Abstract
We investigated the expression of T helper (Th)1/Th2 regulatory cytokine receptors on lymphocytes from patients with common variable immunodeficiency (CVID), a disorder associated with raised Th1 cytokine production, comparing the results with those from healthy individuals and atopic asthmatics, the latter generally considered to have a Th2-driven disease. We proposed that alterations in some of the relevant receptors might be related to the observed imbalances in Th1/Th2 cytokines. Cells from CVID patients showed an increase in the percentages of CD212 [interleukin (IL)-12Rβ1] cells within the CD4+ CD45RA+ and CD8+ CD45RA+ subsets (24% and 41%, respectively), as compared to CD4+ CD45RA+ and CD8+ CD45RA+ in healthy subjects (6% and 23%, respectivey). Approximately 21% of the CD4+ CD45RA+ naïve cells expressed IL-18Rα, compared with 11% in healthy subjects. In contrast, the cytokine-receptor expression in asthmatics was similar to that of controls. In spite of the above differences, after 72 h of stimulation with anti-CD3 and anti-CD28, cytokine receptor up-regulation was similar in all three groups, with up to 80% of both CD45RA+ and CD45RO+ lymphocytes expressing CD212 (IL-12Rβ1) and IL-18Rα. Approximately 50% of the ‘naïve’, and 25% of the ‘memory’ subpopulations up-regulated IL-12Rβ2. These findings provide further evidence of a polarization towards a Th1 immune response in CVID, the mechanism possibly involving up-regulation of IL-12-mediated pathways.
Keywords: IL-12 receptor, IL-18 receptor, CVID, lymphocytes human
INTRODUCTION
There are several factors implicated in the differentiation of naïve T helper (Th)1 and Th2 cells, such as the intensity and nature of the coordinated T-cell receptor (TCR) stimuli and costimulatory signals, and also the cytokine environment in which T cells are primed. There are two main groups of regulatory cytokines: the Th1-promoting cytokines [interleukin (IL)-12 and IL-18] and the Th2-inducing cytokines (IL-4 and IL-10) [1]. It is possible to control the differentiation of cells towards Th1 or Th2 subsets in vitro by stimulating them with IL-12 and neutralizing anti-IL-4 on the one hand, or by stimulation with IL-4 and neutralizing anti-IL-12 on the other [2].
IL-12 is a heterodimeric cytokine composed of a 40 000-molecular weight (MW) subunit (p40) and a 35 000-MW subunit (p35), predominantly produced by activated monocytes/macrophages, dendritic cells [2,3] and neutrophils [4]. Production is increased after infection with intracellular organisms and by the microbial cell-wall lipopolysaccharide (LPS) [5]. The ligand for IL-12 is the IL-12 receptor, which is composed of two subunits: the 73 000-MW IL-12Rβ1 polypeptide chain (CD212), which binds IL-12, and the 130 000-MW IL-12Rβ2 signal-transducing polypeptide chain. Although not always coexpressed on T cells, both of these subunits are necessary for a functional receptor [6–8]. Significantly, IL-12Rβ2 is not expressed on naïve T cells, but is induced at low levels after antigen stimulation of the TCR on Th1, but not Th2, clones [9,10].
The main sources of IL-18 [11–13] are macrophages and antigen-presenting cells. Although IL-18 enhances the IL-12-induced Th1 response, it cannot induce interferon-γ (IFN-γ) production on its own. Transcription of IFN-γ can be also achieved by the addition of IL-12 and IL-18, in the absence of signalling, through the TCR [13]. The IL-18 receptor (65 000–100 000 MW) belongs to the family of IL-1R/Toll-like receptors [11–13] and consists of a ligand-binding α chain subunit (IL-1Rrp) and a signal-transducing β chain subunit.
