than one illness and examples include anti-U1-snRNP which is present in lupus and mixed connective tissue disease, and anti-SS-A/Ro and anti-SS-B/La which are present in lupus and Sjögren's syndrome. However, such cross-over autoantibodies rarely involve more than two diseases. These findings have evolved into the idea of profiles of autoantibodies characteristic of separate illnesses and have become useful aids in differential diagnosis [1].
The situation in cancer appears to be different. Anti-p53 is present in a wide variety of cancers, including esophageal, bladder, colon, ovarian, lung, breast, gastric cancers and hepatocellular carcinoma [67] and the frequency varies from 14% to 31%. The frequency in healthy adults was 1·5%. Certain other cancers such as lymphomas, prostate, testicular cancers and melanomas have frequencies not differing significantly from healthy adults. Such wide distribution of antibody responses in cancer has also been found with respect to other TAAs, including c-myc, IMP2/p62, IMP3/Koc, IMP1, cyclin B1 and survivin [68]. It would be important to determine the pattern of antibody responses to TAAs of more restricted cell expression such as the onconeural antigens in PND syndromes and melanoma antigens to conclude whether this is the general paradigm of cancer autoantibodies.
The frequency of antibodies to an individual TAA averages 15–20% and although the specificity for cancer could be high, such as 95% specificity for antip53 [67], the low sensitivity of individual autoantibodies make them poor diagnostic indicators. In a study involving 777 patients with 10 different types of cancer, it was noted that 11·6% had antibodies to p62/IMP2 and 12·2% had antibodies to Koc/IMP3 [69]. When the data were analysed for frequency of antibodies to the combination of the two TAAs, the frequency of antibodies was 20·5%, a figure much higher than the result with one TAA alone. These data indicated that the majority of antibody positive sera reacted with either one or the other TAA and that a only small fraction contained antibodies reactive with both TAAs.
This notion was examined in a follow-up study of 527 sera from hepatocellular carcinoma and from breast, lung, colorectal, gastric and prostate cancers. Full-length recombinant proteins were purified from cDNA clones expressing c-myc, p53, cyclin B1, p62/IMP2, Koc/IMP3, IMP1 and survivin and used as antigens in enzyme immunoassay with controls consisting of normal human sera and 103 sera from lupus and Sjögren's syndrome [68]. For every type of cancer sera examined, there were an increasing number of positive reactions with the sequential addition of each TAA [Table 2]. In the case of lung cancer, 67·9% were positive for antibody in the seven TAA mini-array [68]. The selection of the seven TAAs was based on their known association with tumours and previously documented autoantibody responses. It could be optimized and be designed with panels having greater specificity for certain types of tumours. Gene abnormalities, such as mutations or overexpression tend to occur in combinations that vary from tissue to tissue [70], so that it is possible that the antibody responses would be different from one cancer to another. It would be of interest to determine if addition of HER2/neu to the mini-array of TAAs would enhance antibody positivity for breast over other types of cancer [71].
Table 2.
Frequency of autoantibodies in relationship to number of TAAs in mini-array
| Type of cancer | ||||||
|---|---|---|---|---|---|---|
| Antigen(s) | Breast 64 | Lung 56 | Colorectal 45 | Prostate 206 | Gastric 91 | HCC 65 |
| IMP1 | 5 (7·8)a | 4 (7·1) | 6 (13·3) | 18 (8·7) | 15 (16·5) | 10 (15·4) |
| IMP1 or p62 | 8 (12·5) | 15 (26·8) | 8 (17·8) | 64 (31·1) | 18 (19·8) | 15 (23·1) |
| IMP1 or p62 or Koc | 14 (21·9) | 19 (33·9) | 10 (22·2) | 72 (35·0) | 28 (30·8) | 20 (30·8) |
| IMP1 or p62 or Koc or p53 | 17 (26·6) | 25 (44·6) | 16 (35·6) | 78 (37·9) | 37 (40·7) | 24 (36·9) |
| IMP1 or p62 or Koc or p53 or c-myc | 25 (39·1) | 27 (48·2) | 17 (37·8) | 81 (39·3) | 40 (44·0) | 32 (49·2) |
| IMP1 or p62 or Koc or p53 or c-myc or cyclin B1 | 26 (40·6) | 36 (64·3) | 23 (51·1) | 95 (46·1) | 47 (51·6) | 35 (53·8) |
| IMP1 or p62 or Koc or p53 or c-myc or cyclin B1 or survivin | 28 (43·8) | 38 (67·9) | 23 (51·1) | 95 (46·1) | 48 (52·7) | 37 (56·9) |
Numbers in parenthesis are percentage of positive reactors in that cancer category. Positive reactors are defined as above the mean of 82 NHS ± 3 s.d. (from [68]).
