Abstract
In most individuals minute amounts of food proteins pass undegraded across the intestinal mucosa and trigger antibody formation. Children with coeliac disease have enhanced antibody production against gliadin as well as other dietary antigens, e.g. β-lactoglobulin, in cow's milk. Antibody avidity, i.e. the binding strength between antibody and antigen, often increases during antibody responses and may be related to the biological effectiveness of antibodies. The aim of the present study was to determine the avidity of serum IgG antibodies against β-lactoglobulin and gliadin in healthy children during early childhood and compare these avidities to those found in children with coeliac disease. The average antibody avidity was analysed using a thiocyanate elution assay, whereas the antibody activity of the corresponding sera was assayed by ELISA. The avidity of serum IgG antibodies against β-lactoglobulin as well as gliadin increased with age in healthy children, even in the face of falling antibody titres to the same antigens. Children with untreated coeliac disease had IgG anti-β-lactoglobulin antibodies of significantly higher avidity than healthy children of the same age, and the same trend was observed for IgG antigliadin antibodies. The present data suggest that the avidities of antibodies against dietary antigens increase progressively during early childhood, and that this process seems to be accelerated during active coeliac disease.
Keywords: antibody avidity, children, coeliac disease, dietary antibodies
INTRODUCTION
A small fraction of food proteins passes undegraded across the gut mucosa into the circulation [1], which enables antibodies to be produced against protein antigens in the diet. This is probably a physiological phenomenon, as most exposed individuals have low levels of antibodies in serum against cow's milk proteins, including β-lactoglobulin, as well as cereal proteins such as gliadin [2–4].
Certain diseases are characterized by enhanced antibody formation against dietary antigens, e.g. coeliac disease [5,6], cow's milk protein intolerance of delayed onset [7,8] and inflammatory bowel disease [9,10]. T cells are held to play a central role in these conditions. Thus, cell-free supernatants from gluten-specific T cell clones can cause an enteropathy identical to coeliac disease in normal small intestine in vitro, and this can be blocked by anti-interferon (IFN)-γ[11]. Further, a case report of coeliac disease in a patient with panhypogammaglobulinaemia suggests that antibodies are not necessary to trigger the disease process [12]. On the other hand, IgG antibodies might activate complement [13] or trigger cytolysis of antigen-coated cells via an ADCC (antibody-dependent cell-mediated cytotoxicity) reaction [14,15], thereby contributing to mucosal damage.
An important characteristic of an antibody is affinity, the binding strength of a single antigen-binding site of an antibody to the corresponding antigenic epitope. A closely related measure is avidity, or functional affinity, which is the overall strength of the interaction between an antibody and its antigen [16] that depends on both antibody affinity and valency [17]. A progressive rise in antibody avidity, termed avidity maturation [18], usually characterizes an immune response. This results from point mutations in the variable region of the immunoglobulin genes of B cells participating in the response, followed by selection of high-affinity B cells by competition for antigen [19,20]. However, the affinity may also remain constant during an antibody response [21,22], oscillate after an initial increase [23] or even decrease. For example, hyposensitization against bee venom by repeated injections leads to decreased antibody avidity [24]. Thus, constant exposure to dietary antigens might result either in increased or decreased antibody avidity.
High-avidity antibodies are more effective than those of low avidity in a number of immune reactions [25,26]. Antibodies of high avidity directed to dietary antigens could be protective by their superior capacity to eliminate food antigens from the circulation compared to low-avidity antibodies. Conversely, they might be pathogenic, e.g. by effectively fixing complement. In autoimmune diseases high-avidity antibodies may play a pathogenic role; in patients with systemic lupus erythematotosus, appearance of high-avidity antibodies against double-stranded DNA heralds disease exacerbations and prognosticate a severe disease course [27], and in autoimmune thyroiditis symptoms appear when IgG1 antithyroglobulin antibodies progress from low to high affinity[28].
The aim of the present study was to measure the avidity of serum IgG antibodies against the dietary antigens β-lactoglobulin and gliadin in early childhood, both in healthy children and in children with coeliac disease.