Common variable immunodeficiency (CVID) is the commonest severe primary immunodeficiency disorder, affected patients having low serum levels of all immunoglobulin classes and frequently having low numbers of circulating CD4+ T cells [14]. Although a minority of ‘CVID’ patients may have an undiagnosed single-gene disorder, a substantial proportion appear to have a complex disorder of immune regulation, a major susceptibility locus being in the major histocompatibility complex (MHC) on chromosome 6 [15]. The evidence that immunity in most CVID patients is skewed towards a Th1 response is based mainly on in vitro studies, which show a relative increase in the number of circulating lymphocytes producing IFN-γ, and monocytes producing IL-12, after stimulation [16,17], as well as an increase in the levels of IL-12 p40 in plasma (N. Matamoros, personal communication). At a clinical level, these abnormalities probably play some part in the development of granulomatous inflammation in organs such as the lungs, liver, spleen and lymph nodes, in at least 20% of patients with CVID. On the other hand, allergic asthma is considered a prototype Th2 disease, characterized by increased antibody production to allergens, including high levels of specific immunoglobulin E (IgE) antibodies, the latter being promoted by IL-4, IL-5, IL-10 and IL-13 cytokines produced by Th2 cells [18]. This dysregulation is associated with chronic infiltration of the bronchial wall by Th2 cells, monocytes/macrophages and eosinophils [19].
In this study we explored whether CVID is associated with a particular pattern of cytokine-receptor expression that might lead to a polarized Th1 response. Patients with allergic asthma were similarly investigated so that the control population encompassed a spectrum from normal individuals with balanced Th1/Th2 cytokine profiles to a polarized Th2 disease.
MATERIALS AND METHODS
Patient groups
Heparinized blood was obtained from 31 patients with CVID (of whom nine had granulomatous disease) (median age 51 years, range: 25–75 years), 27 patients with asthma (median age 39 years; range: 19–53 years) and 12 healthy adult volunteers. CVID was defined using the criteria of the International Union of Immunological Society (IUIS) [14]. Of the 31 patients with CVID, 27 had the typical immunoglobulin pattern [immunoglobulin A (IgA) < 0·1, immunoglobulin M (IgM) < 0·1 g/l, and very low immunoglobulin G (IgG)] and normal numbers of circulating B cells seen in CVID. Two females had high serum IgM, and three had normal IgM (one female and two males). Three patients had low numbers of circulating B cells; only one male had a clinical phenotype compatible with XLA, although mutation screening of btk was negative. None of these ‘atypical’ CVID patients had affected relatives.
All 27 asthma patients fulfilled the American Thoracic Society Criteria for the diagnosis of asthma [20]. The study was approved by the ethics committee of the Royal Free Hospital and all subjects gave informed consent.
Isolation and stimulation of T-lymphocyte subpopulations
Two aliquots of mononuclear CD45RA or CD45RO cells were isolated by magnetic antibody cell sorting (MACS) beads, according to the manufacturer's instructions (Milteny Biotec, Bisley, Surrey). Total mononuclear cells and the depleted populations were stimulated with anti-CD28 (1 µg/ml), in 96-well anti-CD3-coated plates. Control cells were cultured without stimulation. Cells were removed from the cultures after 0, 24, 48 and 72 h, labelled with appropriate antibodies (see below) and analysed by flow cytometry.
Detection of cytokine receptors
Cytokine receptors were detected on whole blood or isolated cells, using direct immunofluorescence and the desired combination of antibodies, and read with a Facscalibur (Becton-Dickinson, Cowle, Oxford, UK). Anti-IL-12Rβ1 (CD212)-conjugated phycoerythrin (PE) (Pharmingen, Becton-Dickinson, Oxford, UK) and anti-IL-18Rα-conjugated PE (R & D Systems, Abingdon, UK), were combined with anti-CD3 to analyse the expression on T cells. The resting CD3+ population were further analysed by costaining with either anti-CD4 or anti-CD8, and with CD45RA, CD45RO or CD45RB, to assess their expression within naïve, primed and terminally differentiated CD4 and CD8 cells.
