Abstract
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease characterized by the predominant infiltration of T cells, eosinophils and macrophages in lesional skin. Recently, eotaxin-2/CCL24 and eotaxin-3/CCL26 were identified as CC chemokines that signal exclusively via the CCR3 receptor and have eosinophil-selective chemoattractant activity, as does eotaxin/CCL11. We previously reported that serum levels of thymus and activation-regulated chemokine (TARC)/CCL17 and macrophage-derived chemokine (MDC)/CCL22 were correlated with the severity of AD. In this report, we investigated the participation of eotaxin-2/CCL24 and eotaxin-3/CCL26 in AD, first measuring the serum levels of eotaxin-2/CCL24 and eotaxin-3/CCL26 in 30 patients with AD, 20 patients with psoriasis vulgaris and 20 healthy controls. The serum levels of eotaxin-3/CCL26 (but not eotaxin-2/CCL24) were significantly higher in patients with AD than in either healthy controls or patients with psoriasis vulgaris; furthermore, the eotaxin-3/CCL26 levels in patients with moderate and severe AD were significantly higher than eotaxin-3/CCL26 levels in patients with mild AD. The serum eotaxin-3/CCL26 levels tended to decrease after treatment, but there was no significant difference between groups. Moreover, the serum eotaxin-3/CCL26 levels were significantly correlated with the serum TARC/CCL17 and MDC/CCL22 levels, eosinophil numbers in peripheral blood and the scoring AD (SCORAD) index. Our study strongly suggests that serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, have a notable correlation with disease activity of AD and that eotaxin-3/CCL26, as well as TARC/CCL17 and MDC/CCL22, may be involved in the pathogenesis of AD.
Keywords: eotaxin-2/, CCL24 eotaxin-3/, CCL26, atopic dermatitis, disease activity
INTRODUCTION
Atopic dermatitis (AD) is an inflammatory skin disease that is characterized by pruritic and eczematous lesions which chronically persist. It is often associated with elevated serum immunoglobulin E (IgE) levels and peripheral blood eosinophilia. Histopathologically, the skin lesions in AD show infiltration of eosinophils, T lymphocytes and macrophages [1,2]. We previously showed that serum thymus and activation-regulated chemokine (TARC/CCL17) levels and serum macrophage-derived chemokine (MDC/CCL22) levels are significantly correlated with the disease activity of AD, as well as with eosinophil numbers in peripheral blood [3,4].
Both eotaxin-2/CCL24 and eotaxin-3/CCL26 are CC chemokines and functional ligands for CC chemokine receptor 3 (CCR3), which is preferentially expressed on eosinophils. After identification of eotaxin/CCL11 as a relatively selective chemokine for eosinophils [5,6], eotaxin-2/CCL24 was cloned and showed chemotactic activity for eosinophils in a range similar to that of eotaxin/CCL11 [7,8]. More recently, a new member of the eotaxin family – eotaxin-3/CCL26 – has been cloned by two different research groups [9,10]. Only 34% of the amino acids in eotaxin-2/CCL24 and eotaxin-3/CCL26 are identical [10], but these two chemokines have a common characteristic feature in terms of chemotaxis for eosinophils via the CCR3 [7–11]. However, the precise role of these chemokines in AD remained unclear.
To clarify the relationship between AD, eotaxin-2/CCL24 and eotaxin-3/CCL26, we measured the serum levels of eotaxin-2/CCL24 and eotaxin-3/CCL26 and compared them in patients with AD, patients with psoriasis vulgaris and healthy controls. We also compared them with the clinical and laboratory data that reflected the disease activity of AD.
SUBJECTS AND METHODS
Subjects
Thirty patients [mean age ± standard deviation (s.d.): 28·7 ± 7·1 years] with AD, 20 patients (28·2 ± 6·1 years) with psoriasis vulgaris, and 20 control subjects (30·4 ± 10·3 years) were enrolled in this study. All patients with AD were given diagnoses according to the criteria of Hanifin & Rajka [1], and were treated with topical corticosteroids in combination with oral antihistamines. Disease activity of AD was determined by the modified scoring atopic dermatitis (SCORAD) index [12,13]. We divided the patients with AD into three categories, based on severity of the condition, using only objective criteria of SCORAD (mild, < 15; moderate, 15–40; severe, > 40) [13]. In eight patients with AD, we collected the sera before and after treatment. The 20 healthy controls had no history of allergy or psoriasis. All sera were stored at −20°C until use.
Enzyme-linked immunosorbent assay (ELISA)
TARC/CCL17 and MDC/CCL22 immunoassay kits were obtained from Genzyme TECHNE (Minneapolis, MN). Eotaxin-2/CCL24 and eotaxin-3/CCL26 immunoassay kits were from R & D systems (Minneapolis, MN). Serum levels of eotaxin-2/CCL24, eotaxin-3/CCL26, TARC/CCL17 and MDC/CCL22 were measured according to the manufacturer's instructions. Optical densities were measured at 450 nm using a Bio-Rad Model 550 microplate reader (Bio-Rad Laboratories Inc., Hercules, CA). The concentrations were calculated from the standard curve generated by a curve-fitting program. Data obtained from the ELISA are presented as mean ± s.d.