PATIENTS AND METHODS
Patients
Sera from healthy children, as well as from children with coeliac disease, were studied with respect to the avidity of antibodies against β-lactoglobulin and gliadin. Some children yielded more than one sample, permitting longitudinal analyses of avidity progression, whereas only one sample was obtained from others. If the serum quantity was large enough, antibodies to both antigens were assayed. In some cases, especially for the coeliac children, this was not possible.
The initial feeding pattern of the children was uniform, following the recommendations of the Swedish National Board of Health and Welfare. Thus, after initial exclusive breast feeding, cow's milk was introduced into the diet at 3–6 months of age and gluten-containing food at the age of 4–6 months. The diagnostic groups had the following characteristics.
Healthy children
The children were followed because of asymptomatic bacteriuria, but at the time of serum sampling they were healthy and abacteriuric. All children were fed a diet containing cow's milk protein as well as gluten.
Thirty-three individuals were examined for their serum IgG anti-β-lactoglobulin antibody levels and avidities. Their median age was 11 months (range 6–36). From 21 of the 33 children a second sample was collected at a median age of 35 months (range 26–48). Thirty children were examined for activities and avidities of serum IgG antigliadin antibodies. Their median age at initial sampling was 12 months (range 7–37). A second sample was taken from 17 of the 30 children at a median age of 36 months (range 26–48). Twenty-nine of the children were analysed for both antigens, 16 of whom yielded two consecutive serum samples.
Children with coeliac disease
The diagnosis of coeliac disease was based on the criteria of European Society for Paediatric Gastroenterology and Nutrition (ESPGAN) [29]. All coeliac children were on a cow's milk-containing diet. Serum samples were obtained when the first biopsy was taken (untreated coeliac disease), in remission on a gluten-free diet (treated), and/or at relapse after reintroduction of gluten (challenged). Anti-β-lactoglobulin antibody levels and avidities were analysed in sera from 16 children with untreated disease (median age 12 months, range 10–25) and nine with treated disease (median age 35 months, range 17–48). Antigliadin antibody levels and avidities were measured in 16 untreated coeliac children (median age 15 months, range 10–24), 11 treated cases (median age 32 months, range 17–57) and in eight coeliac children at relapse on gluten challenge (median age 45 months, range 32–53). In many coeliac patients the small amount of sera permitted analysis of antibodies to only either of the antigens. However, 12 coeliac children were analysed for antibodies to β-lactoglobulin as well as gliadin.
Determination of IgG antibody levels against b-lactoglobulin or gliadin
Serum IgG antigliadin and anti-β-lactoglobulin antibody levels were analysed by enzyme-linked immunosorbent assay (ELISA) as described previously [14,15]. Briefly, microtitre plates [Enzyme Immunoassay (EIA)/radioimmunoassay (RIA), Costar Corp., Cambridge, MA, USA] were coated overnight at room temperature with 5 µg/ml gliadin (crude, Sigma Chemicals, St Louis, MO, USA) or 5 µg/ml of equal proportions of β-lactoglobulin A and B (Sigma). Serum samples, diluted in PBS-Tween, were added and incubated overnight. A peroxidase-conjugated rabbit antihuman IgG antibody (Dakopatts A/S, Copenhagen, Denmark), diluted 1/500, was used for detection. The antibody levels were expressed either in relation (%) to a reference serum (for IgG anti-β-lactoglobulin antibodies) or as arbitrary ELISA units calculated with the aid of a standard curve derived from a high titred serum (for IgG antigliadin antibodies).
