Abstract
C57Bl6 mice sensitized to Dermatophagoides pteronyssinus and challenged with D. pteronyssinus allergen extract given intranasally followed by treatment with intranasal applications of a 60-kDa truncated, trimeric recombinant form of human SP-D (rfhSP-D) showed a significant reduction in serum IgE, IgG1, peripheral blood eosinophilia and airway hyperresponsiveness compared to saline or bovine serum albumin-treated controls. Intracellular cytokine staining of lung and spleen homogenates showed increases in interleukin (IL)-12 production in lung tissue and normalization of IL-12 and interferon (IFN)-γ in spleen tissue. In previous studies we demonstrated the effectiveness of native SP-D and rfhSP-D in down-regulating allergic responses to allergens of Aspergillus fumigatus. The results reported here indicate that rfhSP-D can suppress the development of allergic symptoms in sensitized mice challenged with allergens of the common house dust mite.
Keywords: airway hyperresponsiveness, house dust mite, IL-12, plethysmography, SP-D
INTRODUCTION
The house dust mite, Dermatophagoides pteronyssinus, is one of the leading causes of allergic asthma in the industrialized world [1–3]. In some studies as many as 85% of asthmatics responded with a positive skin prick test to house dust mite allergen extract [4]. Many studies have shown the importance of interleukin (IL)-12 in inhibiting Th2 cytokine production and IgE both in vitro and in vivo [5–7], and administration of IL-12 by intraperitoneal injection in the mouse was shown to reduce D. pteronyssinus-specific IgE and airway eosinophilia [8]. However, there are toxicity and dosage problems with the direct administration of cytokines. Another approach is to use natural up-regulators to elevate endogenous IL-12 or interferon (IFN)-γ. Many microbial products, including heat-killed bacteria and CpG motifs, up-regulate Th1 cytokines, including IL-12 [9,10], but there is a concern that such microbial products could overstimulate the Th1 cytokine profile leading to Th1-mediated pathology such as autoimmunity [11].
Surfactant protein D (SP-D), like surfactant protein A (SP-A), is a member of the collectin family of proteins found in the alveolar lining layer of the lung. Both SP-A and SP-D have important roles in innate host defence against inhaled pathogens [12]. There is also mounting evidence that the surfactant proteins (in particular SP-D) are able to modulate the process of allergic inflammation to airborne allergens [13,14]. While the mechanism of their immunomodulatory effect remains to be clarified, it is probably due in part to the ability of the of the carbohydrate recognition domain (CRD) to recognize and bind to glycosylated residues of allergen molecules, such as those of the common house dust mite [14].
Both SP-D and SP-A exist as higher-order multimers of non-covalent trimers of polypeptides which consist of a cysteine-rich N-terminal region, a long collagen-like ‘tail’, an alpha-helical ‘neck’ region and a C-terminal CRD. The hydrophobic neck region is essential for trimerization of the whole molecule [15].
We have demonstrated previously that native human SP-D, as well as the truncated 60 kDa rfhSP-D trimer, consisting of the α-helical coiled coil neck region and CRD, has immunomodulatory properties in a mouse model of allergic hypersensitivity to allergen extracts of Aspergillus fumigatus [16,17]. These effects appear to be mediated through the CRD [18]. It was shown that treatment of Afu allergic mice with native human SP-D or rfhSP-D produced a significant reduction in IgE and peripheral blood eosinophilia. There was a concomitant decrease in IL-2, IL-4 and IL-5 and an increase in IFN-γ in spleen cell culture supernatants, which suggested that the truncated fragment of human surfactant protein D (rfhSP-D) was effective in down-regulating allergic hypersensitivity against fungal allergens, by promoting Th-1 responses.
The aim of the present study was to investigate the effect of treatment with the same recombinant fragment of human SP-D in mice sensitized to and then challenged with allergens of the common house dust mite, D. pteronyssinus.
MATERIALS AND METHODS
Recombinant SP-D (rfhSP-D)
The 60 kDa trimeric fragment of human SP-D was expressed in Escherichia coli and purified as described elsewhere [16] and dissolved in endotoxin-free phosphate buffered saline (PBS). Endotoxin contamination was removed by passing over a column of polymyxin beads. Residual endotoxin levels were measured by the limulus amoebocyte lysate assay (BioWhitaker, UK) and only preparations containing less than 5 pg endotoxin/µg rfhSP-D were used.
Allergen extracts
Standardized D. pteronyssinus extract (Greer Laboratories, Lenoir, NC, USA) containing 10 000 allergy units (AU)/ml was diluted into sterile endotoxin-free PBS.
Sensitization
Female C57BL/6 mice were given 4-weekly i.p. injections of a mixture of allergen extract (63 AU D. pteronyssinus) with alum suspension (1 : 4 v/v Pierce Imject) in 100 µl of sterile PBS.