Statistical analysis
Data were analysed using the Mann–Whitney U-test. A P-value of <0·05 was considered to be statistically significant. Correlation coefficients were determined by using the Spearman rank correlation test. Statistical analysis was also performed, and a P-value of <0·05 was considered statistically significant.
RESULTS
Serum eotaxin-2/CCL24 and eotaxin-3/CCL26 levels in patients with AD, patients with psoriasis vulgaris and healthy controls
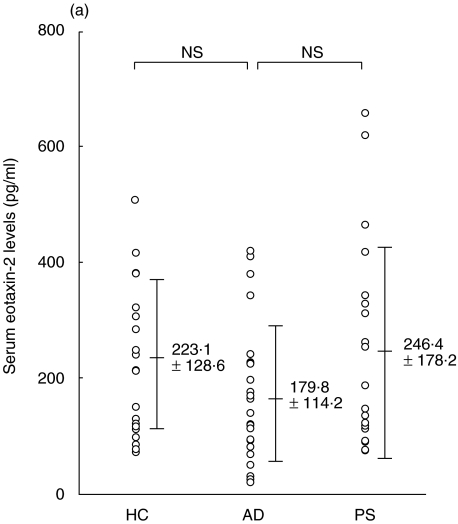
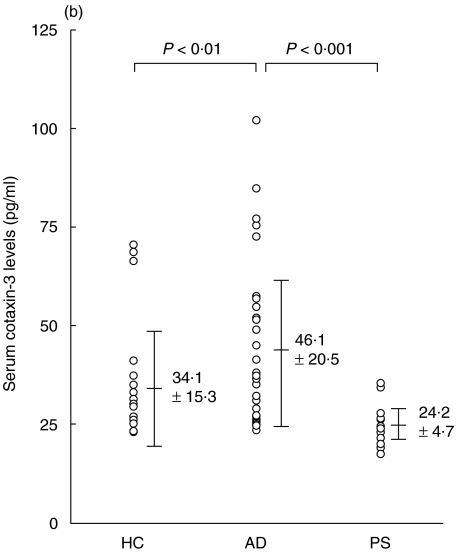
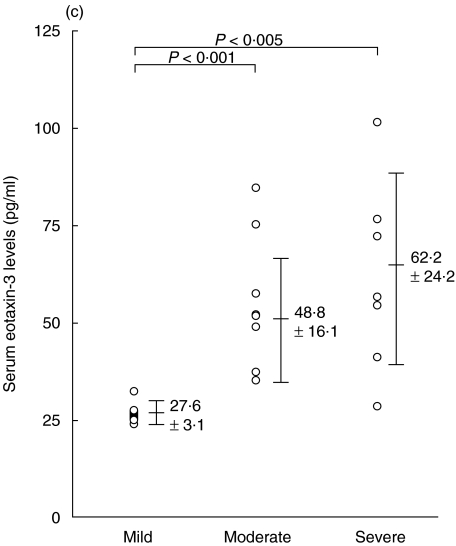
The mean serum eotaxin-2/CCL24 levels of patients with AD, patients with psoriasis vulgaris and control subjects were 179·8 ± 114·2 pg/ml, 246·4 ± 178·2 pg/ml, and 223·1 ± 128·6 pg/ml, respectively; there was no significant difference between these groups (Fig. 1a). In contrast, the mean serum level of eotaxin-3/CCL26 of patients with AD was 46·1 ± 20·5 pg/ml, which was significantly higher than that of patients with psoriasis vulgaris (24·2 ± 4·7 pg/ml, P < 0·001), or controls (34·1 ± 15·3 pg/ml, P < 0·01) (Fig. 1b). In AD patients, in the categories of mild, moderate and severe, the serum eotaxin-3/CCL26 levels were 27·6 ± 3·1 pg/ml, 48·8 ± 16·1 pg/ml and 62·2 ± 24·2 pg/ml, respectively (Fig. 1c). The levels in the groups with moderate and severe AD were significantly higher than those in the group with mild AD (P < 0·001, P < 0·005, respectively).
Fig. 1.
Enzyme-linked immunosorbent assay (ELISA) results of eotaxin-2/CCL24 (a) and eotaxin-3/CCL26 (b) using sera of patients with atopic dermatitis (AD), patients with psoriasis vulgaris and control subjects. (c) Serum eotaxin-3/CCL26 levels of the three groups (mild, moderate and severe) of patients with AD.