Many studies have shown that antip53 antibodies can appear before clinical detection of cancer. These include reports on two smokers who developed lung cancer years after detection of antip53 antibodies and one who has enjoyed long remission because the cancer was detected early [67,72]. Anti-p53 has also been detected before diagnosis of cancer in obstructive pulmonary disease [73] and in workers exposed to vinyl chloride who are at high risk for cancer development [74]. In uranium miners who are at high risk for developing lung cancer, antip53 was detected 17–47 months before clinical tumour manifestation in some patients [75]. Liver cirrhosis and chronic hepatitis are conditions with high predisposition to developing hepatocellular carcinoma, and such patients developed novel autoantibodies several months preceding cancer detection [76].
DISCUSSION
There are similarities and differences between autoantibodies in lupus and certain autoimmune rheumatic diseases on the one hand and autoantibodies in cancer on the other. Each lupus or related autoimmune disease is characterized by the presence of some autoantibody of unique specificity such as antidouble-strand DNA and/or anti-Sm for lupus and antitopoisomerase I for systemic scleroderma, but this appears not to be the case for cancer autoantibodies. Anti-p53, antic-myc, anticyclin B1 and other antibodies are present in many different types of cancer. Although it may be said that molecules such as p53, c-myc and cyclin B1 are ubiquitous in all cells, this is also true of antigens such as the Sm proteins which are involved in splicing of precursor mRNAs. It is still possible that further studies may reveal autoantibodies specifically related to some cancers due to aberrations of unique oncogenes or tumour suppressor genes. At the present time the reasons for this difference between lupus and cancer are unexplained. Nevertheless, identification of cancer autoantibodies could become useful in diagnosis because the likelihood of detecting antibody to a TAA can be increased substantially by using a mini-array of several TAAs as the immunoassay platform (Table 2). An array of seven TAAs detected antibody in 67·9% of lung cancer and to a somewhat lower percentage in other types of cancer [68], but as discussed earlier these numbers could be increased by increasing the numbers of TAAs in the mini-array and the use of custom-made antigen arrays for different cancers. Furthermore, there is already evidence that in high-risk individuals or certain occupations, autoantibodies may be predictive of incipient tumours.
Although many antigens and their molecular structures and functional properties have been characterized extensively in lupus and other autoimmune rheumatic diseases, there has been little understanding as to why these ubiquitous intracellular proteins or nucleic acids become immunogenic. In cancer, there is good evidence that immunogenicity could be due to gene mutations as in p53 or to abnormal regulation of accessory oncogenic factors such as the IGFII due to interaction with mRNA binding proteins. Thus in cancer, immunogenicity of TAAs could be related to genetic or epigenetic aberrations, which are not limited to the two examples described. These observations in cancer could be used as clues to ascertain whether immunogenicity of antigens in lupus and other disorders may have a similar basis.