Measurement of antibody avidity
The average avidity of serum IgG anti-β-lactoglobulin and antigliadin antibodies was assessed using a thiocyanate elution enzyme immunoassay, used previously in a number of studies [30–33]. In principle, wells containing antibody bound to antigens are exposed to various concentrations of the chaotropic agent potassium thiocyanate (KSCN) and resistance to elution is taken as a measure of avidity. Microtitre plates (Costar EIA/RIA plates) were coated at room temperature with 5 µg/ml gliadin (crude, Sigma) or 5 µg/ml of equal quantities of β-lactoglobulin A and B (Sigma). After three washes in phosphate buffered saline (PBS) with 0·05% Tween (Kebo Laboratory AB, Stockholm, Sweden), 100 µl of each serum sample, diluted to give an absorption of 0·6 in the ELISA, were added and incubated for 3 h at room temperature. After three washes, 100 µl of KSCN (Kebo Laboratory AB) ranging from 0·1 m to 4 m were added and the plates were incubated for another 30 min at room temperature, whereupon the wells were washed six times with PBS-Tween. The amount of residual bound antibody was determined by the addition of 100 µl of rabbit antihuman IgG conjugated to alkaline phosphatase (diluted 1/500 in PBS-Tween). After incubation overnight at room temperature and three washes with PBS-Tween, 1 g/l of p-nitrophenyl phosphate (Sigma) in 1 m diethanolamine buffer (pH 9·8) was added and the absorbance at 405 nm was read in a spectrophotometer (Titertek Multiscan MCC/340, Flow Laboratories, Solna, Sweden) after 100 min. The molar KSCN concentration, giving an absorbance of 50% of that in the absence of KSCN, was used as a relative measure of average antibody avidity.
Ethics
Informed consent was obtained from the parents. The study was approved by the Ethics Committee of Göteborg University.
Statistical analysis
In order to evaluate the influence of age on antibody avidity, linear regression analyses were performed separately for each diagnostic group, with avidity as the dependent variable and age as the independent one. The slopes of the regression lines were compared using Student's t-test. Most of the healthy children contributed with more than one serum sample. The degrees of freedom in the analyses were, however, based on the number of individuals and not on the number of observations. The avidity values of different diagnostic groups were compared using Fisher's permutation test [34]. A comparison where the influence of age was eliminated was performed with Mantel's test [35]. Correlations were assessed using Spearman's rank correlation test.
RESULTS
Avidity of serum IgG antibodies against β-lactoglobulin in relation to age and diagnostic group
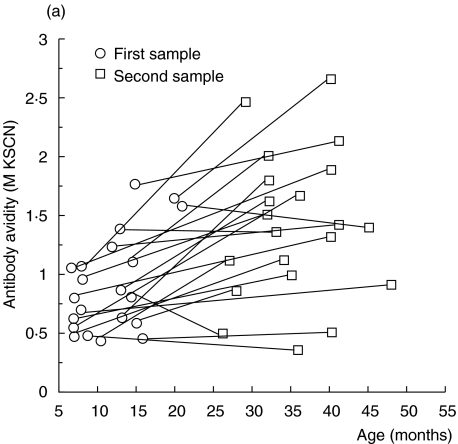
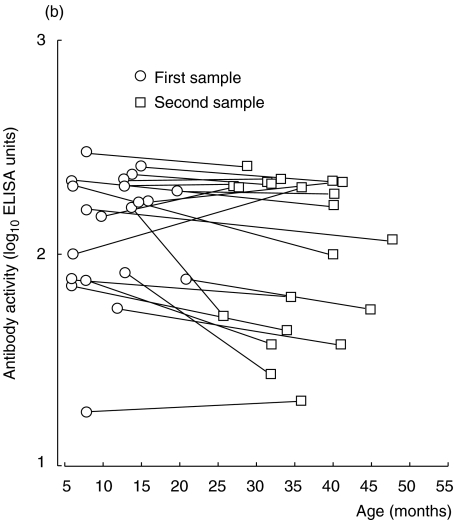
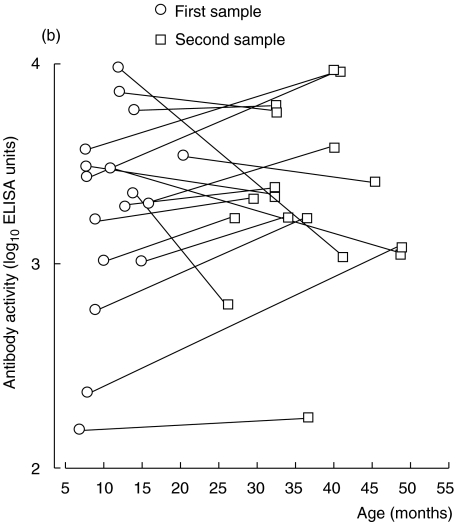
Twenty-one healthy children contributed two consecutive serum samples during their first years of life; serum-IgG anti-β-lactoglobulin antibody activity in these samples was measured by ELISA and the avidity of the antibodies was assessed by KSCN elution. In these children, the avidity of IgG anti-β-lactoglobulin antibodies increased with time (P < 0·001) (Fig. 1a), despite the fact that their IgG antibody levels did not rise during the same period (Fig. 1b). An increased avidity with age was also observed using linear regression on data from all 33 children (P < 0·001, cross-sectional data) (Fig. 2).
