Abstract
We previously described the requirement of tumour necrosis factor-alpha (TNF-α) and the role of β2 integrins in the Fc-gamma receptor IIa (FcγRIIa)-mediated mechanism of neutrophil activation by antiproteinase-3 (anti-PR3) or anti-myeloperoxidase (anti-MPO) antibodies. In the present study, we assessed the involvement of FcγRIIIb by studying the respiratory burst activation of completely FcγRIIIb-deficient neutrophils primed by TNF-α and exposed to anti-PR3 or anti-MPO. Activation of the NADPH oxidase occurred normally in these neutrophils, which indicates that engagement of FcγRIIIb is not essential in our model. Experiments performed with neutrophils from severe leucocyte adhesion deficiency (LAD) patients confirmed that β2 integrins play a pivotal role in this activation. We next studied whether adhesion per se, β2-integrin-mediated adhesion, or β2-integrin ligation without adhesion is necessary or sufficient for this activation. Anti-PR3 or anti-MPO induced an FcγRIIa-dependent burst in TNF-primed neutrophils incubated in wells coated with poly-l-lysine, known to induce β2-integrin-independent adhesion, but this reaction was still inhibited by blocking CD18 antibodies. In a system with granulocyte-macrophage colony-stimulating factor (GM-CSF)-primed neutrophils, which did not enhance adhesion, we measured a similar activation by anti-PR3 or anti-MPO and inhibition by CD18. We also noticed that treatment with the β2-integrin-activating CD18 MoAb KIM185 per se is insufficient for neutrophil activation by anti-PR3 or anti-MPO. We therefore conclude that ligation of β2 integrins rather than adherence per se is essential for this activation, and that TNF-α or GM-CSF is needed for priming but not for adherence.
Keywords: ANCA, Fcγ receptors, β2 integrins, neutrophil activation, vasculitis
INTRODUCTION
Anti-neutrophil cytoplasm autoantibodies (ANCA) have been detected in the circulation of patients with vasculitides, such as Wegener's granulomatosis (WG) [1]. Proteinase-3 (PR3) and myeloperoxidase (MPO) are the two main target antigens of ANCA [2,3].
It has been suggested that ANCA are involved directly in the pathogenesis of WG, because of the relationship of ANCA titres with disease activity [4]. Indeed, it has been well established that in vitro, ANCA are capable of activating neutrophils pretreated with tumour necrosis factor-alpha (TNF-α), which is known to bring neutrophils in a preactivated state through a process called ‘priming’ [5].
In a previous study [6], we focused on the mechanisms involved in this neutrophil activation. We demonstrated that neutrophil activation by anti-PR3 or anti-MPO antibodies proceeds through an Fc-gamma receptor IIa (FcγRIIa)-dependent mechanism, in accordance with data from other groups [7,8]. Nevertheless, the partial independence of this neutrophil activation from FcγRIIa ligation is still a matter of discussion [5,9,10]. We also demonstrated that β2 integrins are instrumental in provoking neutrophil activation by anti-PR3 or anti-MPO antibodies [6]. The family of leucocyte adhesion receptors called CD11/CD18 integrins or β2 integrins, used by leucocytes to interact with the endothelium, enables them to transmit a signal to the inside of the cell [11].
In the present study, we further characterized the involvement of Fcγ receptors and β2 integrins in neutrophil activation by anti-PR3 or anti-MPO antibodies.
MATERIALS AND METHODS
Reagents and antibodies
Human fibronectin (FN), hydrobromide poly-l-lysine, sodium azide, N-formyl-methionyl-leucyl-phenylalanine (fMLP), phorbol-myristate acetate (PMA), sodium barbital, Triton X-100 (TX-100), l-cysteine, ethylene-diamine-tetra-acetic acid (EDTA), N-ethyl maleimide, papain and protein A were obtained from Sigma-Aldrich, St Louis, MO, USA. Dihydro-rhodamine-1,2,3 (DHR) was purchased from Molecular Probes, Eugene, OR, USA. Human recombinant TNF-α and p-nitrophenyl phosphate were from Roche Diagnostics, Mannheim, Germany. Human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) was from PeproTech, Rocky Hill, NJ, USA. All other reagents were of analytical grade purity.
Monoclonal antibodies (MoAbs) 12·8 against PR3 (mIgG1), MoAb 4·15 against MPO (mIgG1), CD2 (mIgG1) (CLB-T11/1), and MoAb IV.3 (CD32, mIgG2b) against FcγRIIa were from the Central Laboratory of the Netherlands Blood Transfusion Service (CLB), Amsterdam, the Netherlands. MoAb MHM23 (LFA-1, β-chain, mIgG1) was from Dako, Glostrup, Denmark. The β2-activating MoAb KIM185 was kindly provided by Dr N. Hogg, Leucocyte Adhesion Laboratory, Imperial Cancer Research Fund, London, UK [12].