Serum eotaxin-2/CCL24 and eotaxin-3/CCL26 levels in patients with AD, before and after treatment
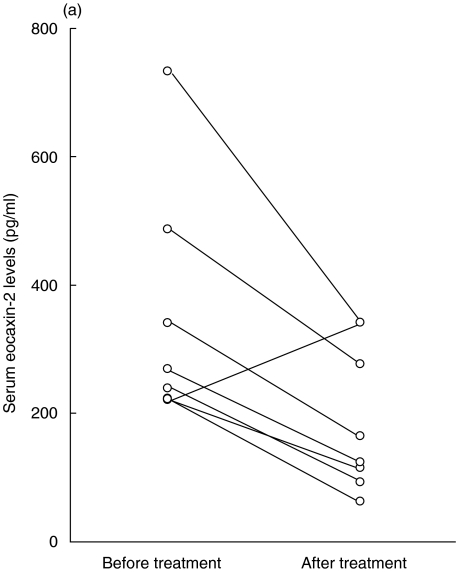
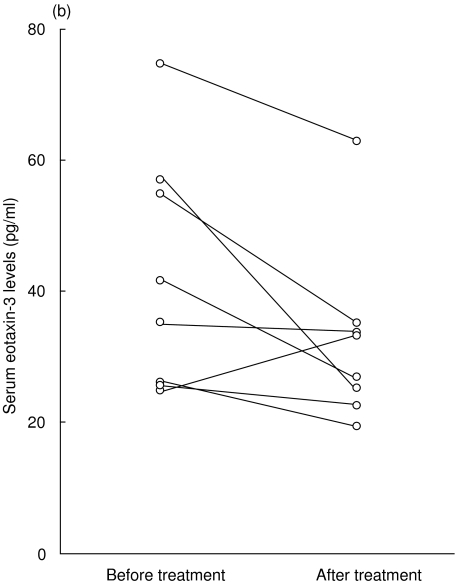
In eight patients with AD, we evaluated serum eotaxin-2/CCL24 and eotaxin-3/CCL26 levels before and after topical corticosteroid treatment in combination with oral antihistamines. The serum eotaxin-2/CCL24 levels decreased from 343·0 ± 182·6 pg/ml to 194·8 ± 112·5 pg/ml after the treatment (Fig. 2a) and the serum eotaxin-3/CCL26 levels decreased from 42·6 ± 18·2 pg/ml to 32·8 ± 13·5 pg/ml (Fig. 2b). However, there was no significant difference between these levels.
Fig. 2.
Serum eotaxin-2/CCL24 (a) and eotaxin-3/CCL26 (b) levels of eight patients with atopic dermatitis (AD) were measured before and after treatment with topical corticosteroids and oral antihistamines.
Correlation between serum eotaxin-2/CCL24 and eotaxin-3/CCL26 levels and other clinical or laboratory data
Because the serum eotaxin-3/CCL26 levels in patients with AD were significantly higher than those in patients with psoriasis vulgaris and in healthy controls, we next compared them with other clinical or laboratory data: serum TARC/CCL17 and MDC/CCL22 levels; eosinophil numbers in peripheral blood; and SCORAD. The serum eotaxin-3/CCL26 levels correlated significantly with the serum TARC/CCL17 levels (r = 0·50, P < 0·05), the serum MDC/CCL22 levels (r = 0·46, P < 0·05), eosinophil numbers in peripheral blood (r = 0·44, P < 0·05) and SCORAD (r = 0·55, P < 0·01) (Table 1). In addition, the serum TARC/CCL17 and MDC/CCL22 levels were significantly correlated with the SCORAD, eosinophil numbers in peripheral blood and each other, consistent with our previous reports (data not shown) [3,4]. In contrast, serum eotaxin-2/CCL24 levels were not correlated with these clinical or laboratory data (data not shown).
Table 1.
Correlation coefficient (r) among serum eotaxin-3/CCL26 levels and other clinical and laboratory data in patients with atopic dermatitis (AD)
| Eotaxin-3 | ||
|---|---|---|
| r | P-value | |
| TARC | 0·50 | < 0·05 |
| MDC | 0·46 | < 0·05 |
| Eosinophils | 0·44 | < 0·05 |
| SCORAD | 0·55 | < 0·01 |
| Eotaxin-2 | 0·21 | 0·25 |
MDC, macrophage-derived chemokine; SCORAD, scoring atopic dermatitis index; TARC, thymus and activation-regulated chemokine.
DISCUSSION
Eosinophils are involved in allergic diseases, such as asthma, rhinitis and AD [14]. Elucidation of the mechanisms underlying the accumulation of eosinophils in inflamed tissues is of critical importance for understanding the onset and progress of these eosinophilic diseases. Previous studies reported that the serum eotaxin/CCL11 levels were significantly correlated with the disease activity of AD [15] and that immunoreactivity and transcripts of eotaxin/CCL11 and CCR3 were significantly increased in lesional skin from AD [16]. Furthermore, a previous study reported that rhinovirus infection up-regulated eotaxin-2/CCL24 expression in bronchial epithelial cells [17]. However, little is known about the relationship between eotaxin-2/CCL24, eotaxin-3/CCL26 and AD.
