Abstract
In systemic lupus erythematosus (SLE), autoantibodies directed against complement components of the classical pathway, especially against C1q, are associated with severe disease and are of prognostic value for flares of lupus nephritis. Mannose-binding lectin (MBL), the recognition unit of the MBL pathway of complement activation, has structural similarities to C1q. Deficiencies of MBL have been shown to predispose to the development of SLE and to influence the course of the disease. We hypothesized that the presence of autoantibodies to MBL, analogous to autoantibodies to C1q in patients with SLE, may contribute to disease development. The occurrence of anti-MBL autoantibodies was assessed by enzyme-linked immunosorbent assay (ELISA) of 68 serum samples from 20 patients with SLE and in serum from 70 healthy controls. Levels of antibodies directed against MBL were significantly higher in patients with SLE compared to healthy subjects. No significant difference was found between patients with active disease compared to those with inactive disease. While the occurrence of anti-C1q autoantibodies was associated with renal involvement, no such relationship was found for anti-MBL autoantibodies. A significant correlation was found between anti-MBL and anti-C1q antibody levels. The level of anti-MBL antibodies was negatively correlated with MBL–complex activity of circulating MBL. Anti-MBL autoantibodies were of the immunoglobulin G (IgG) isotype and the binding site of IgG anti-MBL was located in the F(ab′)2 portion. We conclude that anti-MBL are present in sera from SLE patients and influence the functional activity of MBL.
Keywords: SLE, autoantibodies, MBL, C1q
INTRODUCTION
The innate immune system plays a crucial role in the pathogenesis of systemic lupus erythematosus (SLE) [1,2]. In the pathogenesis of SLE the complement system has a dual role. On the one hand, activation of complement may cause tissue injury; on the other hand, genetic deficiencies of complement components, especially the early components of the classical pathway, are strongly associated with the occurrence of SLE [3]. Furthermore, the presence of antibodies to C1q is associated with hypocomplementaemia and nephritis in patients with SLE [4–7]. Recent studies indicate a possible role for the lectin pathway of complement activation in the pathogenesis of SLE [8,9].
Mannose-binding lectin (MBL), structurally homologous to C1q, activates the lectin pathway of complement. Like C1q, MBL consists of trimeric subunits with a collagen-like tail. These subunits assemble to higher-order structures consisting of up to six trimers [10]. Binding of the carbohydrate-recognition domain of MBL to different carbohydrates, in a calcium-dependent manner, activates the MBL-associated serine proteases MASP-1, MASP-2 and MASP-3 that are strongly related to the serine proteases of the classical pathway, C1r and C1s. Evidence has been provided that activation of MASP-2 is responsible for activation of the complement cascade [11].
MBL deficiency or low serum MBL levels are frequently found in the general population owing to point mutations in the structural portion or promoter region of the MBL gene [9–12]. The three known mutations in the structural domain of the MBL gene are located in codons 52 [13], 54 [14] and 57 [15] of exon 1, whereas mutations at positions −550 (H/l alleles), −221 (X/Y alleles) and +4 (P/Q alleles), upstream of the MBL gene, are located in non-coding regions [16,17]. Circulating concentrations of MBL have been shown to be associated with recurrent bacterial and fungal infections in both children and adults [18–21]. Variant alleles, leading to reduced serum concentrations of MBL, may be associated with a predisposition for SLE [8,22–25]. Association of a promotor polymorphism with the development of SLE has been reported [26–28]. Furthermore, complications caused by bacterial infections in patients with SLE occur more frequently if these individuals carry mutations in the MBL gene [24,29].
Autoantibodies to C1q are found in 30–45% of patients with SLE [4–7,24]. The presence of anti-C1q autoantibodies is associated with hypocomplementaemia and glomerulonephritis. In the presence of anti-C1q the C1q levels are reduced [6]. Stabilization of deposited C1q in the kidney, by these antibodies, has been suggested to cause ongoing complement activation and thereby damage to the kidney [30]. Increase of anti-C1q levels in serum of patients with SLE is strongly associated with flares of lupus nephritis [5–7,31].
Low MBL concentrations may result from gene polymorphisms, consumption of MBL during disease activity or possibly because of autoantibodies directed against MBL. Because of the structural similarities between C1q and MBL, and the role of MBL in patients with SLE, we investigated if, analogous to the presence of anti-C1q autoantibodies, autoantibodies to MBL occur.
PATIENTS AND METHODS
Patients
All 20 patients with SLE (five males and 15 females; mean age 33 years) visited the out-patient clinic of the department of Nephrology. The patients fulfilled the American Rheumatological Association (ARA) criteria for SLE. The clinical data were recorded from the patient files. From each patient at least two serum samples were obtained over time, the maximum number of serum samples/patient was seven. The total number of serum samples from the 20 patients was 68. Samples were taken (after obtaining informed consent from the patient) during inactive and active phases of disease. The autoantibody titres were measured in different serum samples from each patient during active and inactive disease. Active disease was considered to be present when patients had disease activity (as defined by clinical criteria) or when laboratory results indicated active nephritis, i.e. proteinuria>0·5 g/24 h and erythrocytes (or red cell casts) on urinalysis. The serum aliquots were stored at −20°C until required for further use. From 70 healthy individuals, working in our hospital, serum samples were obtained and used as controls.