Statistical methods
Data were analysed using the prism statistical package and, if not stated otherwise, were normally distributed and expressed as mean ± standard error of the mean (s.e.m.) (95% confidence interval). When comparing three or more groups, the analysis of variance (anova) Krusallis test was used, and results were confirmed by a conservative Bonferroni post test. Spearman's correlation test was used to test the relationship between two sets of data. All tests of significance were two tailed.
RESULTS
Expression of IL-12 and IL-18 receptors in CVID and asthmatic patients
In CVID there was a marked increase (P < 0·001) in the T-cell expression of both CD212 (IL-12Rβ1) and IL-18Rα receptors [CD212 (IL-12Rβ1): 52·4 ± 2·43; IL-18Rα: 47·4 ± 2·32] compared with healthy controls [CD212 (IL-12Rβ1): 35·5 ± 3·28; IL-18Rα: 38·5 ± 3·19]. (The results for the five XLA patients were not significantly different from the control group and therefore these data are not included.) The numbers of inflammatory cytokine-receptor positive cells in the asthmatics were very similar to those of controls [CD212 (IL-12Rβ1): 31·8 ± 2·52; IL-18Rα: 34·9 ± 2·36]. The expression of IL-12Rβ2 was not detectable on resting cells.
There was a twofold increase in the percentage of CD4+ CD212+ (IL-12Rβ1) cells in CVID patients (43·3 ± 3·36) compared with controls (24·8 ± 2·28) P < 0·001, with a lesser, but statistically significant, increase in the IL-18Rα-positive sub-population (controls: 37·5 ± 2·12; CVID: 46·2 ± 3·27; P = 0·04). In asthmatics the expression of CD212 (IL-12Rβ1) on CD4+ cells was similar to that of healthy controls.
In the CD8 populations there was only a slight increase in the percentage of CD8+ T cells that expressed CD212 (IL-12Rβ1) in CVID (57·4 ± 3·04) compared with controls (43·3 ± 3·27), while the proportion of IL-18Rα cells was similar to that of controls. However, the number of CD8+ T cells expressing CD212 (IL-12Rβ1) was significantly decreased in the asthmatics (controls: 43·7 ± 3·26; asthmatics: 31·1 ± 2·24; P = 0·02); there was also a small decrease in the numbers of cells expressing IL-18Rα.
The percentage of CD212+ (IL-12Rβ1) and IL-18Rα+ T cells in blood is increased in patients with CVID, owing to an increase in the number of CD4 CD45RA+ cells
Twelve CVID patients and controls were tested for cytokine receptor expression on CD45RA and RO subpopulations (Fig. 1, Table 1). The CD45RA+ (naïve) CD4+ population in the CVID patients showed a fourfold increase in the percentage of CD212+ (IL-12Rβ1) cells (23·6 ± 5·34) when compared with healthy controls (6·5 ± 1·71; P < 0·01), while the percentage of CD45RO+ cells expressing CD212 (IL-12Rβ1) was increased only minimally (Fig. 2). The three patients in this cohort with granulomatous disease had the highest percentage of CD212+ (IL-12Rβ1) and IL-18Rα+ cells. Within the CD8 population, there was a small increase in the percentage of CD45RO cells expressing CD212 (IL-12Rβ1), but a twofold increase in the percentage of CD45RA cells expressing this receptor chain (P < 0·01) (Fig. 2).
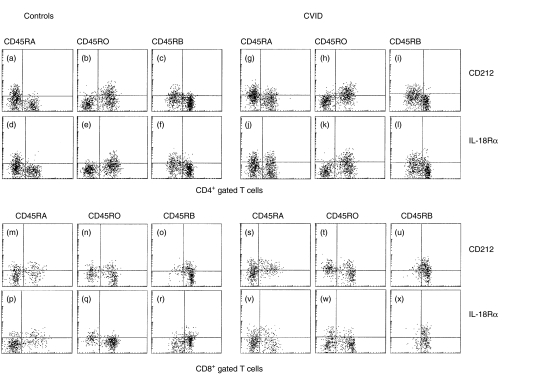
Fig. 1.