In this study, we clearly showed that the serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, in patients with AD were significantly higher than those in patients with psoriasis vulgaris and healthy controls, although the serum level of eotaxin-3/CCL26 was lower than that of eotaxin-2/CCL24. Moreover, the serum eotaxin-3/CCL26 levels significantly increased in accordance with the clinical severity of AD. However, there was no significant difference before and after treatment for AD, although the elevated serum eotaxin-3/CCL26 levels tended to decrease after the treatment.
To clarify the relationship between eotaxin-3/CCL26 and AD in detail, we next compared the serum eotaxin-3/CCL26 levels with the disease activity of AD and found that these levels are significantly correlated with SCORAD, the serum TARC/CCL17 levels, the serum MDC/CCL22 levels and eosinophil numbers in peripheral blood. This indicates that the serum levels of eotaxin-3/CCL26 are also correlated with the disease activity of AD. Because we found a wide range in the eotaxin-3/CCL26 levels of patients with AD, we analysed the association between serum eotaxin-3/CCL26 levels and disease duration of AD. However, we did not find any significant association (data not shown). This is the first report describing the serum eotaxin-3/CCL26 and its possible role in the disease activity of AD. We also found that serum eotaxin-3/CCL26 levels were not significantly correlated with serum eotaxin-2/CCL24 levels, although both chemokines have similar biological functions in terms of recruitment of eosinophils via the CCR3. An in vitro study reported that eotaxin-3/CCL26 is strongly expressed and produced in vascular endothelial cells by stimulation with interleukin-4 (IL-4) [10], whereas eotaxin-2/CCL24 is not produced in vascular endothelial cells. Thus, the production of eotaxin-3/CCL26 is distinct from that of eotaxin-2/CCL24, which might explain the results of our in vivo data.
In conclusion, we have clearly shown that serum levels of eotaxin-3/CCL26, but not of eotaxin-2/CCL24, are significantly correlated with the disease activity of AD. This strongly suggests that eotaxin-3/CCL26 might have an important role in the pathogenesis of AD in conjunction with TARC/CCL17 and MDC/CCL22.
REFERENCES
- 1.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venerol (Stock) 1980;92(Suppl.):44–7. [Google Scholar]
- 2.Leung DYM. Atopic dermatitis: immunobiology and treatment with immune modulators. Clin Exp Immunol. 1997;107(Suppl.):25–30. [PubMed] [Google Scholar]
- 3.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–41. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 4.Kakinuma T, Nakamura K, Wakugawa M, et al. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin Exp Immunol. 2002;127:270–3. doi: 10.1046/j.1365-2249.2002.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jose PJ, Griffiths Johnson DA, Collins PD, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponath PD, Qin S, Ringler DJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, Baggiolini M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171–6. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JR, Imuburgia C, Dul E, et al. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol. 1997;62:667–75. doi: 10.1002/jlb.62.5.667. [DOI] [PubMed] [Google Scholar]
- 9.Kitaura M, Suzuki N, Imai T, et al. Molecular cloning of a novel human CC chemokine (eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 1999;274:27975–80. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 10.Shinkai A, Yoshisue H, Koike M, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–10. [PubMed] [Google Scholar]
- 11.Elsner J, Petering H, Kluthe C, Kimmig D, Smolarski R, Ponath P, Kapp A. Eotaxin-2 activates chemotaxis-related events and release of reactive oxygen species via pertussis toxin-sensitive G proteins in human eosinophils. Eur J Immunol. 1998;28:2152–8. doi: 10.1002/(SICI)1521-4141(199807)28:07<2152::AID-IMMU2152>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Europian Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis. The SCORAD Index. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 13.Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–9. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 14.Butterfield JH, Lieferman KM. Eosinophil-Associated Diseases. In: Smith H, Cook RM, editors. Immunopharmacology of Eosinophils. San Diego, CA: Academic Press; 1993. [Google Scholar]
- 15.Kaburagi Y, Shimada Y, Nagaoka T, Hasegawa M, Takehara K, Sato S. Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1alpha, MIP-1beta, and eotaxin) in patients with atopic dermatitis. Arch Dermatol Res. 2001;293:350–5. doi: 10.1007/s004030100230. [DOI] [PubMed] [Google Scholar]
- 16.Yawalkar N, Uguccioni M, Scharer J, Braunwalder J, Karlen S, Dewald B, Braathen LR, Baggiolini M. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol. 1999;113:43–8. doi: 10.1046/j.1523-1747.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, Johnston SL. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–6. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]