Allergen challenge and treatment with rfhSP-D
Five days after the last i.p. injection, sensitized mice were anaesthetized with isoflurane and challenged with 50 AU of D. pteronyssinus extract in PBS given intranasally followed by intranasal doses of PBS or rfhSP-D or BSA, given within 1–2 h. This protocol of allergen challenge followed by treatment was repeated on a daily basis for 4–5 days as indicated.
Peripheral blood eosinophils
Blood was collected from the tail vein of mice for estimation of peripheral eosinophils. The total leucocytes were counted with an automatic cell counter and the proportion of eosinophils determined by differential counting of May–Grunwald–Giemsa-stained blood smears. Results are expressed as 106 cells/ml.
Serum IgE and D. pteronyssinus-specific IgG1
Attempts to measure D. pteronyssinus-specific IgE were unsuccessful, due probably to competitive blocking by D. pteronyssinus-specific IgG, which occurs at a much higher concentration than IgE and the low concentration of D. pteronyssinus proteins in the extract. Total serum IgE was measured by capture ELISA using a kit and following the manufacturer's instructions (BD PharMingen, Cowley, UK). Tail-vein blood serum was diluted serially to give values which were linear with respect to a purified mouse IgE standard. Results are expressed in µg/ml. D. pteronyssinus-specific IgG1 was measured by first coating microwell plates with a 1/1000 dilution of the D. pteronyssinus extract diluted in sodium carbonate buffer overnight at 4°C, followed by blocking with 1% BSA (w/v) for 2 h. Serum was added at a dilution of 1/250–1/32 000 and incubated at 37°C for 2 h followed by washing and incubation with antimouse-IgG1-HRP conjugate (1/1000) for 1 h. Colour was developed by adding HRP substrate (Pierce & Warriner) and absorbance measured at 450 nm. Results are expressed as relative absorbance (A450 nm) after subtraction of background.
Intracellular cytokine staining
After treatment, mice were sacrificed humanely by CO2 asphyxiation and their lungs and spleens removed and homogenized in PBS. The homogenate was filtered and red blood cells lysed with ammonium chloride lysing reagent (BD Pharmingen) and fixed with 4% (v/v) paraformaldehyde for 20 min. The cells were washed with PBS supplemented with 3% (v/v) heat-inactivated fetal calf serum with 0·1% (w/v) sodium azide (FSB), re-suspended in 10% (v/v) DMSO in FSB and stored at −80°C.
Cells were permeabilized with Cytoperm wash buffer (CPB, BD Biosciences, Cowley, UK) for 15 min at 4°C and aliquots of 106 cells were blocked by incubation for 30 min at 4°C with CPB supplemented with 50 µg/ml rat IgG. Intracellular cytokines were stained with 1 µg phycoerythrin (PE)-conjugated antimouse cytokine monoclonal antibody (BD Biosciences) incubated for 30 min at 4°C. The cells were washed with CPB followed by FSB and re-suspended in 0·5 ml FSB.
Flow cytometry was performed with a FACScan flow cytometer (Beckton Dickinson, Mountain View, CA, USA) using CellQuest software. Data were collected for 20 000 cells. The average FSC of cells was 100 in all cases. Stained cells (FSC > 100, FL2 > 100) were gated and the proportion of these cells staining intensely for PE (PE > 1000) was calculated. Results are expressed as the percentage of intensely stained cells after subtraction of background fluorescence for unstained cells incubated with rat IgG (% PE > 1000).
Endogenous mouse SP-D and SP-A in the lung
Endogenous levels of SP-A and SP-D were measured in the BAL of mice as described previously [16]. Briefly, immediately after sacrifice by CO2 asphyxiation, bronchoalveolar lavage was performed with 3 × 1 ml PBS, which were pooled and the total volume BAL was noted for all samples. After centrifugation to remove cells, SP-D and SP-A were measured by ELISA using polyclonal antibodies raised previously against recombinant mouse SP-D and SP-A (provided by Dr P. Lawson). These antibodies were shown not to cross-react with human SP-D or SP-A and were specific for murine SP-D and SP-A. Results are expressed as µg/ml of BAL fluid.
Lung histology
Immediately after treatment, the lungs of mice from each treatment group were fixed in 10% (v/v) neutral buffered formalin, embedded in paraffin, sectioned and stained with haematoxylin and eosin and the degree of peribronchial inflammation scored independently on a scale of 0–4 (corresponding to a score of normal to severe, respectively) by a veterinary histologist blinded to the experimental details, as described previously [16].
Whole body plethysmography
In this study, airway hyperresponsiveness was measured using unrestrained whole body plethysmography [19] with a four-chamber system (Buxco, Sharon, CT, USA). Mice were first challenged with intranasal antigen and allowed to recover for 2 h before being placed into the chambers and their breathing monitored for 10 min. When acclimatized, their baseline response was measured for 5 min. The mice were then subjected to 1 min of aerosolized PBS, followed by nebulized methacholine. Responses are recorded for 5 min in every case with a short interval between to allow return to baseline Penh. Each group contained four to eight mice. Results are presented as the average percentage elevation in Penh above baseline after a challenge of methacholine.