Expression of the regulatory cytokine receptors CD212 [interleukin (IL)-12Rβ1] and IL-18Rα on CD4+ and CD8+ T-cell subpopulations expressing the three different CD45 isoforms. Comparative dot-plots are shown depicting the expression of CD212 (IL-12Rβ1) (a–c, g–i, m–o and s–u) and IL-18Rα (d–f, j–l, p–r and v–x) in CD4+ gated (top panel) and CD8+ gated (bottom panel) T-cell subpopulations, defined by their expression of CD45RA, CD45RO and CD45RB on resting T cells from a healthy control sample (left panels) and a patient with common variable immunodeficiency (CVID) (right panels). The figure shows that the majority of CD212+ (IL-12Rβ1) and IL-18Rα+ cells are within the CD45RO and CD45RB low subpopulations. An increase was observed in the percentage of CD4+ CD45RA+ cells that express CD212 (IL-12Rβ1) and IL-18Rα in the CVID (g and j) patient, in comparison with the healthy individual.
Table 1.
Relative expression of CD212 [interleukin (IL)-12Rβ1] and IL-18Rα within the CD4+/CD8+ ‘naïve’ and ‘memory’ subpopulations (CD45RA/CD45RO)
| CD4/CD45RA (95% CI) | CD4/CD45RO (95% CI) | CD8/CD45RA (95% CI) | CD8/CD45RO (95% CI) | |
|---|---|---|---|---|
| CD212 (IL-12Rβ1) | ||||
| Controls (n = 12) | 6·5 ± 1·71 | 34·3 ± 3·80 | 23·6 ± 3·80 | 51·0 ± 2·94 |
| (2·46–10·03) | (25·9–42·7) | (15·3–32·0) | (44·6–57·8) | |
| CVID (n = 31) | 23·6 ± 5·34 | 39·0 ± 2·87 | 40·0 ± 5·14 | 57·1 ± 4·58 |
| (11·9–35·2) | (32·9–45·4) | (28·8–51·4) | (47·6–67·8) | |
| Asthma (n = 20) | 7·1 ± 1·23 | 33·9 ± 3·01 | 22·1 ± 3·29 | 41·8 ± 3·96 |
| (4·51–9·69) | (27·6–40·3) | (15·21–28·9) | (33·5–50·1) | |
| IL-18Rα | ||||
| Controls (n = 12) | 10·8 ± 1·51 | 47·0 ± 2·32 | 15·7 ± 1·81 | 40·9 ± 1·96 |
| (7·43–14·2) | (41·7–52·2) | (11·7–19·7) | (22·9–51·8) | |
| CVID (n = 31) | 20·6 ± 3·80 | 46·6 ± 3·78 | 14·6 ± 1·73 | 27·5 ± 3·10 |
| (12·3–29·0) | (35·3–52·0) | (10·8–18·4) | (17·7–31·3) | |
| Asthma (n = 20) | 11·4 ± 1·20 | 43·8 ± 2·30 | 14·9 ± 1·52 | 36·4 ± 3·69 |
| (8·9–13·9) | (38·9–48·6) | (11·7–18·4) | (28·7–44·1) | |
CI, confidence interval; CVID, common variable immunodeficiency; n, number of patients.
Data are expressed as mean ± standard error of the mean (95% CI).
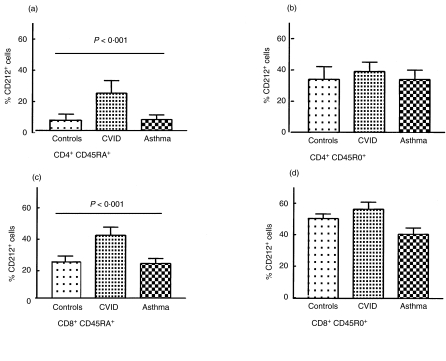
Fig. 2.