Purification of MBL and measurement of MBL serum concentrations
Purification of MBL and the assessment of MBL concentrations was performed exactly as described previously [32].
Detection of immunoglobulin G (IgG) binding to MBL
For detection of IgG antibodies to MBL, Nunc Maxisorb plates (Roskilde, Denmark) were first coated, for 2 h at 37°C, with 0·5 µg/ml MBL in coating buffer (100 mm Na2CO3/NaHCO3, pH 9·6). After incubation, the plates were washed three times with phosphate-buffered saline (PBS) containing 0·05% Tween-20 (PBS/Tw). Unoccupied binding sites were blocked by incubation for 1 h at 37°C with 0·01% gelatin in PBS. Serum samples diluted in PBS/Tw, containing 1% bovine serum albumin (PBS/Tw/BSA) and 10 mm EDTA, were incubated on the plate for 1 h at 37°C. After incubation, bound IgG was detected using digoxigenin (Dig) (Boehringer Mannheim, Mannheim, Germany)-conjugated monoclonal mouse anti-human IgG [HB43, monoclonal antibody (MoAb) anti-IgG, hybridoma obtained from the American Type Culture Collection (ATCC), Manassas, VA], diluted in PBS/Tw/BSA. Subsequently, horseradish peroxidase (HRP)-conjugated Fab rabbit anti-Dig Abs (Boehringer Mannheim) were added and the enzymatic activity of HRP was assessed using 2,2′-azino-bis(3-ethyl benzathioline-6-sulphonic acid) (Sigma, St Louis, MO). The optical density (OD) at 415 nm was measured using a microplate biokinetics reader (EL312e; Biotek instruments, Winooski, VT). The concentration of IgG reactive with MBL is expressed in units/ml of serum (U/ml), related to a standard serum. Values below the detection limit of the assay (OD value <1·5-fold the OD value of background) were given an arbitrary value of 40 U/ml. Pooled sera from patients with SLE were used as standard serum. The concentration of IgG reactive with MBL in the standard serum was arbitrarily set at 1000 U/ml.
Detection of circulating complexes of MBL and IgG binding to MBL
Microtitre plates were coated with monoclonal mouse anti-human MBL (3E7; kindly provided by Dr T. Fujita, Fukushima, Japan) or an isotype-matched control mouse MoAb (IgG1; anti-rat kappa chain). After a blocking step, serum samples were incubated (diluted 1 : 10) and IgG binding was detected as described above.
Detection of anti-C1q
Anti-C1q autoantibodies were assessed as described previously [6,33]. Purified C1q (2 µg/ml) was coated onto 96-well Nunc Maxisorb plates in coating buffer, in a final volume of 100 µl, for 2 h at 37°C. Plates were washed with PBS/Tw three times after each incubation period. Serum samples were diluted in PBS/0·05% Tween/1% fetal calf serum (FCS)/1 m NaCl for 1 h at 37°C. IgG antibodies were detected using Dig-conjugated monoclonal mouse anti-human IgG (HB43), as described above. This assay is used as a routine diagnostic assay in our laboratory for detection of anti-C1q. Values>90 U/ml, compared with a standard serum, are considered as high.
Functional activity of the MBL complex activity
MBL complex activity was assessed using the method described by Petersen et al. [34], with slight modifications. In brief, mannan-coated plates were incubated with serum, diluted in GVB++ [VBS (1·8 mm Na-5,5-diethylbarbital, 0·2 mm 5,5-diethylbarbituric acid, 145 mm NaCl) containing 0·5 mm MgCl2, 2 mm CaCl2, 0·05% Tween-20 and 0·1% gelatin; pH 7·5] containing 1 m NaCl, for 16 h at 4°C. Plates were washed with PBS/Tween containing 5 mm CaCl2, followed by incubation with purified C4 [32] (1 µg/ml), diluted in VBS containing 1 mm MgCl2, 2 mm CaCl2, 0·05% Tween-20 and 1% BSA, pH 7·5, for 1 h at 37°C. Activation of C4 was assessed using MoAb C4-4a (anti-human C4d, from Dr C. E. Hack) conjugated to Dig. Functional activity was expressed in arbitrary units/ml (aU/ml), based on serial dilutions of a human pool serum that was used as a standard on each plate. Activity of this standard was set at 1000 U/ml.
Isolation and characterization of MBL-binding immunoglobulin
To determine the type and size of the immunoglobulin binding to MBL, 3 ml of serum from a patient with SLE was applied to a Superdex HR 200 column (Pharmacia, Uppsala, Sweden) equilibrated with PBS, and fractions of 2·0 ml were collected. Total protein concentration in the fractions was determined in a bicinchoninic acid (BCA) protein assay (Pierce, OMNILAB, Breda, the Netherlands). Size-marker proteins IgM, IgA and IgG, and anti-MBL IgG were assessed by enzyme-linked immunosorbent assay (ELISA) analysis of the fractions.