Fab fragments were made by digestion with 4% (w/w) papain in PBS containing 10 mm cysteine and 5 mm EDTA for 1·5 h at 37°C. The reaction was terminated by addition of 20 mm N-ethyl maleimide. Protein-A affinity chromatography was used to remove Fc fragments and intact antibodies. When Fab fragments were checked on sodium dodecylsulphate-polyacrylamide gel electrophoresis, intact antibodies or Fc fragments were not detectable.
Immunoglobulin G from sera with either PR3-ANCA or MPO-ANCA were purified by passage over HiTrap protein-G sepharose (Amersham Biosciences, Piscataway, NJ, USA). Purity of the IgG preparations as determined by sodium dodecylsulphate-polyacrylamide gel electrophoresis was always greater than 95%.
Isolation of neutrophils
Blood was obtained from healthy donors, from one completely FcγRIIIb-deficient donor [13] and from two severe leucocyte adhesion deficiency type I (LAD-1) patients. Granulocytes were purified from blood anticoagulated with 0·4% (w/v) trisodium citrate (pH 7·4), as described [14]. In short, blood cells were separated by density gradient centrifugation over isotonic Percoll (Amersham Biosciences) with a specific gravity of 1·076 g/ml. The interphase, containing the mononuclear cells, was removed. The pellet fraction, containing erythrocytes and granulocytes, was treated for 10 min with ice-cold isotonic NH4Cl solution (155 mm NH4Cl, 10 mm KHCO3, 0·1 mm EDTA, pH 7·4) to lyse the erythrocytes. The remaining granulocytes were washed twice in phosphate-buffered saline (PBS) and were resuspended in incubation medium containing 132 mm NaCl, 6 mm KCl, 1 mm CaCl2, 1 mm MgSO4, 1·2 mm KH2PO4, 20 mm Hepes, 5·5 mm glucose and 0·5% (w/v) human serum albumin (pH 7·4) and were kept at room temperature (RT) at a final concentration of 2 × 106 cells/ml, unless indicated otherwise. Purity of neutrophils was more than 95% (the contaminating cells were mainly eosinophils), and viability was more than 98%.
Measurement of respiratory burst in dihydro-rhodamine-1,2,3-loaded neutrophils
The assay to measure NADPH-oxidase activity in DHR-loaded neutrophils incubated in polystyrene tubes in a shaking waterbath under gentle agitation was carried out essentially as described [6,15].
Alternatively, wells (15·5 mm diameter) of a flat-bottomed 24-well polystyrene plate (Nunclon Delta, Nunc, Roskilde, Denmark) were pretreated for 1 h at 37°C with FN (10 µg/ml) or poly-l-lysine (10 µg/ml) dissolved in PBS, and were then washed once with PBS and once with incubation medium at RT. Neutrophils (2 × 106/ml) were incubated in polypropylene tubes in a shaking waterbath for 5 min at 37°C, before DHR (0·5 µm) and sodium azide (2 mm) were added. Subsequently, these neutrophils were rapidly distributed at 106 cells per well over the polystyrene plate previously incubated at 37°C. After another 10 min, TNF-α (2 ng/ml) or GM-CSF (20 ng/ml) was added to some of the wells. After 10 min of priming at 37°C, fMLP (1 µm), anti-PR3 MoAb (5 µg/ml), anti-MPO MoAb (5 µg/ml) or purified IgG preparations of either PR3- or MPO-ANCA (75 µg/ml) was added to the wells. Control cells received PBS only. Thirty min after addition of the stimulus, unless indicated otherwise, the supernatant of the wells, including the nonadherent cells, was removed, and the wells were gently washed with ice-cold incubation medium. Thereafter, 500 µl of ice-cold PBS containing 1% (w/v) bovine serum albumin (BSA) with 10 mm EDTA was added to the wells and left for 30 min at 4°C. After this period, neutrophils were resuspended in tubes with 3 ml of ice-cold PBS/BSA 1%. Thereafter, the tubes were centrifuged (400 g) for 5 min at 4°C, and the cells were resuspended in about 100 µl of ice-cold PBS/BSA 1%, and kept on ice in the dark until analysis in the flow cytometer (Epics Profile, Coulter Corporation, Miami, FL, USA). Neutrophils were distinguished by forward–side-scatter pattern, and data were collected from 5000 cells. The results are expressed as mean fluorescence intensity (MFI).