Statistics
Results are the average for four to eight mice/group and error bars are ± s.e.m. Significance was determined by Student's two-tailed t-test. Significance was accepted for P < 0·05.
RESULTS
Treatment with rfhSP-D reduces serum IgE and D. pteronyssinus-specific IgG1 response to allergen challenge
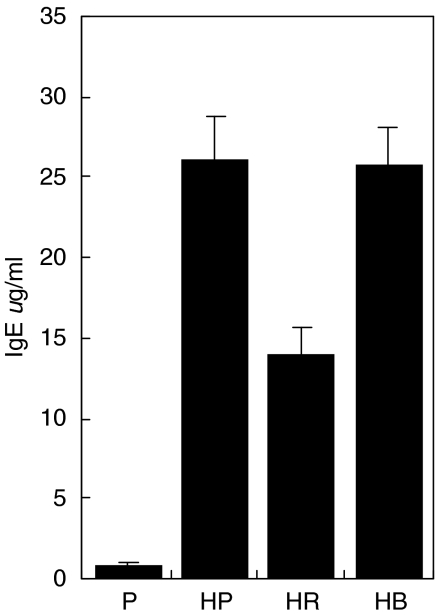
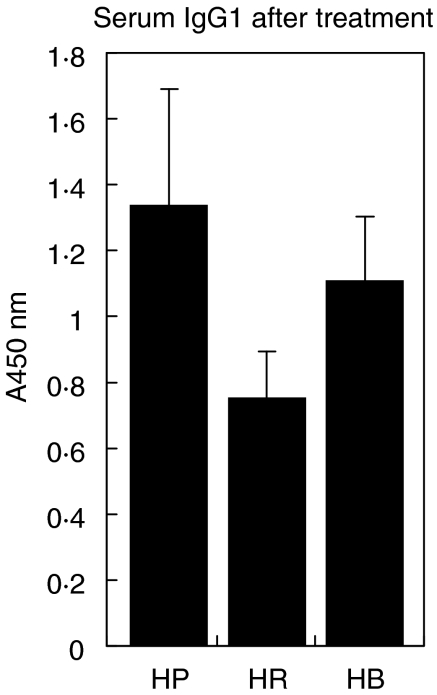
Sensitized mice had elevated serum IgE relative to non-sensitized mice, demonstrating that the sensitization protocol was effective (Fig. 1). Treatment with rfhSP-D of sensitized mice following allergen challenge for 5 days resulted in a significantly lower IgE response (P < 0·01, n = 4–8 mice/group). Treatment with PBS or the same dose of a control protein (BSA) did not lower the IgE. A similar reduction by treatment with rfhSP-D was measured on D. pteronyssinus-specific IgG1 (Fig. 2, P < 0·05, n = 4–8 mice/group). No D. pteronyssinus-specific IgG1 was detectable in non-sensitized animals.
Fig. 1.
Total serum IgE measured after treatment with five daily doses of PBS (HP), 10 µg rfhSP-D (HR) or BSA (HB), given after Der p challenge. P = non-sensitized mice treated with PBS.
Fig. 2.
D. pteronyssinus-specific IgG1 after treatment with five daily doses of PBS (HP), 10 µg rfhSP-D (HR) or BSA (HB), given after allergen challenge.
Treatment with rfhSP-D decreases peripheral blood eosinophilia response to D. pteronyssinus allergen challenge
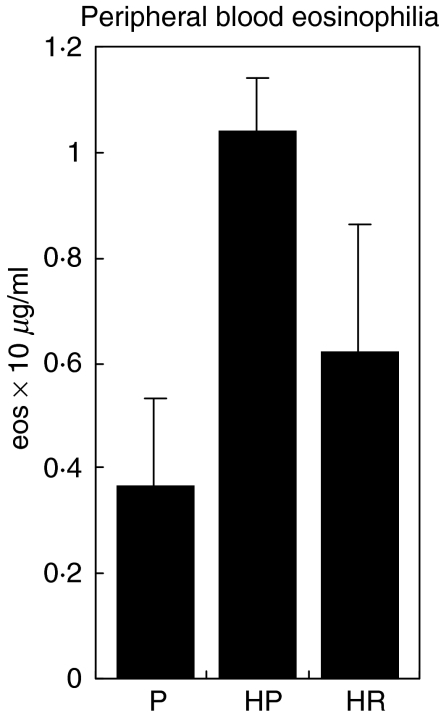
Having established that treatment with rfhSP-D had a specific effect on reducing serum IgE and IgG1, the effect of treatment on peripheral blood eosinophilia was investigated. Sensitized mice were challenged with D. pteronyssinus extract intranasally, followed by treatment with PBS or 10 µg rfhSP-D on 4 successive days. Measurement of eosinophil counts in blood obtained from tail bleeding on the following day showed a 41% fall in eosinophil counts compared to mice treated with PBS, although this difference did not reach statistical significance (Fig. 3).