Fig. 1.
(a, b) IgG anti-β-lactoglobulin antibodies in sera from healthy children followed with two consecutive samples. (a) Avidity as determined by KSCN elution. (b) Activity as measured by ELISA.
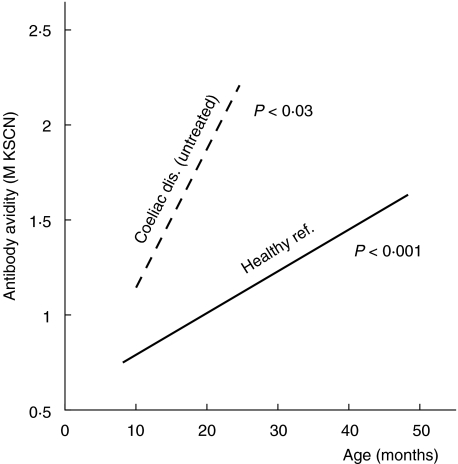
Fig. 2.
Linear regression lines of IgG anti-β-lactoglobulin antibodies as a function of age in healthy and coeliac children. The P-values indicate the significance of the slope of respective regression line.
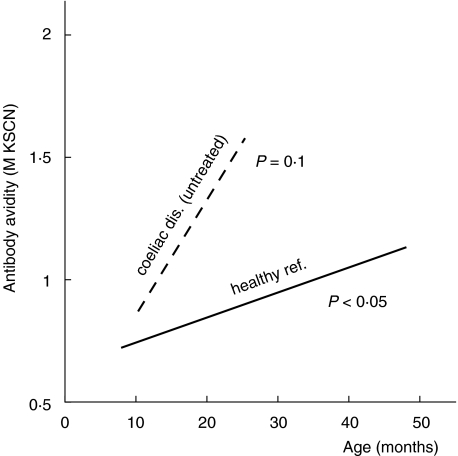
A significant age-dependent increase of the avidity of anti-β-lactoglobulin antibodies was also seen in the untreated coeliac children (cross-sectional data, linear regression analysis, P = 0·02) (Fig. 2). Children with untreated coeliac disease tended to have a more rapid progression of avidity with time than healthy children (P = 0·059 for the difference in regression line slopes, Fig. 2). Accordingly, the IgG anti-β-lactoglobulin antibodies in untreated coeliac children were of significantly higher avidity than the corresponding antibodies in healthy children of the same age (P < 0·05, Mantel's test). In remission, induced by a gluten-free diet, the avidity of anti-β-lactoglobulin antibodies remained at the same level as during the active state of disease, despite decreasing antibody levels (data not shown).
In healthy children, IgG anti-β-lactoglobulin antibody levels and avidities were significantly positively correlated (r = 0·47, P < 0·05). No such relationship was demonstrated in the groups of children with coeliac disease.