Expression of CD212 [interleukin (IL)-12Rβ1] within CD4 and CD8 naïve and memory subpopulations. Comparison of the percentages of the CD4+ CD45RA+ T cells (a) and the CD4+ CD45RO+ lymphocytes (b) and CD8+ CD45RA+ (c) and CD8+ CD45RO+ (d) memory populations expressing CD212 (IL-12Rβ1) in the peripheral blood of normal controls, patients with common variable immunodeficiency (CVID) and asthmatics. Note the statistically significant [using analysis of variance (anova) difference in expression of CD212 (IL-12Rβ1) in the naïve populations between the three groups. Results represent mean ± standard error of the mean (s.e.m.).
Consistent with the increased numbers of CD212+ (IL-12Rβ1) cells, the number of IL-18Rα+ cells in CVID patients was also increased twofold in the CD4+ CD45RA+ subpopulation (20·6 ± 3·80) compared with controls (10·8 ± 1·51, P < 0·01) (Table 1). There was no difference in IL-18Rα expression between CVID and healthy controls within the CD4+ CD45RO+ population. In contrast to the CD4 cells, the percentage of CD8+ CD45RA+ cells expressing IL-18Rα in CVID was similar to controls, but the percentage of CD8+ CD45RO+ cells positive for IL-18Rα (27·5 ± 3·0) was ≈65% of that in the healthy controls (40·9 ± 1·96, P = 0·03) (Table 1, Fig. 2).
In marked contrast, the expression of both receptors on CD4 naïve and primed cells from asthmatics was not altered when compared with healthy controls. The small decrease in the numbers of CD8+ CD45RO+ cells that expressed either CD212 (IL-12Rβ1) and IL-18Rα was not statistically significant (Fig. 2).
The IL-12β1R and IL-18Rα receptors were preferentially expressed on the CD45RB low subpopulations
In CVID, the relative number of CD212+ (IL-12Rβ1) and IL-18Rα cells in the CD4+ CD45RBhigh subpopulation was two- to threefold higher than in controls (P < 0·001), with only a slight increase in the percentage of CD4+ CD45RBlow subpopulations expressing these receptor subunits in both groups (Table 2). There was a higher percentage of CD212+ (IL-12Rβ1) cells in the CD8+ CD45RBhigh subpopulation in CVID, but this was not significant and the percentage of IL-18Rα+ was the same or slightly decreased in the CD8+ CD45RBlow cells (Table 3). There was no difference in the expression of the receptors in any of these subpopulations between asthmatics and healthy controls (Table 2).
Table 2.