Fig. 3.
Effect of treatment with four doses of PBS (HP), 10 µg rfhSP-D (HR) or BSA (HB) on peripheral blood eosinophilia in allergen challenged mice. P = non-sensitized mice treated with PBS.
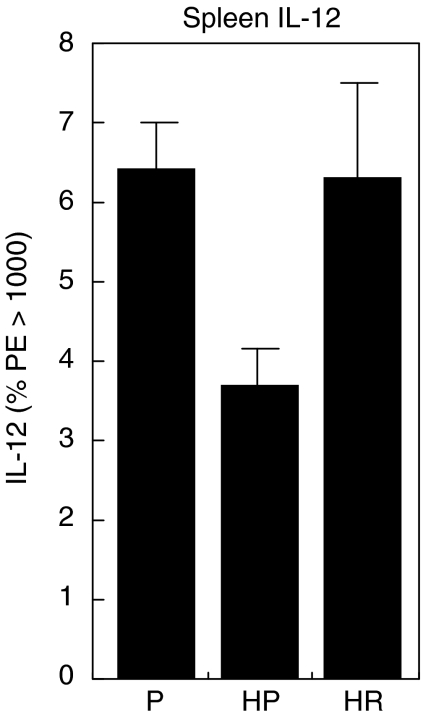
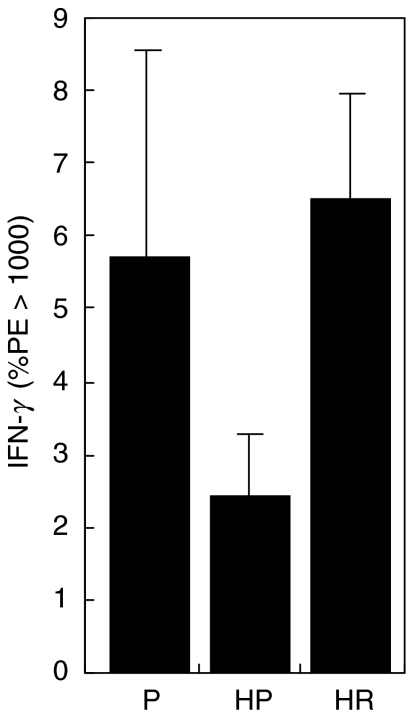
Treatment with rfhSP-D produces an elevation in IL-12 in the lung and reverses the decrease in IL-12 and IFN-γ measured in the spleen
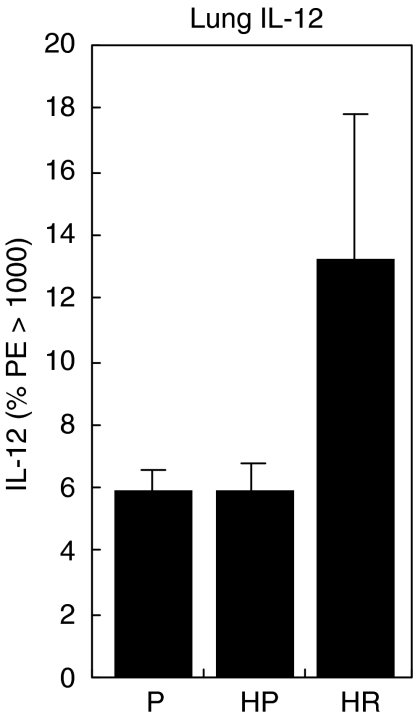
To examine further the effect of rfhSP-D treatment, the cytokines IL-12 and IFN-γ were measured by intracellular cytokine staining of whole cell homogenates of lung and spleen after treatment with 4 daily doses of 10 µg rfhSP-D given after challenge with 50 AU of D. pteronyssinus extract. Mice were re-challenged 4 days after treatment with D. pteronyssinus extract alone and cytokines measured the following day. Treatment with rfhSP-D produced a marked increase, although the difference was not statistically significant, in IL-12 measured in the lungs (Fig. 4) of allergen-challenged mice, while treatment with PBS had no measurable effect when compared to non-sensitized mice treated with PBS. The level of IL-12 measured in the spleen was reduced significantly after allergen challenge (P < 0·005), but was elevated to levels comparable to non-sensitized mice after treatment with rfhSP-D (Fig. 5). Treatment with rfhSP-D but not PBS resulted in a significant elevation in IFN-γ levels measured in the spleen (Fig. 6, P < 0·05).
Fig. 4.
IL-12 measured in the lungs 1 day after allergen re-challenge given 4 days after completion of treatment of allergen-challenged mice with four daily doses of PBS (HP) or 10 µg rfhSP-D (HR). P = non-sensitized mice treated with PBS.
Fig. 5.
IL-12 measured in the spleens taken 1 day after allergen re-challenge given 4 days after completion of treatment of allergen-challenged mice with four daily doses of PBS (HP) or 10 µg rfhSP-D (HR). P = non-sensitized mice treated with PBS.