A question which has long been the focus of much investigation is whether autoantibodies to intracellular antigens have a role in pathogenesis. In a situation where the antigen is at the cell membrane, such as epitopes on the alpha loop of the nicotinic acetylcholine receptor (nAChR), circulating antibodies to nAChR in myasthenia gravis correlate in many ways with clinical symptoms and produce the expected effects of interference at the neuro–muscular junction [77–80]. However, in other cases where the antigens are localized in the cytoplasm, nucleus or nucleolus they would be inaccessible to circulating antibody. If, by some mechanism, circulating antibodies were capable of reacting with intracellular antigens in vitro and have some deleterious effect on function, in lupus one might expect to see abnormalities in splicing (for anti-Sm antibodies) and translation (for antiribosomal RNP antibodies), but these have not been reported. In lupus, the most well-documented pathogenic effect of autoantibodies has been shown for anti-DNA and this is due to antigen–antibody complex formation in the circulation or in tissues such as the glomerular capillaries where antibodies bind to DNA deposited previously at that site. The origin of the extracellular DNA has not been demonstrated conclusively but the most favoured hypothesis is cell death due to necrosis or apoptosis.
A somewhat similar discussion has been ongoing for cancer autoantibodies. The literature on the relationship of antip53 antibodies and clinical outcome in cancer patients is extensive and there are numerous reports of both favourable and poor outcomes. The conflicting studies may be related to biased patient populations or to variables in the immunoassay systems [81–85]. A study using native p53 recombinant protein and a large number of patients with ovarian tumours showed that antip53 was predictive of invasive cancer and poor survival [86]. In paraneoplastic neurological disorder syndromes, there have been some instances of spontaneous tumour regression [87] which may be related to the presence of killer T cells [88]. Many factors have to be considered in investigating the possible pathogenetic role of circulating autoantibodies, including whether the autoantigens are accessible, whether cell necrosis might be occurring with release of intracellular antigens into the extracellular environment and whether helper T cells, cytotoxic T lymphocytes or NK cells have been activated. Autoantibodies are pathogenetically uncommitted and whether they are protective or deleterious is due to a combination of its interaction with other immune or inflammatory factors, as has been shown in the eradication of established HER2/neu carcinoma in an experimental model [89].
Cancer immunotherapy based on the use of peptide antigens to enhance immune responses has received intensive attention in recent years [90–93]. The candidate antigens can now be identified readily either by looking for target antigens of antibodies or of T cells. A major problem is the selection of a candidate peptide which would be strongly immunogenic and induce the desired T cell responses in vivo. Some strategies have been directed at modifying amino acid residues on putative autoepitopes such as a recent study on a proinsulin peptide which resulted in the induction of either regulatory CD4+ or cytotoxic CD8+ T cells in the non-obese diabetic mouse [94,95]. The experiments described earlier on the nature of autoepitopes on the lupus autoantigens SS-B/La and PCNA show that autoepitopes can be complex in structure and be conformation-dependent discontinuous regions of an antigen. Because we do not have any clear ideas as to how intracellular antigens are processed and how these lead to autoimmune responses, whether at the B or T cell level, it might be advisable to use the intelligence conveyed by the immune system with respect to the nature of autoepitopes [11]. This type of information could be useful in the design of immunotherapeutic peptides as the immune system has shown how it has been able to respond to the self-antigen.
Acknowledgments
This study was supported in part by NIH grant CA56956. Fu-Dong Shi is a recipient of a Development Grant from Muscular Dystrophy Association. This is manuscript 15879–MEM from The Scripps Research Institute.