Neutrophil adherence assay
Neutrophil adherence to FN or poly-l-lysine was measured under conditions identical to those used for the activation of the neutrophil respiratory burst in flat-bottomed 24-well plates. The non-adherent cells of coated wells were removed by washing the plate twice with ice-cold incubation medium. Neutrophil adherence to FN or poly-l-lysine was measured by alkaline phosphatase activity of adherent cells [16]. Briefly, 500 µl of buffer containing 50 mm sodium barbital (pH 10·5), 1 mm MgCl2, 0·1% TX-100 and 1 mg/ml of p-nitrophenyl phosphate were added to the wells. After an incubation period of 30 min at 37°C, the supernatant was transferred to a microplate for determination of the optical density at 410 nm (Labsystems iEMS reader MF, Helsinki, Finland). The percentage of adherent cells was calculated from appropriate standard curves, obtained by incubating known numbers of neutrophils with alkaline phosphatase substrate in the same microplate.
Statistical analysis
Results are expressed as mean ± s.e.m. of (n) independent experiments. Statistical significance was evaluated by means of analysis of variance (anova) to assess whether there was a significant overall effect of treatment. When significant effects were found, individual analyses were performed with the two-sided t-test. A value of P < 0·05 was considered significant.
RESULTS
Involvement of Fcγ receptors and β2 integrins in neutrophil activation by anti-PR3 or anti-MPO antibodies
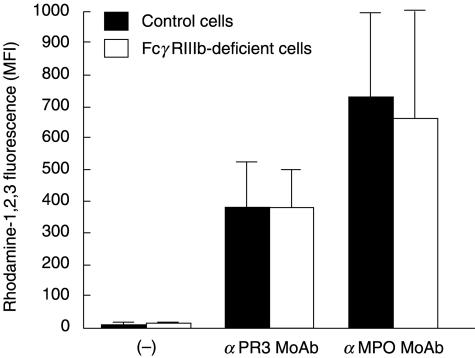
In our previous study [6], we observed that neutrophil activation by anti-PR3 or anti-MPO antibodies occurs only when TNF-α is present, and that binding of anti-PR3 or anti-MPO antibodies via their Fc region to the FcγRIIa of the neutrophil is instrumental in provoking the respiratory burst activation. Moreover, the addition of F(ab′)2 fragments of 3G8 MoAb, which blocks the binding of IgG to FcγRIIIb, did not have a significant effect [6]. In the present study, respiratory burst experiments were conducted with TNF-primed neutrophils from one completely FcγRIIIb-deficient donor exposed to anti-PR3 or anti-MPO MoAbs. The cells were gently shaken in polystyrene tubes to allow cell adhesion to the plastic, similar to the conditions used in [6]. As a sensitive method to detect neutrophil activation, we used the conversion of added DHR to the fluorescent rhodamine, which is dependent on NADPH-oxidase activity [6,15]. Activation of NADPH oxidase by anti-PR3 or anti-MPO MoAbs occurred normally in these FcγRIIIb-deficient neutrophils (Fig. 1).
Fig. 1.
Respiratory burst in TNF-treated neutrophils from one completely FcγRIIIb-deficient donor versus control donors. Neutrophils (2 × 106/ml) from the FcγRIIIb-deficient donor and from control donors were incubated at 37°C with dihydro-rhodamine-1,2,3 in polystyrene tubes in a shaking waterbath under gentle agitation, as described in Materials and methods, in the presence of TNF-α (2 ng/ml). After 10 min of priming, the cells were stimulated for 30 min with anti-PR3 or anti-MPO MoAbs at a final concentration of 5 µg/ml. Control cells (–) received PBS only. Samples were processed for flow cytometry to measure rhodamine-1,2,3 fluorescence. Results (MFI) are the mean ± s.e.m. of results from three independent experiments obtained with one completely FcγRIIIb-deficient donor and three control donors. The difference between results obtained with FcγRIIIb-deficient cells and control cells was not significant for anti-PR3 or anti-MPO antibodies.
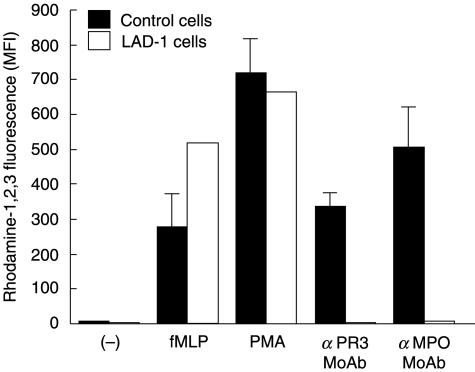
We also observed [6] that activation of TNF-primed neutrophils by anti-PR3 or anti-MPO antibodies is strongly impaired when neutrophil adhesion is prevented by continuous stirring of the cells or by addition of CD18 antibodies. To confirm that β2 integrins are involved in the signal transduction from the FcγRIIa to the NADPH oxidase induced by anti-PR3 or anti-MPO antibodies, experiments were conducted with neutrophils from two severe LAD-1 patients. In the TNF-primed neutrophils that lack the β2 (CD18) integrins, anti-PR3 or anti-MPO MoAbs were not able to induce any NADPH-oxidase activation, in contrast to the strong reaction induced in control neutrophils (Fig. 2). The LAD-1 neutrophils expressed normal amounts of PR3 and MPO on their surface (not shown) and were normally activated by fMLP and by PMA.