Fig. 6.
IFN-γ measured in the spleens taken 1 day after allergen re-challenge given 4 days after completion of treatment of allergen-challenged mice with four daily doses of PBS (HP) or 10 µg rfhSP-D (HR). P = non-sensitized mice treated with PBS.
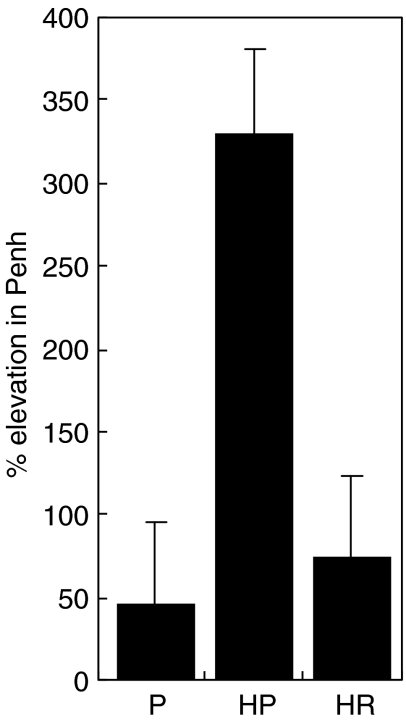
Effect of treatment with rfhSP-D on airway hyperresponsiveness
In order to find a dose of methacholine that would show a maximal difference between allergic and non-sensitized mice, mice were subjected to 1 min of nebulized methacholine over a range of increasing concentrations. This indicated that a concentration of 20 or 40 mg/ml would be appropriate to elicit a strong response. Sensitized mice responded with a significantly elevated Penh after allergen challenge (Fig. 7). Intranasal treatment of allergen challenged sensitized mice with rfhSP-D at a concentration of 10 µg/ml for 4 days resulted in a substantial and significant (P < 0·001) reduction in Penh when compared to treatment with PBS.
Fig. 7.
Comparison of Penh response in groups challenged with 20 mg/ml methacholine after 4 days of treatment. P = non-sensitized mice treated with PBS. HP = allergen-challenged mice treated with PBS, HR = allergen-challenged mice treated with 10 µg rfhSP-D (n = 4–8/group).
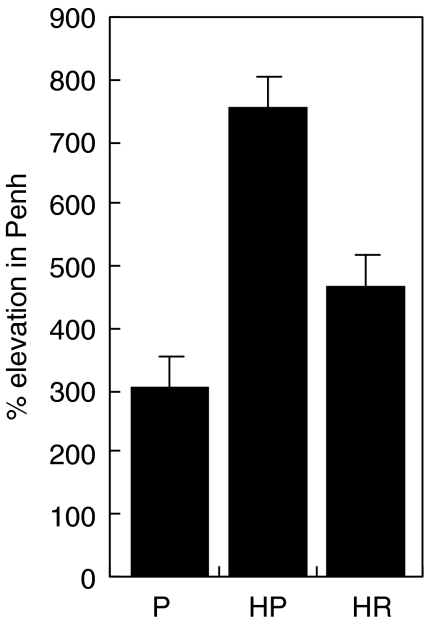
These mice were re-challenged with allergen alone given 8 days later and the Penh response measured after provocation with 40 mg/ml methacholine (Fig. 8). The rfhSP-D treated mice responded with 30% less airway hyperresponsiveness compared to mice treated with PBS, although the difference was not statistically significant.
Fig. 8.
Comparison of Penh response in groups challenged with 40 mg/ml methacholine on re-challenge with allergen given 4 days after the end of treatment. P = non-sensitized, treated with PBS. HP = allergen-challenged mice treated with PBS, HR = allergen-challenged mice treated with 10 µg rfhSP-D (n = 4–8/group).
Endogenous mouse SP-D and SP-A
Endogenous levels of SP-D measured in the BAL of allergic mice were elevated approximately 10-fold from a level of 0·53 ± 0·43 µg/ml in BAL from normal mice treated with saline to 4·91 ± 0·65 µg/ml in sensitized mice after challenge with mite allergen. Endogenous SP-A levels were 1·68 ± 0·41 µg/ml in normal mice and were increased two- to threefold in sensitized mice after allergen challenge. Treatment with five daily doses of 10 µg/ml rfhSP-D (or control) did not produce any change in the level of endogenous SP-D or SP-A levels.
Lung histology
H&E-stained lung sections were assessed independently for peribronchial inflammation using a scoring system as previously described [16] and overall showed a reduction in cellular inflammation in the D. pteronyssinus-sensitized and allergen-challenged lung after treatment with rfhSP-D, but not after treatment with either PBS or BSA. Of the sensitized mice in this study six PBS-treated mice had scores of 2 or more, whereas four of the five mice in the rfhSP-D treatment group had lower scores than controls. The score for non-sensitized mice was 0.