REFERENCES
- 1.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 2.Notkins A, Lernmark A. Autoimmune type I diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–52. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posner JB, Dalmau J. Paraneoplastic syndromes. Curr Opin Immunol. 1997;9:723–924. doi: 10.1016/s0952-7915(97)80055-6. [DOI] [PubMed] [Google Scholar]
- 4.Musunuru K, Darnell RB. Paraneoplastic neurologic disease antigens: RNA-binding proteins and signaling proteins in neuronal degeneration. Annu Rev Neurosci. 2001;24:239–62. doi: 10.1146/annurev.neuro.24.1.239. [DOI] [PubMed] [Google Scholar]
- 5.Gershwin ME, Manns MP, Mackay IR. Molecular analysis of cytoplasmic autoantigens in liver disease. In: Rose NR, Mackay IR, editors. The autoimmune diseases, II. San Diego: Academic Press; 1992. pp. 213–34. [Google Scholar]
- 6.Leslie D, Lipsky P, Notkins AL. Autoantibodies as predictors of disease. J Clin Invest. 2001;108:1417–22. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30:403–8. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 8.Boon T, Van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang R-F. CD4+ T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci USA. 2001;98:3964–9. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad K. Autoantibodies in cancer patients and in persons with a higher risk of cancer development. In: Shoenfeld Y, Gershwin ME, editors. Cancer and autoimmunity. Amsterdam: Elsevier Science; 2000. pp. 159–73. [Google Scholar]
- 11.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alspaugh MA, Talal N, Tan EM. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976;19:216–22. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- 13.Rinke J, Steitz JA. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La antibodies. Cell. 1982;29:149–56. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- 14.Chan EKL, Tan EM. Human autoantibody reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987;166:1627–40. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–334. [PubMed] [Google Scholar]
- 16.Prelich G, Tan C-K, Kostura M, et al. Functional identity of proliferating cell nuclear antigen and a DNA polymerase δ auxiliary protein. Nature (Lond) 1987;236:517–20. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- 17.Bravo R, Fey SJ, Bellatin J, Larsen PM, Arevalo J, Celis JE. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981;136:311–9. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- 18.Mathews MB, Bernstein RM, Franza BR, Jr, Garrels JI. Identity of the proliferating cell nuclear antigen and cyclin. Nature (Lond) 1984;309:374–6. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- 19.Takasaki Y, Deng J-S, Tan EM. A nuclear antigen associated with cell proliferation and blast transformation. Its distribution in synchronized cells. J Exp Med. 1981;154:1899–909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olins DE, Olins AL, Cacheiro LH, Tan EM. Proliferating cell nuclear antigen/cyclin in the ciliate Euplotes eurystomus: localization in the replication band and in micronuclei. J Cell Biol. 1989;109:1399–410. doi: 10.1083/jcb.109.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravo R, Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985;4:655–61. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celis JE, Madsen P, Celis A, Nielsen HV, Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase δ) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett. 1987;220:1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- 23.Tan C-K, Sullivan K, Li X, Tan EM, Downey KM, So AG. Autoantibody to the proliferating cell nuclear antigen neutralizes the activity of the auxiliary protein for DNA polymerase delta. Nucl Acids Res. 1987;15:9299–308. doi: 10.1093/nar/15.22.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews MB, Bernstein RM. Myositis autoantibody inhibits histidyl-tRNA synthetase. A model for autoimmunity. Nature (Lond) 1983;304:177–9. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- 25.Dang CV, Tan EM, Traugh JA. Myositis autoantibody reactivity and catalytic function of threonyl-tRNA synthetase. FASEB J. 1988;2:2376–9. doi: 10.1096/fasebj.2.8.2452112. [DOI] [PubMed] [Google Scholar]