Relative expression of CD212 [interleukin (IL)-12Rβ1] and IL-18Rα in both the CD45RBhigh and CD45RBlow within CD4 and CD8 T-cell subsets
| CD4 | CD8 | |||
|---|---|---|---|---|
| CD45RBhigh (95% CI) | CD45RBlow (95% CI) | CD45RBhigh (95% CI) | CD45RBlow (95% CI) | |
| CD212 (IL-12Rβ1) | ||||
| Controls (n = 12) | 10·7 ± 1·59 | 34·6 ± 4·06 | 30·1 ± 3·20 | 50·1 ± 4·12 |
| (7·20–14·3) | (25·5–43·6) | (23·7–38·0) | (40·1–59·2) | |
| CVID (n = 31) | 26·3 ± 5·1 | 42·1 ± 4·06 | 41·8 ± 5·06 | 50·7 ± 4·08 |
| (15·2–37·5) | (33·2–50·1) | (30·7–53·01) | (41·7–59·7) | |
| Asthma (n = 20) | 13·6 ± 2·03 | 29·8 ± 2·54 | 28·9 ± 2·85 | 36·3 ± 3·62 |
| (9·3–17·9) | (24·4–35·2) | (22·4–34·7) | (28·5–44·15) | |
| IL-18Rα | ||||
| Controls (n = 12) | 17·7 ± 1·49 | 48·4 ± 3·21 | 25·7 ± 4·47 | 35·4 ± 3·81 |
| (14·4–21·03) | (41·2–55·6) | (15·6–35·9) | (26·7–44·0) | |
| CVID (n = 31) | 25·7 ± 3·54 | 44·2 ± 3·83 | 21·3 ± 2·64 | 22·6 ± 3·06 |
| (17·9–33·42) | (35·8–52·5) | (15·4–27·1) | (15·9–29·4) | |
| Asthma (n = 20) | 21·3 ± 21·3 | 44·1 ± 2·81 | 25·7 ± 2·84 | 32·3 ± 3·39 |
| (17·8–24·8) | (38·1–50·1) | (19·6–31·8) | (25·0–39·6) | |
CI, confidence interval; CVID, common variable immunodeficiency; n, number of patients.
Data are expressed as mean ± standard error of the mean (95% CI).
Kinetics of up-regulation of cytokine receptors in stimulated T cells
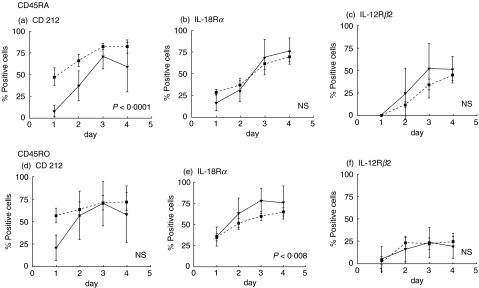
Following stimulation with anti-CD3 and anti-CD28, the number of CD212 (IL-12Rβ1) cells within the CD3 T cells increased from 13·2 ± 2·50 at 0 h to 69·5 ± 4·6 at 72 h. This was paralleled by an increased expression of IL-18Rα (14·7 ± 2 at 0 h to 67·9 ± 4·01 at 72 h). The number of IL-12 Rβ2+ cells also increased, but did not reach the levels of the other receptors (0·2 ± 0·14 at 0 h and 44 ± 5·9 at 72 h). In the CVID group, the kinetics of the up-regulation was the same as that in the control group. However, the number of positive cells was slightly higher at all time-points for CD212 (IL-12Rβ1) and IL-18Rα, although not for IL-12 Rβ2, which remained similar to that in controls. Nevertheless, in the majority of samples there was a population of cells that remained negative.
We then investigated whether CD45RA+ and CD45RO+ populations were capable of up-regulating the expression of CD212 (IL-12Rβ1) and IL-18Rα(Fig. 3). After activation, the percentage of cells positive for each of these receptors was similar in both populations. The number of CD45RA+ cells expressing CD212 (IL-12Rβ1) remained consistently higher in the CVID patients, as compared to controls, at all time-points (P < 0·001). The IL-12Rβ2 chain was also up-regulated in both populations, although a greater number of cells were positive in the CD45RA+ subset.
Fig. 3.
Up-regulation of CD212 (IL-12Rβ1), IL-18Rα and IL-12Rβ2 on stimulated CD45RA and CD45RO T-cell subsets. Naive and memory lymphocytes from normal controls (solid line) or from patients with common variable immunodeficiency (CVID) (dotted line) were cultured over 4 days with anti-CD3 and anti-CD28. Both CD45RA-naïve (a), (b) and (c) and CD45RO-primed (d) and (e) T-cell subsets were able to up-regulate CD212 (IL-12Rβ1) (a) and (d), IL-18Rα (b) and (e) and IL12Rβ2 (c) and (f).