DISCUSSION
D. pteronyssinus is the most abundant species of house dust mite and is a major source of allergens that contribute to allergic asthma, characterized by high serum IgE and D. pteronyssinus-specific IgG1, peripheral and lung eosinophilia and increased airway hyperresponsiveness. We have previously shown the effectiveness of a recombinant fragment of human SP-D in reducing these parameters in mice sensitized to fungal allergens [16,17], and the present study was carried out to test its effects in a mouse model of house dust mite allergy. The possibility that SP-A and SP-D may modulate airway responses to house dust mite allergens was first raised by Wang, who identified an interaction between the proteins and D. pteronyssinus extracts [14]. In a subsequent study he investigated the effect of native hSP-A, hSP-D and rfhSP-D on PHA and D. pteronyssinus extract-induced histamine release and proliferation of peripheral blood mononuclear cells (PBMC) obtained from asthmatic children [20]. Incubation with hSP-A, hSP-D and rfhSP-D reduced proliferation in both non-asthmatic age-matched control and stable asthmatic PBMCs by approximately 50%. The rfhSP-D showed a higher potency than either hSP-A or hSP-D and gave a 77·5% inhibition after only 6 h incubation at equivalent concentrations. The inhibitory effect was abolished by maltose confirming the involvement of the CRD in a carbohydrate-mediated interaction. It was suggested further that the antiproliferative effect is the result of a direct interaction between rfhSP-D and the CR3 integrin as incubation with rfhSP-D reduced FITC-anti-CD11b staining in PBMCs.
The results reported in the current study show that rfhSP-D is effective at reducing serum IgE, D. pteronyssinus-specific IgG1 and peripheral blood eosinophilia in a model in which allergic mice were challenged daily with allergen before treatment. This is a more severe test of the efficacy of rfhSP-D than in our previous model using Afu allergens [16], and mirrors a treatment scenario in which a patient might wish to take rfhSP-D therapeutically to reduce the symptoms of perennial allergy or allergic asthma to continuous exposure to house dust mite. Similarly, the effect of treatment with rfhSP-D on lung function was assessed by whole body plethysmography performed after a course of allergen challenge followed by treatment. There was a dramatic reduction in airway hyperresponsiveness, indicating a significant improvement in lung status, consistent with the biochemical, cellular and histological parameters. This effect lasted into the second week, with decreased AHR following re-challenge with allergen up to 8 days later (Fig. 8).
To investigate possible mechanisms, intracellular cytokines were assayed. There was an increase in IL-12 response, even after allergen challenge. IL-12 is a heterodimeric cytokine that is produced by macrophages and antigen-presenting cells and plays an important role in the regulation of both innate and adaptive immune responses. IL-12 stimulates the proliferation of Th1 lymphocytes while inhibiting proliferation of Th2 lymphocytes. Several studies have shown that IL-12 inhibits airway hyperresponsiveness [21,22], consistent with the present findings. IL-12 stimulates the production of IFN-γ by Th1 lymphocytes and natural killer cells and the present study also shows an increase in IFN-γ after rfhSP-D treatment. The two cytokines have a synergistic effect on reducing allergic hypersensitivity and reducing Th2 cytokines, including IL-4 [23]. IL-4 acts upon B cells, promoting class switching to IgG1 in mice (IgG4 in humans) and IgE [23,24] and IL-4 is directly implicated in the immunopathogenesis of asthma [25–27] and the development of airway hyperresponsiveness [28].
Consistent with the role of IL-12 in redirecting the immune response to Th1, it exerts a powerful regulatory effect on the humoral immune response by promoting IgG2a, IgG2b and IgG3 by reducing IgG1 [29] and also suppressing IL-4-induced IgE production [30]. This effect is enhanced further by the up-regulation of IFN-γ by IL-12, which also suppresses IgE production. Kiniwa showed that administration of as little as 1 µg/day of recombinant IL-12 inhibited D. pteronyssinus-specific IgE production by 88% and IgG1 production by 93% [30]. Other studies have demonstrated clearly a link between IgE and airway hyperresponsiveness in asthmatic patients [31].
The reduction in peripheral blood eosinophilia observed in the present study is consistent with an up-regulation of IL-12 and IFN-γ. Sur et al. showed that administration of IL-12 decreased allergen-induced lung eosinophilia in a murine model of ragweed allergy [32]. Similar effects were found when as little as 0·1 µg/day was given to D. pteronyssinus-sensitized mice [8]. Treatment with IL-12 resulted in an increase in IFN-γ and a decrease in IL-5 in splenocytes from allergic mice after stimulation with D. pteronyssinus extract. Additionally, IL-12 up-regulation correlates with a reduction in airway hyperresponsiveness [19,33].