Isolation of F(ab′)2 fragments
IgG was purified from 2 ml of serum obtained from a pool of five sera from patients with known reactivity against MBL as well as from serum from a healthy volunteer. Pepsin digestion was performed using pepsin at a 1 : 10 (w/w) pepsin/protein ratio for 18 h at 37°C in Whalpole buffer. The reaction was stopped by addition of 1-m Tris base, 250 µl/ml of digest. The mixture was applied on a G150 Sepharose column (Pharmacia) to separate the F(ab′)2 fragments from the undigested IgG. Binding of F(ab′)2 fragments to MBL was assessed using a rabbit anti-human light-chain (kappa and lambda) antibody.
Statistics
The Mann–Whitney U-test, the Kendall tau-b non-parametric correlation coefficient and the Fisher exact test were used. P-values of <0·05 were considered statistically significant.
RESULTS
Detection of anti-MBL in patients with SLE
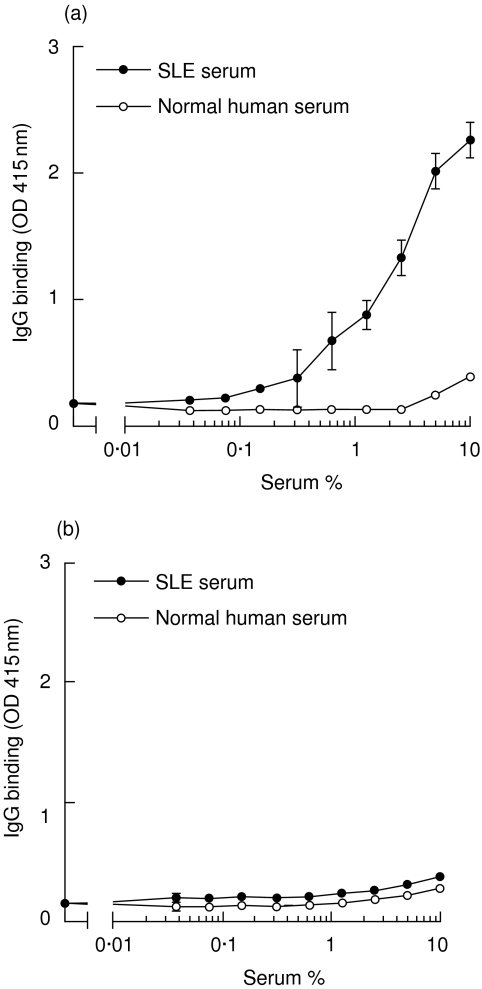
First, the presence of anti-MBL was investigated in sera obtained from patients with SLE. A pool of sera with demonstrable activity was made and further examined. Using this serum pool, a dose-dependent binding of IgG to solid-phase MBL was demonstrated, while serum from a healthy control did not show significant reactivity (Fig. 1a). This assay was performed in the presence of EDTA to prevent MBL binding via its C-type lectin domains. This serum pool was used as a standard for further experiments. As a control, ELISA wells were coated with gelatin alone; no detectable IgG binding was observed (Fig. 1b). Binding of IgG to MBL could not be inhibited by addition of an excess of purified MBL in the fluid phase, suggesting that the antibody is primarily binding to solid-phase MBL (results not shown).
Fig. 1.
Binding of immunoglobulin G (IgG) to immobilized mannose-binding lectin (MBL). Microtitre plates were coated with MBL (a) or gelatin (b). Pooled serum from patients with systemic lupus erythematosus (SLE) (standard serum), or a healthy control serum, were added in serial dilutions in the presence of EDTA (10 mm). Binding of IgG was analysed. Results represent the mean value ± standard deviation (s.d.).
Subsequently, IgG anti-MBL autoantibodies were identified in 68 serum samples from 20 patients with SLE and in 70 samples from healthy controls. The frequency of the clinical variables of disease activity that were observed at any period during disease activity are presented in Table 1.
Table 1.
Clinical presentation of disease activity in 20 patients with systemic lupus erythematosus (SLE)
| Clinical variable of disease activity | Number of patients | Occurrence of disease parameters (% of patients) |
|---|---|---|
| General | 10 | |
| Fatigue | 30 | |
| Fever | 10 | |
| Raynaud's phenomenon | 20 | |
| Skin | 11 | |
| Erythema | 35 | |
| Photosensitivity | 30 | |
| Oral ulcers | 25 | |
| Discoid lesions | 15 | |
| Vasculitis | 20 | |
| Alopecia | 25 | |
| Joints | 13 | |
| Arthritis | 50 | |
| Arthralgia | 30 | |
| Tendinitis | 5 | |
| Serosa | 7 | |
| Pericarditis | 25 | |
| Pleuritis | 30 | |
| Peritonitis | 10 | |
| Kidney | 15 | |
| Proteinuria* | 75 | |
| Haematuria† | 75 | |
| Nervous system | 4 | |
| Seizure | 0 | |
| Cva | 5 | |
| Lupus headache | 5 | |
| Mononeuritis multiplex | 5 | |
| Personality disorder | 10 |
> 0·5 g/24 h.