Fig. 2.
Respiratory burst in TNF-treated neutrophils from two leucocyte adhesion deficiency type 1 (LAD-1) patients versus control donors. Neutrophils (2 × 106/ml) from two LAD-1 patients and from control donors were incubated at 37°C with dihydro-rhodamine-1,2,3 in polystyrene tubes in a shaking waterbath under gentle agitation, as described in Materials and methods, in the presence of TNF-α (2 ng/ml). After 10 min of priming, the cells were stimulated for 15 min with fMLP (1 µm), PMA (100 ng/ml) and for 30 min with anti-PR3 or anti-MPO MoAbs, at a final concentration of 5 µg/ml. Control cells (–) received PBS only. Samples were processed for flow cytometry to measure rhodamine-1,2,3 fluorescence. Results (MFI) are the mean of results with two LAD-1 patients and the mean ± s.e.m. of results with three control donors. The difference between the results obtained with control cells and LAD-1 cells was not significant for PBS, fMLP and PMA, but it was significant for anti-PR3 (P < 0·001) or anti-MPO (P < 0·001) antibodies.
To investigate in more detail the nature of the cell adhesion required for the activation by anti-PR3 and anti-MPO antibodies, we performed experiments with neutrophils adhering to FN-coated wells. Similar to the situation in polystyrene tubes [6], the activation in FN-coated wells depended on the presence of both TNF-α (2 ng/ml) and anti-PR3 MoAb, anti-MPO MoAb or purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA (Table 1). This activation was inhibited for 80–90% by CD18 MoAb MHM23 Fab fragments. As in our previous study [6], activation was not detected in TNF-treated neutrophils incubated with an irrelevant antibody (CD2, mIgG1) (not shown). The presence of the blocking CD18 antibodies also inhibited the TNF-induced adherence of the cells to FN (Table 2). The activation of the cells in this system was FcγRIIa-dependent, because MoAb IV.3 against FcγRIIa inhibited the NADPH-oxidase activation (not shown), but this had no effect on the TNF-induced adherence to FN (Table 2). Only the additional adhesion induced by anti-PR3 or anti-MPO MoAbs was inhibited by IV.3 MoAb (Table 2).
Table 1.
Respiratory burst in TNF-untreated and -treated neutrophils incubated in fibronectin (FN)-coated wells or in poly-l-lysine-coated wells and exposed to anti-PR3 MoAb, anti-MPO MoAb or purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA
| Rhodamine–1,2,3 fluorescence (MFI) | |||||
|---|---|---|---|---|---|
| Unstimulated | α PR3 MoAb | α MPO MoAb | PR3-ANCA | MPO-ANCA | |
| FN-coated wells | |||||
| TNF-untreated | 5 ± 1 | 5 ± 1 | 7 ± 1 | 12 ± 1 | 14 ± 1 |
| TNF-treated | 36 ± 10 | 414 ± 99 | 490 ± 82 | 431 ± 116 | 499 ± 74 |
| Poly-l-lysine-coated wells | |||||
| TNF-untreated | 12 ± 1 | 15 ± 1 | 37 ± 3 | 98 ± 15 | 92 ± 26 |
| TNF-treated | 76 ± 18 | 310 ± 36 | 380 ± 79 | 408 ± 52 | 363 ± 75 |
Statistical significance of differences between TNF-untreated and TNF-treated cells: P < 0·05. Neutrophils (1 × 106/well) were incubated at 37°C with dihydro-rhodamine-1,2,3 in polystyrene wells coated with FN (10 µg/ml) or with poly-l-lysine (10 µg/ml), as described in Materials and methods, in the absence or presence of TNF-α (2 ng/ml). After 10 min of priming, the cells were stimulated for 30 min with anti-PR3 MoAb (5 µg/ml), anti-MPO MoAb (5 µg/ml) or with purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA (75 µg/ml). Control cells received PBS only. Samples of adherent cells to FN or to poly-l-lysine were processed for flow cytometry to measure rhodamine-1,2,3 fluorescence. Results, expressed as mean fluorescence intensity (MFI), are the mean ± s.e.m. of three to five experiments.
Table 2.