DISCUSSION
The main focus of this study was to test for aberrations in the expression of IL-12 and IL-18 receptors on T cells in patients with CVID whose circulating monocytes have previously been shown to produce high levels of IL-12 when stimulated in vitro with LPS [17] and increased levels of plasma IL-12 p40. We also investigated asthmatics as a Th2 disease control, but did not find evidence of down-regulation of IL-12R in the circulation. On the other hand, we found evidence for an underlying tendency towards a Th1-driven response in CVID. The control of expression of a functional IL-12R, which requires both the β1 and β2 chains, on naïve T cells, is not yet clear, particularly the regulation of the IL-12β1R chain. In CVID, there was a relative expansion (of approximately fourfold) of CD4+ naïve T cells, and a doubling of CD8+ naïve T cells expressing the IL-12β1R, when compared with cells from healthy subjects. Although these cells do not express the IL-12β2R chain while in the ‘resting’ state within the circulation, it is probable that the recruitment of these cells into the lymphoid apparatus during an immune response will lead to increased numbers of T cells expressing a fully functional IL-12R in the vicinity of a cognate immune reaction. This, in turn, may lead to increased production of IFN-γ by T cells and further up-regulation of IL-12 and IFN-γ receptors. This scenario is probably the cause of inflammation and granuloma formation seen in the lymph nodes and spleens of patients with CVID, the granulomas apparently developing within the primary lymphoid follicles with signs of more general macrophage activation in the red pulp of the spleen. This disruption of germinal centre formation may explain the somatic hypo-mutation of immunoglobulin V-region genes in the circulating B cells of some patients [21,22].
The finding that three of the CVID patients with granulomatous complications had the highest number of CD4+ CD45RA+ cells expressing CD212 (IL-12Rβ1) and IL-18Rα suggests that an increase in these receptors on this particular cell type may be a useful marker of granulomatous disease. CVID patients usually fail to generate circulating antigen-specific CD4+ T cells following in vivo immunization, at least when tested in vitro[22]. No studies have yet been published on the generation of specific CD8+ T cells, but the clinical phenotype in most patients does not suggest a severe T-cell immunodeficiency. When stimulated in vitro with phorbol 12-myristate 13-acetate (PMA) and ionomycin, many more circulating CD4 and CD8 T cells from CVID patients express IFN-γ[17], suggesting that there may be priming, and possibly persistent activation of T cells in the periphery. Further work is now needed to investigate any correlation between IL-12R expression and priming for IFN-γ production.
Our data are compatible with repeated attempts in CVID to generate primary responses to common microbial antigens, the naïve T cells being exposed to IL-12 and IFN-γ, which may up-regulate the IL-12β1R, following which these cells re-circulate in relatively high numbers [23]. As these cytokines are pivotal in protection against mycobacteria [24], CVID patients should have an ‘innate’ resistance to these organisms; this is supported at the clinical level by the extreme rarity of mycobacterial disease in CVID, despite the fact that many patients have low absolute and relative numbers of circulating CD4+ T cells [25].
The possibility that the observed dysregulation of IL-12 and IL-18 receptors in CVID is secondary to infection or regular immunoglobulin therapy, needs to be considered. However, it is unlikely that the naïve T-cell subset would be preferentially affected, and these receptors were not up-regulated in XLA patients who suffer from the same infections and have the same therapy (data not shown).
In conclusion, we have shown an up-regulation of certain IL-12 and IL-18 receptor chains on a subpopulation of ‘naïve helper’ T cells in CVID. This complements other evidence of dysregulation of inflammatory cytokines, particularly IL-12. The relative numbers of circulating ‘naïve’ T cells expressing these receptor chains may correlate with granulomatous complications, and be a useful marker for using to predict clinical problems.
Acknowledgments
This work was supported by Contract Grant Sponsor: Special Trustees of the Royal Free Hospital and Fundació Irsicaixa, Barcelona Spain. We would like to thank Dr Creer, Sister Cilla Freud and staff nurse Irene Wahlberg for providing blood samples.