> 10 red blood cells (RBC)/high-power field.
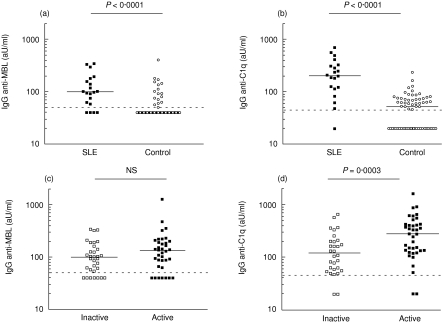
The results of anti-MBL measurements were expressed in units relative to the standard serum. Statistical analysis was performed on the mean level of anti-MBL IgG of the different serum samples from one patient. Levels of anti-MBL autoantibodies were significantly higher in SLE patients than in healthy controls (Fig. 2a). When the anti-MBL titres were compared between sera obtained during inactive and active phases of disease, no significant difference was found (Fig. 2c). In the same samples, anti-C1q levels were also determined (Fig. 2b). A significant difference in anti-C1q titre was found between sera of patients with SLE as compared to controls. As expected, significantly higher anti-C1q levels were found in SLE sera during active disease compared with inactive disease (Fig. 2d).
Fig. 2.
Anti-mannose-binding lectin (anti-MBL) and anti-C1q autoantibodies in different serum samples. (a) Anti-MBL reactivity was measured in 68 serum samples of 20 patients with systemic lupus erythematosus (SLE) and in 70 samples from healthy controls. (c) Anti-MBL autoantibody levels of samples obtained during inactive and active phases of disease. (b) Anti-C1q levels in SLE patients and healthy controls. (d) Anti-C1q autoantibody levels of samples obtained during inactive and active phases of disease. The median of each group is indicated by the solid line. The dashed line indicates the detection limit. aU, arbitrary units.
Association of anti-MBL autoantibodies with disease activity and correlation with the presence of anti-C1q
Anti-C1q antibody levels are elevated in patients with active SLE and predictive for renal disease [4–7,31]. We investigated whether the presence of high anti-MBL levels was associated with disease activity and predictive for renal involvement.
In the sera studied (n = 68), we detected a significant association with active disease for high levels of anti-C1q, but not for anti-MBL antibodies (Table 2). Furthermore, levels of anti-MBL in patients with SLE with renal involvement were statistically not different from levels in patients without renal involvement. For anti-C1q, as expected, antibody levels were significantly higher in patients with renal involvement (Table 3).
Table 2.
Number of sera from systemic lupus erythematosus (SLE) patients with high or low anti-C1q and anti-mannose-binding lectin (anti-MBL) levels during inactive and active phases of disease
| Number of sera with high or low anti-C1q levels | Number of sera with high or low anti-MBL levels | |||
|---|---|---|---|---|
| Disease activity | > 90 aU/ml | < 90 aU/ml | > 100 aU/ml | < 100 aU/ml |
| Inactive | 17 | 13 | 15 | 17 |
| Active | 34 | 4 | 22 | 14 |
| Fisher exact test P = 0·005 | Fisher exact test P = 0·3 | |||
The value for high levels of anti-MBL was set at a level at which 10% of the healthy control group were considered as having high anti-MBL levels. Thereby, anti-MBL levels>100 arbitrary units (aU)/ml were defined as high.
Table 3.
The mean antibody level of anti-mannose-binding lectin (anti-MBL) and anti-C1q per patient measured in sera from systemic lupus erythematosus (SLE) patients, with or without renal involvement
| Anti-MBL Ab levels | Anti-C1q Ab levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| With renal involvement | Without renal involvement | With renal involvement | Without renal involvement | ||||||
| Median | Range | Median | Range | P* | Median | Range | Median | Range | P* |
| 139 | 40–345 | 98 | 63–140 | 0·41 | 291 | 98–690 | 112 | 48–149 | 0·04 |
Mann–Whitney U-test.
Statistical analysis was performed on the mean level of immunoglobulin G (IgG) anti-MBL of the different serum samples from one patient.
As anti-C1q are directed mostly against the collagenous tail of C1q [35], we investigated whether there was any correlation with the occurrence of anti-MBL antibodies. The presence of anti-C1q was significantly associated with the presence of anti-MBL (Table 4) and a statistically significant correlation was found between the anti-MBL and anti-C1q levels (R = 0·21, P = 0·004).
Table 4.
Number of serum samples from patients with high or low levels of anti-C1q and anti-mannose-binding lectin (anti-MBL)
| Anti-C1q | ||
|---|---|---|
| > 90 aU/ml | < 90 aU/ml | |
| Anti-MBL | ||
| > 100 aU/ml | 33 | 4 |
| < 100 aU/ml | 18 | 13 |
| Fisher's exact test P = 0·05 | ||
aU, arbitrary units.