Effect of CD18 MoAb MHM23 and MoAb IV.3 (anti-FcγRIIa) on adherence of TNF-treated neutrophils to fibronectin (FN)-coated wells
| Neutrophil adherence to FN (%) | |||
|---|---|---|---|
| Unstimulated | anti-PR3 MoAb | anti-MPO MoAb | |
| TNF-untreated | 7 ± 1 | 7 ± 1 | 3 ± 1 |
| TNF-treated | 32 ± 2 | 40 ± 2 | 48 ± 4 |
| + CD18 MHM23 | 6 ± 1a | 9 ± 2a | 13 ± 3a |
| + anti-FcγRIIa | 32 ± 4b | 30 ± 5c | 30 ± 4c |
Statistical significance of differences between TNF-untreated and TNF-treated cells (in the absence of CD18 MoAb or anti-FcγRIIa): P < 0·001. Statistical significance of differences between TNF-treated unstimulated and stimulated cells (in the absence of CD18 MoAb or anti-FcγRIIa): P < 0·05. Statistical significance of differences between TNF-treated cells in the absence or presence of CD18 MoAb or of anti-FcγRIIa:
P < 0·001
n.s.
P < 0·05.
Neutrophils (1 × 106/well) were incubated at 37°C in polystyrene wells coated with FN (10 µg/ml) in the absence or presence of CD18 MoAb MHM23 (10 µg/ml) or of MoAb IV.3 (anti-FcγRIIa) (10 µg/ml), for 5 min prior to addition of TNF-α (2 ng/ml). After 10 min of priming, the cells were stimulated with anti-PR3 or anti-MPO MoAbs, used at a final concentration of 5 µg/ml. Part of the cells was left untreated, as indicated. Control cells received PBS only. After 30 min of stimulation, neutrophil adherence to FN (%) was detected, as described in Materials and methods. Results are the mean ± s.e.m. of three to four experiments.
β2-integrin ligation rather than adherence per se is essential for activation of the respiratory burst by anti-PR3 or anti-MPO antibodies
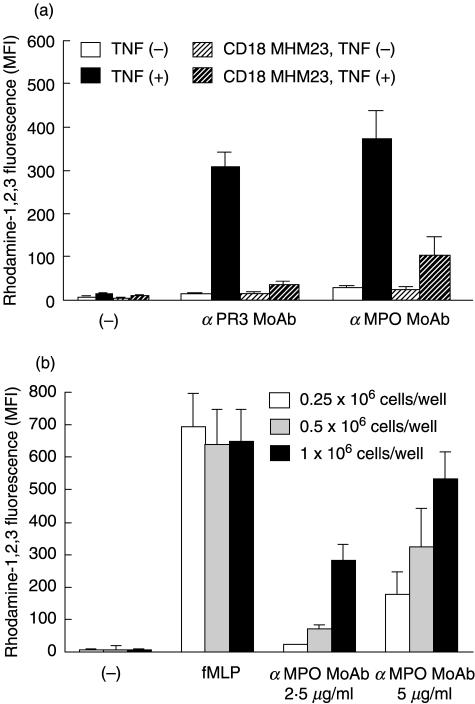
We then investigated whether the adhesion step as such or the β2-integrin ligation was essential for the oxidase activation by anti-PR3 or anti-MPO antibodies. We tested whether adherence of neutrophils to poly-l-lysine, known not to be β2-integrin-mediated, also provided sufficient conditions for oxidase activation by anti-PR3 or anti-MPO antibodies. Under these conditions, anti-PR3 or anti-MPO MoAbs as well as purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA, indeed induced a respiratory burst in TNF-primed neutrophils (Table 1 and Fig. 3a). The activation of the cells in this system was also FcγRIIa-dependent (not shown). The reaction induced by anti-PR3 or anti-MPO MoAbs was still inhibited by CD18 MoAb MHM23 (Fig. 3a), and that induced by PR3-ANCA or MPO-ANCA was inhibited for 75% by this MoAb. Nevertheless, the CD18 MoAb had no effect on the adhesion of the cells to the poly-l-lysine (Table 3). TNF-α priming was still necessary for the induction of a respiratory burst, although it had no effect on the adherence in this system. In another experimental set-up with GM-CSF (20 ng/ml), instead of TNF-α to prime the cells we observed efficient activation of the respiratory burst in neutrophils incubated in FN-coated wells by anti-PR3 MoAb, anti-MPO MoAb or by purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA, and inhibition of this process by CD18 MoAb MHM23 (not shown). However, no increase in neutrophil adhesion to FN by GM-CSF was detected (Table 4).
Fig. 3.