REFERENCES
- 1.Del Prete G. The concept of type-1 and type-2 helper T cells and their cytokines in humans. Int Rev Immunol. 1998;16:427–55. doi: 10.3109/08830189809043004. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 3.Gately M-K, Renzetti L-M, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 4.Cassatella M-A, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 5.Hilkens C-M, Vermeulen H, van Neerven R-J, Snijdewint F-G, Wierenga E-A, Kapsenberg M-L. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 6.Presky D-H, Minetti L-J, Gillessen S, et al. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. J Immunol. 1998;160:2174–9. [PubMed] [Google Scholar]
- 7.de Jong R, Altare F, Haagen I-A, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 8.Verhagen C-E, de Boer T, Smits H-H, et al. Residual type 1 immunity in patients genetically deficient for interleukin 12 receptor beta1 (IL-12Rbeta1): evidence for an IL-12Rbeta1-independent pathway of IL-12 responsiveness in human T cells. J Exp Med. 2000;192:517–28. doi: 10.1084/jem.192.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogge L, Papi A, Presky D-H, et al. Antibodies to the IL-12 receptor beta 2 chain mark human Th1 but not Th2 cells in vitro and in vivo. J Immunol. 1999;162:3926–32. [PubMed] [Google Scholar]
- 10.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello C-A. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga K, Yoshimoto T, Torigoe K, et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–60. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 14.International Union of Immunological Societies. Primary immunodeficiency diseases. Report of an UIIS Scientific Committee. Clin Exp Immunol. 1999;118(Suppl. 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorechovsky I, Cullen M, Carrington M, Hammarstrom L, Webster A-D. Fine mapping of IGAD1 in IgA deficiency and common variable immunodeficiency: identification and characterization of haplotypes shared by affected members of 101 multiple-case families. J Immunol. 2000;164:4408–16. doi: 10.4049/jimmunol.164.8.4408. [DOI] [PubMed] [Google Scholar]
- 16.North M-E, Webster A-D, Farrant J. Primary defect in CD8+ lymphocytes in the antibody deficiency disease (common variable immunodeficiency). abnormalities in intracellular production of interferon-gamma (IFN-gamma) in CD28+ (‘cytotoxic’) and CD28– (‘suppressor’) CD8+ subsets. Clin Exp Immunol. 1998;111:70–5. doi: 10.1046/j.1365-2249.1998.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambronero R, Sewell W-A, North M-E, Webster A-D, Farrant J. Up-regulation of IL-12 in monocytes: a fundamental defect in common variable immunodeficiency. J Immunol. 2000;164:488–94. doi: 10.4049/jimmunol.164.1.488. [DOI] [PubMed] [Google Scholar]
- 18.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate G-F. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Pouw Kraan T-C, Boeije L-C, de Groot E-R, et al. Reduced production of IL-12 and IL-12-dependent IFN-gamma release in patients with allergic asthma. J Immunol. 1997;158:5560–5. [PubMed] [Google Scholar]
- 20.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987. pp. 225–44. [This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986]. [DOI] [PubMed]
- 21.Bonhomme D, Hammarstrom L, Webster A-D, et al. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J Immunol. 2000;165:4725–30. doi: 10.4049/jimmunol.165.8.4725. [DOI] [PubMed] [Google Scholar]
- 22.Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen-specific memory T cells. MRC Immunodeficiency Group. Clin Exp Immunol. 1997;108:9–13. doi: 10.1046/j.1365-2249.1997.d01-993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenner CA, Guler ML, Macatonia SE, O'Garra A, Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–7. [PubMed] [Google Scholar]
- 24.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 25.Webster ADB. Common variable immunodeficiency. In: Roifman C, editor. Humoral Immunodeficiencies. Vol. 21. Immunology and Allergy Clinics of North America; 2001. pp. 1–22. [Google Scholar]