The data presented above indicate that IgG autoantibodies directed against MBL are present in patients with SLE, but are not clearly associated with disease activity in the patients examined in the present study.
Biochemical characterization of anti-MBL autoantibodies
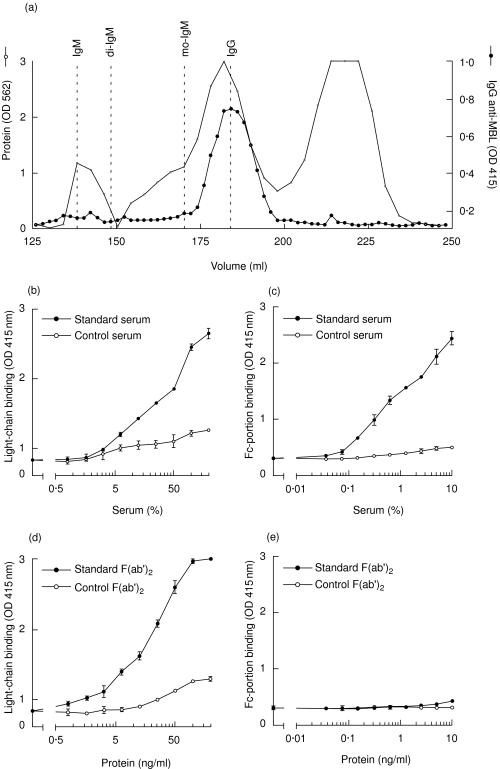
To examine whether binding of anti-MBL antibodies to MBL occurs via the antigen-recognition domain of IgG, we studied the antibody in more detail. Serum containing anti-MBL was fractionated using a gel-filtration column. The elution of IgG, IgA and IgM was assessed by ELISA (Fig. 3a). Anti-MBL IgG co-eluted from the column with monomeric IgG, thus excluding that IgG which binds to MBL is part of a larger (immune) complex. No binding of IgM and IgA to MBL was observed (data not shown).
Fig. 3.
Biochemical characterization of anti-mannose-binding lectin (anti-MBL) autoantibodies. After gel filtration, on Superdex HR 200, of serum from a patient with systemic lupus erythematosus (SLE), fractions were analysed for the presence of immunoglobulin (Ig)G anti-MBL (indicated by the black circles). The protein pattern, as well as the position of filtration of IgM, dimeric IgA (di-IgA), monomeric IgA (mo-IgA) and IgG are depicted (a). Anti-MBL reactivity was detected in serial dilutions of whole serum (b) and (c), and purified F(ab′)2 fragments (d) and (e), using antibodies against the light chains (b) and (d) and the Fc portion (monoclonal antibody HB43) (c) and (e), respectively.
To analyse the binding site of IgG involved in the binding to MBL, F(ab′)2 fragments were generated from IgG isolated from pooled serum of SLE patients with known reactivity against MBL, as well as from IgG of healthy donors without anti-MBL autoantibodies, using pepsin digestion. Using a polyclonal antibody against kappa and lambda light chains (Fig. 3b) or a MoAb directed against the Fc portion of IgG (HB43) (Fig. 3c), it was demonstrated that IgG from the SLE serum, but not IgG from the control serum, binds dose-dependently to coated MBL. A strong dose-dependent binding of F(ab′)2 fragments from SLE serum, but not of control F(ab′)2 fragments, was demonstrated to MBL, using the polyclonal anti-light chain antibody (Fig. 3d). In contrast, binding of the Fc portion of IgG anti-MBL was not detectable after digestion of IgG (Fig. 3e), indicating the complete digestion of IgG. Binding of F(ab′)2 fragments to MBL strongly suggests specific recognition of MBL by the antigen-recognition domain.