While these cytokine changes are consistent with the reduction in allergic responses in treated mice, the precise mechanism by which rfhSP-D produces these effects on immune cells remains to be established. The up-regulation of IL-12 suggests an interaction with macrophages and dendritic cells, which are the sole producers of this cytokine. SP-D is known to bind directly to alveolar macrophages [34,35]. An interaction of SP-D with dendritic cells and involvement of SP-D in antigen presentation has been demonstrated recently [36]. SP-D bound preferentially to immature rather than mature dendritic cells in a calcium-dependent carbohydrate-mediated manner and it was shown that this interaction enhanced antigen presentation. Recent studies from our group have shown an interaction between rfhSP-D and dendritic cells. The recombinant fragment binds preferentially to immature dendritic cells and interestingly, the expression of gp-340, a putative receptor for SP-D, was higher on immature rather than mature dendritic cells [37]. We have also shown recently that rfhSP-D reduces lung inflammation by promoting the clearance of apoptotic alveolar macrophages in the lung [38] and the possibility that this mechanism is also involved in promoting removal of apoptotic eosinophils in the allergic lung is intriguing, and merits further investigation. The obvious question of why the addition of exogenous rfhSP-D should produce an effect over and above that resulting from endogenous SP-D is puzzling. However, it has been observed by our group and others that SP-D levels are markedly up-regulated in the lung during A. fumigatus-induced allergic inflammation [16,39] and it is possible that the addition of exogenous SP-D augments this protective effect. Treatment with rfhSP-D did not increase endogenous SP-D or SP-A expression further but exogenous rfhSP-D provides an additional pool detectable in lung lavage after intranasal administration [16]. The current study is consistent in showing up-regulation of SP-A and SP-D in BAL fluid after challenge with house dust mite allergen in sensitized mice. Again the SP-D response was more dramatic than that of SP-A, increasing 10-fold compared to 2-fold increases in SP-A levels. At first sight these results appear to conflict with those of Wang et al., who reported decreased levels of SP-A and SP-D in a murine model of asthma [40]. This is not likely to be due to the different strain of mice employed in the studies (BALB/c versus C57Bl/6 used in this study), as it has been shown that SP-A and SP-D were consistently up-regulated in both BALB/c and C57Bl/6 strains in a murine model of asthma after ovalbumin challenge [13] and after challenge with Afu allergens [16,39]. Similarly, SP-D expression was markedly up-regulated in a rat model of ovalbumin sensitivity [41]. In Wang's study collectin levels were, however, increased at 24 h after challenge with house dust mite allergens and immunohistochemistry showed increased expression of collectins in areas of eosinophilic or monocytic infiltration for up to 3 days after challenge [40]. These findings are consistent with other studies reported and with the current study, in which collectin levels were assessed within 6 h of an intranasal allergen challenge. In clinical studies, both low levels of SP-A [42] and high collectin levels have been found in asthma sufferers [43]. An explanation for this variability may be that measured levels of collectins in the airways of patients are also related to the timing of sampling in relation to allergen challenge.
In conclusion, we have demonstrated that there are increased levels of both SP-A and SP-D after challenge with allergens of D. pteronyssinus during allergen-induced lung inflammation in mice. Treatment with a recombinant fragment of human SP-D down-regulated airway hyperresponsiveness, serum eosinophilia, D. pteronyssinus-specific IgG1 and total serum IgE after allergen challenge and was accompanied by increased expression of IL-12 and IFN-gamma. The results provide further support for the developing hypothesis that the lung collectins, especially lung surfactant protein D, have an important protective role in the response of the lung to allergens. The efficacy of a recombinant fragment of human SP-D in down-regulating allergic responses in this murine model and in our previous work using a model of allergic hypersensitivity to fungal allergens suggests that recombinant collectins may have therapeutic potential in modulating pulmonary allergic responses.
Acknowledgments
This work was supported by the Medical Research Council (UK), by the EU (QLK-CT-2000–00325) and by a grant from the British Lung Foundation (KBMR and HC). Howard Clark holds a Beit Memorial Fellowship for Medical Research.