(a) Effect of CD18 MoAb MHM23 on the respiratory burst in TNF-untreated and -treated neutrophils incubated in poly-l-lysine-coated wells. Neutrophils (2 × 106/ml) were incubated at 37°C with dihydro-rhodamine-1,2,3 in polystyrene wells coated with poly-l-lysine (10 µg/ml), as described in Materials and methods, in the absence or presence of CD18 MoAb MHM23 (Fab fragments, 20 µg/ml) for 5 min prior to the addition of TNF-α (2 ng/ml). Part of the cells was left untreated, as indicated. After 10 min of priming, the cells were stimulated for 30 min with anti-PR3 or anti-MPO MoAbs, at a final concentration of 5 µg/ml. Control cells (–) received PBS only. Samples of adherent cells to poly-l-lysine were processed for flow cytometry to measure rhodamine-1,2,3 fluorescence. Results (MFI) are the mean ± s.e.m. of four experiments. With both stimuli, TNF-α had a significant enhancing effect in the absence of CD18 (P < 0·05), and CD18 had a significant inhibiting effect in the presence of TNF-α (P < 0·05). (b) Effect of cell concentration on the respiratory burst in TNF-treated neutrophils incubated in poly-l-lysine-coated wells. Neutrophils (0·25 × 106, 0·5 × 106 and 1 × 106/well) were incubated at 37°C with dihydro-rhodamine-1,2,3 in polystyrene wells coated with poly-l-lysine (10 µg/ml), as described in Materials and methods, in the presence of TNF-α (2 ng/ml). After 10 min of priming, the cells were stimulated for 15 min with fMLP (1 µm) and for 30 min with anti-MPO MoAb, at a final concentration of 2·5 µg/ml or 5 µg/ml. Control cells (–) received PBS only. Samples of adherent cells to poly-l-lysine were processed for flow cytometry to measure rhodamine-1,2,3 fluorescence. Results (MFI) are the mean ± s.e.m. of three experiments. With fMLP, increasing the cell concentration did not significantly change the results (n.s.). With anti-MPO MoAb used at both concentrations, increasing the cell concentration from 0·25 × 106 per ml to 1 × 106 per ml, had a significant enhancing effect (P < 0·05).
Table 3.
Effect of CD18 MoAb MHM23 on adherence of TNF-untreated or -treated neutrophils to poly-l-lysine-coated wells
| Neutrophil adherence to poly-l-lysine (%) unstimulated | |
|---|---|
| TNF-untreated | 48 ± 2 |
| + CD18 MHM23 | 39 ± 6a |
| TNF-treated | 39 ± 3 |
| + CD18 MHM23 | 28 ± 6a |
Statistical significance:
n.s. compared to the situation without CD18 MoAb.
Neutrophils (1 × 106/well) were incubated at 37°C in polystyrene wells coated with poly-l-lysine (10 µg/ml) in the absence or presence of CD18 MoAb MHM23 (10 µg/ml), for 5 min prior to addition of TNF-α (2 ng/ml). Part of the cells was left untreated, as indicated. Control cells received PBS only. After 40 min, neutrophil adherence to poly-l-lysine (%) was detected as described in Materials and methods. Results are the mean ± s.e.m. of seven experiments.
Table 4.
Effect of CD18 MoAb MHM23 on adherence of GM-CSF-untreated or -treated neutrophils to fibronectin (FN)-coated wells
| Neutrophil adherence to FN (%) unstimulated | |
|---|---|
| GM-CSF-untreated | 4 ± 1 |
| GM-CSF-treated | 7 ± 2a |
| + CD18 MHM23 | 2 ± 1b |
Statistical significance:
n.s. compared to GM-CSF-untreated
n.s. compared to GM-CSF-treated.
Neutrophils (1 × 106/well) were incubated at 37°C in polystyrene wells coated with FN (10 µg/ml) in the absence or presence of CD18 MoAb MHM23 (10 µg/ml), for 5 min prior to addition of GM-CSF (20 ng/ml). Part of the cells was left untreated, as indicated. Control cells received PBS only. After 40 min, neutrophil adherence to FN (%) was detected as described in Materials and methods. Results are the mean ± SEM of four experiments.
The question remains which ligand binds to the β2 integrins on the neutrophil surface if it is not poly-l-lysine (or FN in case of GM-CSF-primed cells). We noted that activation by anti-MPO MoAb of TNF-treated neutrophils incubated in poly-l-lysine-coated wells, but not in FN-coated wells, was clearly dependent on cell concentration (Fig. 3b). A similar cell concentration-dependent effect was seen with GM-CSF-primed cells in FN-coated wells (not shown).