- 26.Yang VW, Lerner MR, Steitz JA, Flint SJ. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci USA. 1981;78:1371–5. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fradin A, Jove R, Hemenway C, Kaiser HD, Marley JC, Prives C. Splicing pathways of SV40 mRNAs in X. laevis oocytes differ in their requirements for snRNPs. Cell. 1954;37:927–32. doi: 10.1016/0092-8674(84)90427-6. [DOI] [PubMed] [Google Scholar]
- 28.Shero JH, Bordwell B, Rothfield NF, Earnshaw WC. Autoantibodies to topoisomerase I are found in sera from scleroderma patients. Science. 1986;231:737–40. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- 29.Reimer G, Rose KM, Scheer U, Tan EM. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987;79:65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huff JP, Roos G, Peebles CL, Houghten R, Sullivan KF, Tan EM. Insights into native epitopes of proliferating cell nuclear antigen using recombinant DNA protein products. J Exp Med. 1990;172:419–29. doi: 10.1084/jem.172.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muro Y, Tsai W-M, Houghten R, Tan EM. Synthetic compound peptide simulating antigenicity of conformation-dependent autoepitope. J Biol Chem. 1994;269:18529–34. [PubMed] [Google Scholar]
- 32.Laver WG, Air GM, Webster RG, Smith-Gil SJ. Epitopes on protein antigens: misconceptions and realities. Cell. 1990;61:553–6. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- 33.Parry NR, Barnett PV, Ouldridge EJ, Rowlands DJ, Brown F. Neutralizing epitopes of type 0 foot and mouth disease virus. II. Mapping three conformational sites with synthetic peptide reagents. J General Virol. 1989;70:1493–503. doi: 10.1099/0022-1317-70-6-1493. [DOI] [PubMed] [Google Scholar]
- 34.Bidart JM, Troalen F, Ghillani P, et al. Peptide immunogen mimicry of a protein-specific structural epitope on human choriogonadotropin. Science. 1990;248:736–9. doi: 10.1126/science.1692160. [DOI] [PubMed] [Google Scholar]
- 35.Richter W, Shi Y, Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci USA. 1993;90:2832–6. doi: 10.1073/pnas.90.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers AC, Bavik K, Tremble J, Daw K, Scherbaum A, Banga JP. Comparative analysis of epitope recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different autoimmune disorders. Clin Exp Immunol. 1999;118:349–56. doi: 10.1046/j.1365-2249.1999.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High titer HER-2/neu protein-specific antibody can be detected in patients with early stage breast cancer. J Clin Oncol. 1997;15:3363–7. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 38.Takahasi M, Chen W, Byrd DR, et al. Antibody to ras proteins in patients with colon cancer. Clin Cancer Res. 1995;1:1071–7. [PubMed] [Google Scholar]
- 39.Covini G, Chan EKL, Nishioka M, Morshed SA, Reed SI, Tan EM. Immune response to cyclin B1 in hepatocellular carcinoma. Hepatology. 1997;25:75–80. doi: 10.1002/hep.510250114. [DOI] [PubMed] [Google Scholar]
- 40.Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lennon VA. Calcium channel and related paraneoplastic disease autoantibodies. In: Peter JB, Shoenfeld Y, editors. Autoantibodies. Amsterdam: Elsevier; 1996. pp. 139–46. [Google Scholar]
- 42.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finin OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 43.Chambers JC, Keene JD. Isolation and analysis of cDNA clones expressing the human lupus La antigen. Proc Natl Acad Sci USA. 1985;82:2115–21. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome p450db1. J Clin Invest. 1989;83:1284–92. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Chetrit E, Gandy BJ, Tan EM, Sullivan KF. Isolation and characterization of a cDNA clone encoding the 60 kDa component of the human SS-A/Ro RNP autoantigen. J Clin Invest. 1989;83:1284–92. doi: 10.1172/JCI114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai H, Chan EKL, Kiyosawa K, Fu X-D, Tan EM. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J Clin Invest. 1993;92:2419–26. doi: 10.1172/JCI116848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahin U, Tûreci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdor AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–74. [PubMed] [Google Scholar]
- 49.Labrecque S, Naftaly N, Thomson D, Matlas K. Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993;53:3468–71. [PubMed] [Google Scholar]