REFERENCES
- 1.Peat JK, Tovey E, Toelle BG, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–6. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA, Mitchell EB, Chapman MD, Heymann PW. Dust mite allergy: its clinical significance. Hosp Pract (Off, Eds) 1987;22•(91–3):97–100. doi: 10.1080/21548331.1987.11703307. [DOI] [PubMed] [Google Scholar]
- 3.Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19:419–24. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarsfield JK. Role of house-dust mites in childhood asthma. Arch Dis Child. 1974;49:711–5. doi: 10.1136/adc.49.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall JD, Secrist H, DeKruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–7. [PubMed] [Google Scholar]
- 6.Morris SC, Madden KB, Adamovicz JJ, et al. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–56. [PubMed] [Google Scholar]
- 7.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–7. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 8.Lee YL, Fu CLYeYL, Chiang BL. Administration of interleukin-12 prevents mite Der p 1 allergen-IgE antibody production and airway eosinophil infiltration in an animal model of airway inflammation. Scand J Immunol. 1999;49:229–36. doi: 10.1046/j.1365-3083.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–13. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998;161:4146–52. [PubMed] [Google Scholar]
- 11.Tsunoda I, Tolley ND, Theil DJ, Whitton JL, Kobayashi H, Fujinami RS. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 1999;9:481–93. doi: 10.1111/j.1750-3639.1999.tb00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark HW, Reid KB, Sim RB. Collectins and innate immunity in the lung. Microbes Infect. 2000;2:273–8. doi: 10.1016/s1286-4579(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 13.Haley KJ, Ciota A, Contreras JP, Boothby MR, Perkins DL, Finn PW. Alterations in lung collectins in an adaptive allergic immune response. Am J Physiol Lung Cell Mol Physiol. 2002;282:L573–84. doi: 10.1152/ajplung.00117.2001. [DOI] [PubMed] [Google Scholar]
- 14.Wang JY, Kishore U, Lim BL, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106:367–73. doi: 10.1046/j.1365-2249.1996.d01-838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe HJ, Barlow PN, Reid KB. A parallel three stranded alpha-helical bundle at the nucleation site of collagen triple-helix formation. FEBS Lett. 1994;344:191–5. doi: 10.1016/0014-5793(94)00383-1. [DOI] [PubMed] [Google Scholar]
- 16.Strong P, Reid KB, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol. 2002;130:19–24. doi: 10.1046/j.1365-2249.2002.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan T, Kishore U, Singh M, et al. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001;107:467–75. doi: 10.1172/JCI10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark H, Reid KB. Structural requirements for surfactant protein D function in vitro and in vivo. therapeutic potential of recombinant SP-D. Immunobiology. 2002;205:619–31. doi: 10.1078/0171-2985-00159. [DOI] [PubMed] [Google Scholar]
- 19.Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 20.Wang JY, Shieh CC, You PF, Lei HY, Reid KB. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–8. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 21.Bryan SA, O'Connor BJ, Matti S, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2149–53. doi: 10.1016/S0140-6736(00)03497-8. [DOI] [PubMed] [Google Scholar]
- 22.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–36. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988;85:6880–4. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 27.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 28.Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26:202–8. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 29.Germann T, Bongartz M, Dlugonska H, et al. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–9. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 30.Kiniwa M, Gately M, Gubler U, Chizzonite R, Fargeas C, Delespesse G. Recombinant interleukin-12 suppresses the synthesis of immunoglobulin E by interleukin-4 stimulated human lymphocytes. J Clin Invest. 1992;90:262–6. doi: 10.1172/JCI115846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 32.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173–80. [PubMed] [Google Scholar]
- 33.Keane-Myers A, Wysocka M, Trinchieri G, Wills-Karp M. Resistance to antigen-induced airway hyperresponsiveness requires endogenous production of IL-12. J Immunol. 1998;161:919–26. [PubMed] [Google Scholar]
- 34.Kuan SF, Persson A, Parghi D, Crouch E. Lectin-mediated interactions of surfactant protein D with alveolar macrophages. Am J Respir Cell Mol Biol. 1994;10:430–6. doi: 10.1165/ajrcmb.10.4.8136158. [DOI] [PubMed] [Google Scholar]
- 35.Miyamura K, Leigh LE, Lu J, Hopkin J, Lopez Bernal A, Reid KB. Surfactant protein D binding to alveolar macrophages. Biochem J. 1994;300:237–42. doi: 10.1042/bj3000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinker KG, Martin E, Borron P, et al. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1453–63. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- 37.Desai S. MSc Thesis. University of Oxford; 2001. Studies on the interaction of a recombinant fragment of human lung surfactant protein D with dendritic cells. [Google Scholar]
- 38.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein d reduces alveolar macrophage apoptosis in vivo. J Immunol. 2002;169:2892–9. doi: 10.4049/jimmunol.169.6.2892. [DOI] [PubMed] [Google Scholar]
- 39.Haczku A, Atochina EN, Tomer Y, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- 40.Wang JY, Shieh CC, Yu CK, Lei HY. Allergen-induced bronchial inflammation is associated with decreased levels of surfactant proteins A and D in a murine model of asthma. Clin Exp Allergy. 2001;31:652–62. doi: 10.1046/j.1365-2222.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- 41.Kasper M, Sims G, Koslowski R, et al. Increased surfactant protein D in rat airway goblet and Clara cells during ovalbumin-induced allergic airway inflammation. Clin Exp Allergy. 2002;32:1251–8. doi: 10.1046/j.1365-2745.2002.01423.x. [DOI] [PubMed] [Google Scholar]
- 42.Van de Graaf EA, Jansen HM, Lutter R, et al. Surfactant protein A in bronchoalveolar lavage fluid. J Laboratory Clin Med. 1992;120:252–63. [PubMed] [Google Scholar]
- 43.Cheng G, Ueda T, Numao T, et al. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16:831–5. doi: 10.1183/09031936.00.16583100. [DOI] [PubMed] [Google Scholar]