β2-integrin activation per se is insufficient for neutrophil activation by anti-PR3 or anti-MPO antibodies
The previous experiments indicate that priming of neutrophils by TNF-α or GM-CSF for NADPH-oxidase activation by anti-PR3 or anti-MPO antibodies proceeds via activation of β2 integrins. Indeed, it is well known that TNF-α induces β2-integrin activation [17]. We investigated therefore whether β2-integrin activation by other means also primed the cells for oxidase activation. Although Fab fragments of the β2-activating MoAb KIM185 (20 µg/ml) did induce increased neutrophil adherence to FN (not shown), these Fab fragments did not prime the cells for oxidase activation by anti-PR3 MoAb, by anti-MPO MoAb or by purified IgG preparations from sera with either PR3-ANCA or MPO-ANCA (not shown), for which TNF-α was still needed for a good respiratory burst.
DISCUSSION
Previous work from our laboratory [6] and from others [7,8] has shown that anti-PR3 and anti-MPO antibodies activate neutrophils via binding of the Fc region of these antibodies to the FcγRIIa. In our previous work [6], we found that addition of antibodies against FcγRIIIb did not block the burst induced by anti-PR3 or anti-MPO antibodies. However, Kocher et al. [18] have described that ANCA preferentially engage FcγRIIIb on neutrophils. Anti-PR3 or anti-MPO antibodies have been shown by these investigators to decrease significantly the binding of several anti-FcγRIIIb antibodies to neutrophils, under conditions that limit activation-induced FcγRIIIb shedding. In the present study, we first assessed the involvement of FcγRIIIb by studying the respiratory burst activation of completely FcγRIIIb-deficient neutrophils (due to a FcγRIIIB gene deletion) [13] primed by TNF-α and exposed to anti-PR3 or anti-MPO MoAbs. Activation of the NADPH oxidase occurred normally in these neutrophils, which indicates that engagement of FcγRIIIb in our model is not essential for neutrophil activation by anti-PR3 or anti-MPO MoAbs.
We next confirmed the involvement of β2 integrins in this cell activation by showing that TNF-primed neutrophils from two severe LAD-1 patients, which lack β2-integrin expression, were not activated by anti-PR3 or anti-MPO MoAbs. Zhou et al. [19] have demonstrated that LAD cells are perfectly competent to mount a respiratory burst when FcγRIIa is engaged. As PR3 or MPO expression on the surface of LAD-1 cells with TNF-α was not different from control cells, the failure of these LAD-1 cells to generate hydrogen peroxide in the presence of anti-PR3 or anti-MPO MoAbs cannot be explained by a decreased expression of the antigens involved.
We then studied the question whether adhesion per se, β2-integrin-mediated adhesion or β2-integrin ligation without adhesion is necessary or sufficient for the priming action of TNF-α and the oxidase activation by ANCA. In the absence of anti-FcγRIIa MoAb, the adherence is caused both by TNF-α and by anti-PR3 or anti-MPO MoAbs (Table 2). In the presence of anti-FcγRIIa MoAb, anti-PR3 and anti-MPO MoAbs can no longer activate neutrophils, and therefore do not promote adherence over the effect of TNF-α alone. TNF-α (2 ng/ml) does not induce increased surface expression of FcγRIIa [6]. We also observed that binding of the cells to poly-l-lysine, which in itself is β2-integrin-independent, suffices for this TNF-ANCA activation. However, although the adhesion in this system was not blocked by CD18 MoAb MHM23, the cell activation was. Thus, adhesion per se is insufficient for TNF-ANCA activation, but CD18-ligand binding is essential. In an alternative system with neutrophils primed by GM-CSF, described by Lopez et al. not to enhance the adherence of cells to endothelium or plastic surfaces [20], we found that adhesion is not even necessary for efficient ANCA activation but here, too, the reaction was still inhibited by CD18 MoAb MHM23. Thus, both experimental set-ups indicate for the first time that β2-integrin ligation rather than adherence per se is essential for activation of the respiratory burst by anti-PR3 or anti-MPO antibodies, and that TNF-α or GM-CSF is needed for priming but not for adherence. Moreover, the cell concentration-dependent effect for neutrophil activation under these conditions suggests that cell–cell interactions might play a role, e.g. mediated by binding of β2 integrins on one cell with intercellular adhesion molecules-1 or -3 (ICAM-1 or ICAM-3) on another cell.
Activation of β2 integrins by MoAb KIM185 did not generate a signal that could replace TNF-α or GM-CSF in the cell priming. Perhaps this MoAb stabilizes an active configuration of the β2 integrins on the cell surface, sufficient for enhanced binding to FN, but the signal induced by binding of anti-PR3 or anti-MPO antibodies to FcγRIIa needs additional TNF- or GM-CSF-mediated signals for efficient oxidase activation. We postulate that TNF-α may facilitate neutrophil activation by anti-PR3 or anti-MPO antibodies via another mechanism than by increasing the expression of PR3 or MPO on the cell surface, e.g. by priming the cells for enhanced NADPH-oxidase activity when activated by anti-PR3 or anti-MPO antibodies by facilitating the signal transduction between FcγRIIa and the oxidase. This may proceed via up-regulation and activation of β2 integrins, via up-regulation of cytochrome b558 (the central component of the NADPH oxidase), via clustering of FcγRIIa on the neutrophil surface, via co-localization of FcγRIIa and β2 integrins, via association of these two surface proteins with the cytoskeleton and/or via a combination of these processes.