Anti-MBL autoantibodies are found in complex with circulating MBL and are associated with decreased MBL function
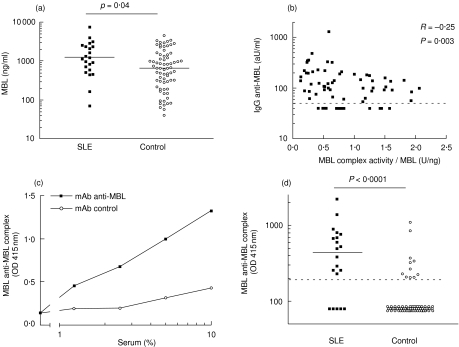
We investigated the influence of anti-MBL autoantibodies on the function of circulating MBL. It is conceivable that anti-MBL may affect the circulating MBL concentration. Patients with SLE had significantly higher MBL concentrations compared with healthy controls (P = 0·04) (Fig. 4a). No significant difference was found between samples obtained during inactive or active phases of disease [median MBL concentration 2175 ng/ml and 2802 ng/ml, respectively (P = 0·20)]. Levels of anti-MBL autoantibodies were higher in patients with high serum levels of MBL (R = 0·26, P = 0002). We further assessed whether the anti-MBL influence the MBL complex activity. As expected [34], a strong correlation between the MBL serum concentration and the MBL complex activity was found (R = 0·65, P < 0·0001). A negative correlation was found between anti-MBL levels and MBL complex activity, adjusted for MBL concentration (Fig. 4b). These data indicate a reduced functional activity of circulating MBL–MASP complexes in the presence of anti-MBL autoantibodies. To examine whether IgG is bound to circulating MBL in vivo, we examined the presence of complexes between MBL and IgG in an ELISA. A strong dose-dependent binding of IgG–MBL complexes was observed following incubation of SLE patient serum to wells coated with MoAb anti-MBL, but not to wells coated with a MoAb of irrelevant specificity (Fig. 4c). Further analysis of the presence of these complexes revealed strongly enhanced levels of IgG–MBL complexes in patients with SLE as compared to healthy controls (Fig. 4d, P < 0·001). The level of these complexes was positively correlated with anti-MBL levels (R = 0·27, P < 0·001) and negatively correlated with the functional activity of MBL (R = −0·17, P = 0·04) Together, these data suggest that IgG anti-MBL interact with MBL in vivo.
Fig. 4.
Interaction of anti-mannose-binding lectin (anti-MBL) with MBL in vivo. The mean MBL concentration per patient is shown in serum samples of 20 patients with systemic lupus erythematosus (SLE) and in 70 samples of healthy controls (a). Statistical analysis was performed on the mean MBL level of the different serum samples from one patient. The correlation between anti-MBL levels and the ratio between the MBL complex activity and the MBL concentration is shown (b). For detection of complexes between immunoglobulin G (IgG) and MBL in serum, plates were coated with monoclonal antibody (MoAb) anti-MBL or control MoAb, as indicated, and incubated with serial dilutions of a systemic lupus erythematosus (SLE) serum containing anti-MBL, followed by detection of IgG binding (c). The number of circulating complexes between IgG and MBL in serum samples from SLE patients and controls (d). aU, arbitrary units.
DISCUSSION
In the present study we demonstrate the presence of anti-MBL autoantibodies in patients with SLE. The data suggest an effect of anti-MBL on the MBL–complex activity of circulating MBL. The presence of anti-MBL did not result in a decrease of the MBL serum concentration. MBL, a member of the collectin family, is a major recognition unit in the most recently described pathway of complement activation. Collectins are proteins with a C-type lectin domain and structurally related to C1q, the first component of the classical pathway of complement activation. Binding of MBL to carbohydrates on certain microbial surfaces can activate the complement system and mediate phagocytosis through receptors on phagocytes. However, binding of C1q to carbohydrate structures has not been found.
The importance of the complement system in the pathogenesis of SLE is recognized in different, contrasting, ways. On the one hand, activation of complement by immune complexes, deposited in tissue, leads to tissue damage. On the other hand, deficiencies of (particularly) the early complement proteins of the classical pathway are associated with SLE. Furthermore, the presence of autoantibodies to neoepitopes on C1q, exposed upon binding to surfaces, is found in one-third of patients with SLE and is associated with renal involvement and hypocomplementaemia [36].
Apoptosis and the role of complement in the clearance of apoptotic material has been implicated in the pathogenesis of SLE [37]. Increased circulating levels of apoptotic material have been demonstrated in patients with SLE [38]. Binding of C1q and MBL to apoptotic products has been postulated to play a role in the clearance of apoptotic debris via phagocytosis [39,40]. Furthermore, autoantibodies associated with SLE are reactive with this apoptotic material [41]. An increased production, and decreased and aberrant clearance, of apoptotic material might result in autoantibody production to antigens present on this material. Because of the presence of C1q on these apoptotic cells, this might also be true for the generation of anti-C1q. In recent literature, binding of MBL to apoptotic material has clearly been shown [42]. Hence, it is possible that because of a greater quantity of apoptotic material in the sera of patients with SLE, and possibly a less efficient clearance of this material, autoantibodies are formed against MBL present on apoptotic cells.
We assessed the presence of anti-MBL autoantibodies in 68 sera of SLE patients during inactive and active phases of disease. A highly significant difference was found between the anti-MBL levels in SLE patients and those in healthy subjects. Because anti-C1q levels are strongly correlated with active kidney disease in SLE, we investigated whether the increased anti-MBL levels in patients with SLE were attributed to disease activity. We found no difference between anti-MBL levels in sera of patients with active disease and inactive disease. As expected, the levels of anti-C1q in the different sera obtained during active disease were significantly higher than those obtained during inactive disease. As we examined only a limited number of patients in the present study, a definite conclusion on the importance of anti-MBL autoantibodies for disease in SLE patients cannot be given and requires more extensive investigations.