- 50.Lubin R, Schlichtholz B, Bengoufa D, Soussi T. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53 on primary structure and exposure on protein surface. Cancer Res. 1993;53:5872–6. [PubMed] [Google Scholar]
- 51.Brenner S, Pepper D, Berns MW, Tan EM, Brinkley BR. Kinetochore structure, duplication and distribution in mammalian cells. Analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981;91:95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Weaver UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1261–70. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cariani E, Lassere C, Seurin D, et al. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors and liver cirrhosis. Cancer Res. 1988;48:6844–9. [PubMed] [Google Scholar]
- 54.Westley BR, May FEB. Insulin-like growth factors: the unrecognized oncogenes. Br J Cancer. 1995;72:1065–6. doi: 10.1038/bjc.1995.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a β-actin mRNA zip code binding protein. Mol Cell Biol. 1997;17:2158–65. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deshler JO, Higkett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by Vera protein and the endoplasmic reticulum. Science. 1997;276:1128–31. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
- 57.Havin L, Git A, Zichrini E, et al. RNA-binding protein conserved in both microtubule and microfilament based RNA location. Genes Dev. 1998;12:1593–8. doi: 10.1101/gad.12.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doyle GAR, Betz NA, Leeds PF, Fleisig AJ, Prokipcak RD, Ross J. The c-myc coding region determinant binding protein; a member of a family of KH domain RNA-binding proteins. Nucl Acids Res. 1998;26:5036–44. doi: 10.1093/nar/26.22.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ioannidis P, Trangas T, Dimitriadis E, et al. C-myc and IGF-II mRNA-binding protein (CRD-BP/IMP1) in benign and malignant mesenchymal tumors. Int J Cancer. 2001;94:480–4. doi: 10.1002/ijc.1512. [DOI] [PubMed] [Google Scholar]
- 60.Doyle GA, Bordeaux-Heller JM, Coulthard S, Meisner LF, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA binding protein. Cance Res. 2000;60:2756–9. [PubMed] [Google Scholar]
- 61.Mueller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly expressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–33. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J-Y, Chan EKL, Peng X-X, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–10. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen FC, Nielsen J, Kristensen MA, Koch G, Christiansen J. Cytoplasmic trafficking of IGF II mRNA binding protein by conserved KH domains. J Cell Sci. 2002;115:2087–97. doi: 10.1242/jcs.115.10.2087. [DOI] [PubMed] [Google Scholar]
- 64.Lu M, Nakamura RM, Dent ED, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Path. 2001;159:945–53. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalmau J, Furneaux HM, Condon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–6. [PMC free article] [PubMed] [Google Scholar]
- 66.Keene JD. Why is Hu where? Shuttling of early response messenger RNA subsets. Proc Natl Acad Sci USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soussi T. p53 antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–88. [PubMed] [Google Scholar]
- 68.Zhang J-Y, Casiano C, Peng X-X, Koziol JA, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epid Biomarkers Prev. 2003;12:136–43. [PubMed] [Google Scholar]
- 69.Zhang J-Y, Chan EKL, Peng X-X, et al. Autoimmune responses to mRNA binding proteins p62 and Koc in diverse malignancies. Clin Immunol. 2001;100:149–56. doi: 10.1006/clim.2001.5048. [DOI] [PubMed] [Google Scholar]
- 70.Bartek J, Lukas J. Are all cancers equal? Nature (Lond) 2001;411:1001–2. doi: 10.1038/35082655. [DOI] [PubMed] [Google Scholar]
- 71.Cheever MA, Disis ML, Bernhard H, et al. Immunity to oncogenic proteins. Immunol Rev. 1995;145:33–59. doi: 10.1111/j.1600-065x.1995.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 72.Lubin R, Zalcman G, Bouchet L, et al. Serum p53 antibodies as early markers of lung cancer. Nature Med. 1995;1:701–2. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]