The present study is an extension of our previous results and emphasizes the pivotal role of FcγRIIa and β2 integrins in neutrophil activation induced by anti-PR3 or anti-MPO antibodies. Association of FcγRIIa with the β2-integrin Mac-1 has been shown by Annenkov et al. [21], who used Mac-1- and p150,95-transfected K562 cells which express endogenous FcγRIIa but not other types of Fc receptors. In conclusion, the role of FcγRIIa and β2 integrins in neutrophil activation induced by anti-PR3 or anti-MPO antibodies suggests that β2 integrins may participate in triggering effector activities induced by FcγRIIa-ligand binding.
Acknowledgments
The authors gratefully thank Daniel Dehay for his technical assistance.
REFERENCES
- 1.Van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: Tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Jenne DE, Tschopp J, Lüdemann J, Utecht B, Gross WL. Wegener's autoantigen decoded. Nature. 1990;346:520. doi: 10.1038/346520a0. [Letter]. [DOI] [PubMed] [Google Scholar]
- 3.Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 4.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Terrel RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reumaux D, Vossebeld PJM, Roos D, Verhoeven AJ. Effect of TNF-induced integrin activation on FcγReceptor II-mediated signal transduction: relevance for activation of neutrophils by anti-proteinase 3 or anti-myeloperoxidase antibodies. Blood. 1995;86:3189–95. [PubMed] [Google Scholar]
- 7.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via FcγRIIa. J Immunol. 1994;153:1271–80. [PubMed] [Google Scholar]
- 8.Mulder AHL, Heeringa P, Brouwer E, Limburg PC, Kallenberg CGM. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–94. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- 10.Kimberly RP. Fcγ receptors and neutrophil activation. Clin Exp Immunol. 2000;120:18–9. [Google Scholar]
- 11.Graham IL, Anderson DC, Holers VM, Brown EJ. Complement receptor 3 (CR3, Mac-1, Integrin αMβ2, CD11b/CD18) is required for tyrosine phosphorylation of paxillin in adherent and nonadherent neutrophils. J Cell Biol. 1994;127:1139–47. doi: 10.1083/jcb.127.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrew D, Shock A, Ball E, Ortlepp S, Bell J, Robinson M. KIM185, a monoclonal antibody to CD18 which induces a change in the conformation of CD18 and promotes both LFA-1 and CR3-dependent adhesion. Eur J Immunol. 1993;23:2217–22. doi: 10.1002/eji.1830230925. [DOI] [PubMed] [Google Scholar]
- 13.De Haas M, Kleijer M, van Zwieten R, Roos D, von dem Borne AEGKr. Neutrophil Fc gamma RIIIb deficiency, nature, and clinical consequences: a study of 21 individuals from 14 families. Blood. 1995;86:2403–13. [PubMed] [Google Scholar]
- 14.Roos D, de Boer M. Purification and cryopreservation of phagocytes from human blood. Meth Enzymol. 1986;132:225–43. doi: 10.1016/s0076-6879(86)32010-x. [DOI] [PubMed] [Google Scholar]
- 15.Van Pelt LJ, van Zwieten R, Weening RS, Roos D, Verhoeven AJ, Bolscher BGJM. Limitations on the use of dihydrorhodamine 123 for flow cytometric analysis of the neutrophil respiratory burst. J Immunol Meth. 1996;191:187–96. doi: 10.1016/0022-1759(96)00024-5. [DOI] [PubMed] [Google Scholar]
- 16.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan C, Srimal S, Farber C, et al. Cytokine-induced respiratory burst of human neutrophils. Dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–9. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kocher M, Edberg JC, Fleit HB, Kimberly RP. Antineutrophil cytoplasmic antibodies preferentially engage FcγRIIIb on human neutrophils. J Immunol. 1998;161:6909–14. [PubMed] [Google Scholar]
- 19.Zhou MJ, Brown EJ. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol. 1994;125:1407–16. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez AF, Williamson DJ, Gamble JR, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annenkov A, Ortlepp S, Hogg N. The beta 2 integrin Mac-1 but not p150,95 associates with Fc gamma RIIA. Eur J Immunol. 1996;26:207–12. doi: 10.1002/eji.1830260132. [DOI] [PubMed] [Google Scholar]