Avidity of serum IgG antibodies against gliadin in relation to age and diagnostic group
Serum IgG antigliadin antibodies were assessed for antibody activity and avidity. In healthy children, from whom two consecutive serum samples were obtained (n = 17), avidity tended to increase with time, although not significantly (Fig. 3a). However, in a cross-sectional linear regression model including data from all 30 healthy children, a significant rise in avidity with age could be demonstrated (P < 0·05) (Fig. 4). Serum antigliadin antibody activity as measured by ELISA also increased with age in most children (Fig. 3b).
Fig. 3.
(a, b) IgG antigliadin antibodies in sera from healthy children followed with two consecutive samples. (a) Avidity as determined by KSCN elution. (b) Activity as measured by ELISA.
Fig. 4.
Linear regression lines of IgG antigliadin antibodies as a function of age in healthy and coeliac children.The P-values indicate the significance of the slope of respective regression line.
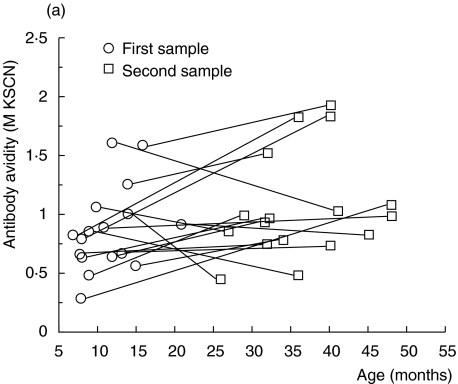
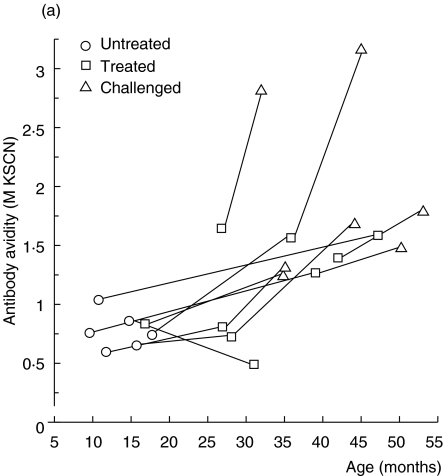
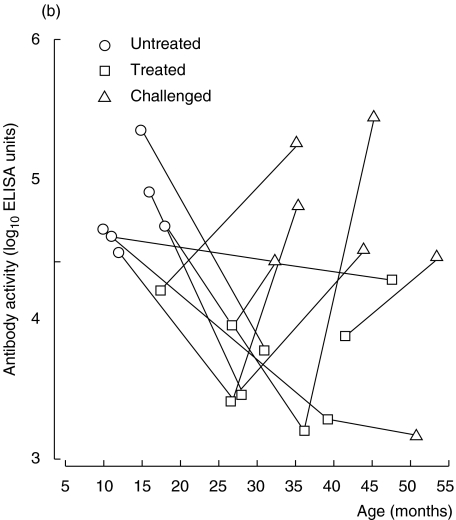
In the nine coeliac children followed consecutively, there was also a trend of increasing antigliadin antibody avidity (Fig. 5a). When in remission on a gluten-free diet (treated) the avidity seemed to be at least preserved, despite a marked decrease in IgG antigliadin antibody activity as measured with ELISA (Fig. 5a,b). Further, when coeliac children relapsed after gluten reintroduction, most showed a marked increase in avidity, in parallel with elevated IgG antigliadin antibody activity (Fig. 5a,b). The avidity seemed to rise more rapidly in children with untreated coeliac disease than in healthy children, although the slope of the regression line did not reach significance in coeliac children (P = 0·10, cross-sectional data) (Fig. 4). In accordance, coeliac children at relapse had significantly higher avidity of their IgG antigliadin antibodies than healthy children of the same age (P < 0·03, Mantel's test).
Fig. 5.
(a, b) IgG antigliadin antibodies in sera from children with coeliac disease followed longitudinally. (a) Avidity as determined by KSCN elution. (b) Activity as measured by ELISA.
A weak positive correlation between serum IgG antigliadin antibody activities and avidities was found in the healthy children (r = 0·35, P < 0·05), but not in any of the groups of coeliac children.