- 73.Trivers GE, De Benedetti VM, Cawley HL, et al. Anti-p53 antibodies in sera from patients with chronic obstructive pulmonary disease can predict a diagnosis of cancer. Clin Cancer Res. 1996;2:1767–75. [PubMed] [Google Scholar]
- 74.Trivers GE, Cawley HL, DeBenebitti VMG, et al. Anti-p53 antibodies in sera of workers occupationally exposed to vinyl chloride. J Natl Cancer Inst. 1995;87:1400–7. doi: 10.1093/jnci/87.18.1400. [DOI] [PubMed] [Google Scholar]
- 75.Royahem J, Conrad K, Frey M, Melhom J, Frank KH. Autoantibodies predictive parameters of tumor development? In: Conrad K, Humbel RL, Meurer M, Shoenfeld Y, Tan EM, editors. Pathogenic and diagnostic relevance of autoantibodies. Berlin: Pabst Science Publications; 1998. pp. 412–4. [Google Scholar]
- 76.Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71:26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 77.Drachman DB, deSilva S, Ramsay D, Pestronik A. Humoral pathogenesis of myasthenia gravis. Ann NY Acad Sci. 1987;505:90–105. doi: 10.1111/j.1749-6632.1987.tb51285.x. [DOI] [PubMed] [Google Scholar]
- 78.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nature Rev. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 79.Shen XM, Ohno K, Tsujino A, et al. Mutation causing severe myasthenia gravis reveals functional asymmetry of AChR signature cystine loops in agonist binding and gating. J Clin Invest. 2003;203:497–505. doi: 10.1172/JCI16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–13. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winter SF, Sekido Y, Minna JD, et al. Antibodies against autologous tumor cell proteins in patients with small cell lung cancer. Association with improved survival. J Natl Cancer Inst. 1993;85:2012–8. doi: 10.1093/jnci/85.24.2012. [DOI] [PubMed] [Google Scholar]
- 82.Bergquist M, Bratstrõm D, Larson A, et al. p53 autoantibodies in non-small cell lung cancer patients can predict increased life expectancy after radiotherapy. Anticancer Res. 1998;18:1999–2002. [PubMed] [Google Scholar]
- 83.Porzsolt F, Schmid M, Hõhes D, Muche R, Gaus W, Montenarh M. Biological relevance of autoantibodies against p53 in patients with metastatic breast cancer. Onkologie. 1994;17:402–8. [Google Scholar]
- 84.Marxsen J, Schmiegel W, Roder C, et al. Detection of anti-p53 antibody response in malignant and benign pancreatic disease. Br J Cancer. 1994;70:1031–4. doi: 10.1038/bjc.1994.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenfeld MR, Malats N, Schramm L, et al. Serum p53 antibodies and prognosis of patients with small cell lung cancer. J Natl Cancer Inst. 1997;89:381–5. doi: 10.1093/jnci/89.5.381. [DOI] [PubMed] [Google Scholar]
- 86.Vogl FD, Frey M, Kreienberg R, Runnebaum IB. Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br J Cancer. 2000;83:1338–43. doi: 10.1054/bjoc.2000.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Byrne T, Mason WP, Posner JB, Dalmau J. Spontaneous neurological improvement in anti-Hu associated encephalomyelitis. J Neurol Neurosurg Psychiatry. 1997;62:276–8. doi: 10.1136/jnnp.62.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–4. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 89.Curcio C, Di Carlo E, Clynes R, et al. Nonredundant roles for antibody, cytokines and perforin in the eradication of established HER2/neu carcinomas. J Clin Invest. 2003;111:1161–70. doi: 10.1172/JCI17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dranoff G. Coordinated tumor immunity. J Clin Invest. 2003;111:1116–8. doi: 10.1172/JCI18359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reeves WH. Tumor immunity and autoimmunity: a case of Dr Jekyll and Mr Hyde. Clin Immunol. 2001;2:129–33. doi: 10.1006/clim.2001.5070. [DOI] [PubMed] [Google Scholar]
- 92.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–94. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris EC, Bendle GM, Stauss HJ. Prospects for immunotherapy of malignant disease. Clin Exp Immunol. 2003;131:1–7. doi: 10.1046/j.1365-2249.2003.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez NR, Augstein P, Moustakas AK, et al. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365–71. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pugliese A. Peptide-based treatment for autoimmune diseases: learning how to handle a double-edged sword. J Clin Invest. 2003;111:1280–2. doi: 10.1172/JCI18395. [DOI] [PMC free article] [PubMed] [Google Scholar]