Because of the similarity of the collagen tails of C1q and MBL, a possible cross-reactivity of anti-C1q with MBL was considered. A positive correlation was found between anti-MBL and anti-C1q levels in sera from patients with SLE. Therefore, one could speculate about the cross-reactivity of anti-C1q with MBL. On the other hand, the presence of autoantibodies directed to a variety of antigens is typically found in SLE patients and therefore anti-C1q and anti-MBL could be directed against different autoantigens. In four sera (6%) we found anti-MBL without anti-C1q reactivity, suggestive for an anti-MBL specific antibody. This is in agreement with the findings of Martensson et al. [33], who found no cross-reactivity of anti-C1q antibodies with MBL.
To examine the nature of anti-MBL autoantibodies, the interaction with MBL was studied in greater detail. We demonstrated that MBL binding was a property of monomeric IgG. Binding to MBL was calcium-independent, as it was established in the presence of EDTA, thus excluding that carbohydrates present on IgG were binding to the C-type lectin domain of MBL. Furthermore, using F(ab′)2 fragments of IgG, we showed that binding was localized in the antigen-recognition domain of IgG. These findings strongly suggest that IgG binding to MBL represents a true antibody–antigen interaction.
During an active phase of renal involvement in SLE, the C1q levels are decreased because of activation of the classical pathway of complement activation, triggered by the interaction of C1q with immune complexes. A second cause of reduced C1q levels is the presence of anti-C1q. Genetic deficiency of C1q is strongly correlated with the development of SLE. Association of reduced MBL levels (as a consequence of gene polymorphisms) with SLE has been reported previously [23]. Anti-MBL, similarly to anti-C1q, could be a cause of low MBL levels in SLE patients. Higher MBL levels were, however, found in sera obtained from SLE patients. A possible explanation could be an increase of MBL levels owing to an acute-phase response, although we found no significant difference in MBL levels in sera obtained from patients during active or inactive phases of disease. Anti-MBL autoantibody levels were higher in patients with high MBL concentrations, suggesting that these autoantibodies do not contribute to increased MBL turnover. Possibly the presence of anti-MBL antibodies, and higher concentrations of MBL, are both a result of disease activity. Furthermore, it could be that anti-MBL autoantibodies are preferentially generated in patients with higher MBL levels owing to an increased exposure of the immune system to (target-bound) MBL.
We continued to examine the effect of the presence of anti-MBL autoantibodies on MBL concentration and function. A significant, negative correlation was observed between the levels of anti-MBL autoantibodies and the MBL complex activity of circulating MBL. The latter parameter represents the ability of the MBL–MASP complexes to activate C4, corrected for the MBL serum concentration. Therefore, the association between anti-MBL and decreased MBL function suggests that these autoantibodies may interfere with the MBL-MASP complex. Such an interference is conceivable in at least three different ways: first, anti-MBL may inhibit MBL binding to mannan; second, these antibodies may interfere with the binding of MASP-2 to MBL; and, third, anti-MBL may bind to the (ligand-bound) MBL–MASP complex and prevent MASP-2 activation. A further argument for the interaction of autoantibodies with MBL in vivo is the presence of circulating complexes between MBL and IgG found in the serum samples of SLE patients in association with the presence of IgG anti-MBL. A negative correlation between MBL complex activity and circulating IgG–MBL complexes was found, supporting the suggestion that anti-MBL bind to MBL in vivo. It is conceivable that these complexes are actually released into the circulation after previous formation in the solid phase. Further experiments are required to establish whether the interaction of IgG with MBL is indeed involved in the association between the presence of anti-MBL and impaired MBL function in vivo.
To our knowledge, this is the first report on the presence of anti-MBL autoantibodies in serum from SLE patients. As far as the results show we cannot predict activity of disease by the presence of anti-MBL autoantibodies. Fascinatingly, these anti-MBL autoantibodies are associated with decreased MBL function. A greater number of patients should be tested for reactivity against MBL before a final conclusion can be made on the role of anti-MBL autoantibodies in the pathogenesis of SLE.
Acknowledgments
The authors thank Ngaisah Klar-Mohamad and Ria C. Faber-Krol for excellent technical assistance, Dr C. E. Hack (Amsterdam, the Netherlands) and Dr T. Fujita (Fukushima, Japan) for their kind gift of reagents used in this study and Dr M. J. K. Mallat for statistical analysis of the data. Part of this work was supported by the Dutch Kidney Foundation (PC 95).
REFERENCES
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 4.Gunnarsson I, Ronnelid J, Huang YH, et al. Association between ongoing anti-C1q antibody production in peripheral blood and proliferative nephritis in patients with active systemic lupus erythematosus. Br J Rheumatol. 1997;36:32–7. doi: 10.1093/rheumatology/36.1.32. [DOI] [PubMed] [Google Scholar]
- 5.Moroni G, Trendelenburg M, Del Papa N, et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am J Kidney Dis. 2001;37:490–8. doi: 10.1053/ajkd.2001.22071. [DOI] [PubMed] [Google Scholar]
- 6.Siegert C, Daha M, Westedt ML, van der Voort, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 7.Trendelenburg M, Marfurt J, Gerber I, Tyndall A, Schifferli JA. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 1999;42:187–8. doi: 10.1002/1529-0131(199901)42:1<187::AID-ANR24>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Davies EJ, Snowden N, Hillarby MC, et al. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–4. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- 9.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiology. 1998;199:327–39. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 10.Hansen S, Holmskov U. Structural aspects of collectins and receptors for collectins. Immunobiology. 1998;199:165–89. doi: 10.1016/S0171-2985(98)80025-9. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165:2637–42. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 12.Garred P, Thiel S, Madsen HO, Ryder LP, Jensenius JC, Svejgaard A. Gene frequency and partial protein characterization of an allelic variant of mannan binding protein associated with low serum concentrations. Clin Exp Immunol. 1992;90:517–21. doi: 10.1111/j.1365-2249.1992.tb05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 14.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 15.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 16.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 17.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–75. [PubMed] [Google Scholar]
- 18.Richardson VF, Larcher VF, Price JF. A common congenital immunodeficiency predisposing to infection and atopy in infancy. Arch Dis Child. 1983;58:799–802. doi: 10.1136/adc.58.10.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 21.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 22.Davies EJ, Teh LS, Ordi-Ros J, et al. A dysfunctional allele of the mannose binding protein gene associates with systemic lupus erythematosus in a Spanish population. J Rheumatol. 1997;24:485–8. [PubMed] [Google Scholar]
- 23.Garred P, Voss A, Madsen HO, et al. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes Immun. 2001;2:442–50. doi: 10.1038/sj.gene.6363804. [DOI] [PubMed] [Google Scholar]
- 24.Ip WK, Chan SY, Lau CS, Lau YL. Association of systemic lupus erythematosus with promoter polymorphisms of the mannose-binding lectin gene. Arthritis Rheum. 1998;41:1663–8. [PubMed] [Google Scholar]
- 25.Sullivan KE, Wooten C, Goldman D, Petri M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:2046–51. doi: 10.1002/art.1780391214. [DOI] [PubMed] [Google Scholar]
- 26.Villarreal J, Crosdale D, Ollier W, et al. Mannose binding lectin and FcgammaRIIa (CD32) polymorphism in Spanish systemic lupus erythematosus patients. Rheumatology (Oxford) 2001;40:1009–12. doi: 10.1093/rheumatology/40.9.1009. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsumi A, Sasaki K, Wakamiya N, et al. Mannose-binding lectin gene: polymorphisms in Japanese patients with systemic lupus erythematosus, rheumatoid arthritis and Sjogren's syndrome. Genes Immun. 2001;2:99–104. doi: 10.1038/sj.gene.6363744. [DOI] [PubMed] [Google Scholar]
- 28.Huang YF, Wang W, Han JY, et al. Increased frequency of the mannose-binding lectin LX haplotype in Chinese systemic lupus erythematosus patients. Eur J Immunogenet. 2003;30:121–4. doi: 10.1046/j.1365-2370.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 29.Garred P, Madsen HO, Halberg P, et al. Mannose-binding lectin polymorphisms and susceptibility to infection in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2145–52. doi: 10.1002/1529-0131(199910)42:10<2145::AID-ANR15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Trouw LA, Seelen MA, Duijs JM, Benediktsson H, Van Kooten C, Daha MR. Glomerular deposition of C1q and anti-C1q antibodies in mice following injection of antimouse C1q antibodies. Clin Exp Immunol. 2003;132:32–9. doi: 10.1046/j.1365-2249.2003.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegert CE, Daha MR, Tseng CM, Coremans IE, van Es LA, Breedveld FC. Predictive value of IgG autoantibodies against C1q for nephritis in systemic lupus erythematosus. Ann Rheum Dis. 1993;52:851–6. doi: 10.1136/ard.52.12.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos A, Bouwman LH, Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001;167:2861–8. doi: 10.4049/jimmunol.167.5.2861. [DOI] [PubMed] [Google Scholar]
- 33.Martensson U, Thiel S, Jensenius JC, Sjoholm AG. Human autoantibodies against Clq: lack of cross reactivity with the collectins mannan-binding protein, lung surfactant protein A and bovine conglutinin. Scand J Immunol. 1996;43:314–20. doi: 10.1046/j.1365-3083.1996.d01-48.x. [DOI] [PubMed] [Google Scholar]
- 34.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–16. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 35.Antes U, Heinz HP, Loos M. Evidence for the presence of autoantibodies to the collagen-like portion of C1q in systemic lupus erythematosus. Arthritis Rheum. 1988;31:457–64. doi: 10.1002/art.1780310401. [DOI] [PubMed] [Google Scholar]
- 36.Coremans IE, Spronk PE, Bootsma H, et al. Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis. 1995;26:595–601. doi: 10.1016/0272-6386(95)90595-2. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–92. [PubMed] [Google Scholar]
- 39.Nauta AJ, Trouw LA, Daha MR, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–36. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Nauta AJ, Daha MR, Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–54. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 41.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–9. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]