Covariation of dietary antibody avidities
The relationship between the avidities of antibodies directed to β-lactoglobulin and gliadin was studied in the 29 control children whose sera were analysed for both antigens. A significant correlation was found between these food-specific antibody avidities (r = 0·47, P < 0·02). In the 12 coeliac children investigated, the avidities of the two antibody specificities also correlated, although not significantly (r = 0·51, P = 0·09).
Sixteen control children yielded two consecutive serum samples of sufficient quantity to permit analysis of antibodies to both β-lactoglobulin and gliadin. In 14 of these children, the avidities of both antibody types changed in parallel (in 11 children both increased while in three children both decreased between the first and second sample). Only in two children did the avidity of antibodies to β-lactoglobulin increase, while the avidity of antibodies to gliadin decreased. Thus, avidity progression between antibodies against the two dietary antigens was significantly associated (P < 0·02, Fisher's exact test).
DISCUSSION
In the present study the avidity of serum IgG antibodies against two common food antigens, β-lactoglobulin and gliadin, was studied in healthy children as well as in children with coeliac disease. In both groups of children, antibody avidity progressed with age. This suggests that persistent exposure to dietary antigens during the first years of life drives avidity maturation. Accordingly, 1-year-old bottle-fed infants have IgG1 anticasein antibodies of higher avidity than breast- or mixed-fed infants [36]. The increasing avidity of IgG antibodies against β-lactoglobulin and gliadin with age in healthy children may be a physiological process that could be beneficial by enabling the immune system to eliminate these antigens effectively.
Persistent exposure to dietary antigens is considered to result in oral tolerance, a process that has been documented mainly in animals, but also occurs in humans [37]. Oral tolerance manifests in decreasing antibody levels and a paralysis of the T helper cell response to the antigen in question. Whether such tolerance results only in lower antibody levels, with persistence or even increased avidity, or if the avidity will eventually fall following decreasing antibody titres can only be speculated upon. Our results demonstrate clearly that antibody avidity could increase in the face of constant or decreasing antibody activity. This was particularly well demonstrated in healthy children whose anti-β-lactoglobulin antibodies increased in avidity with age but decreased in antibody activity. Similarly, patients with coeliac disease exhibited increased or preserved avidity of IgG antigliadin antibodies during remission on a gluten-free diet, in parallel with falling antibody levels. In accordance, a number of studies carried out previously have shown no relation between antibody levels and affinity [30,38]. In animal models, antibody affinity is controlled by mechanisms independent of those governing antibody levels [39].
The avidity progression of both anti-β-lactoglobulin and antigliadin antibodies seemed to be enhanced during active coeliac disease. Thus, coeliac children not yet treated had anti-β-lactoglobulin antibodies of higher avidity than healthy children of the same age. There are several alternative explanations of this phenomenon. The enhanced avidity may be the result of increased intestinal permeability to food antigens, which accompanies the mucosal damage [1,40]. Theoretically, however, an increased antigen load is not linked directly to avidity progression; affinity maturation results from preferential selection of high-affinity B cells by antigen, a process promoted by low rather than high concentrations of antigen [18,20,41]. Another possibility is that, because avidity maturation is a T cell-driven process, the abundance of activated T cells directed to food antigens in children with coeliac disease [42] might promote avidity maturation, e.g. by secreting large amounts of IFN-γ, a cytokine proposed to be involved in affinity maturation [43]. Besides, avidity may be linked to the IgG antibody subclass [44,45]. Patients with active coeliac disease have antigliadin antibodies predominantly of the IgG1 and IgG3 subclass [6,46]. However, this would not explain the increased avidity of anti-β-lactoglobulin antibodies in coeliac children, as their anti-β-lactoglobulin antibodies have a subclass pattern similar to that found in healthy children [47]. Finally, there is a possibility that coeliac patients could be inherently predisposed to produce high-avidity antibodies to dietary antigens, as antibody avidity is genetically influenced [48].
High-avidity antibodies against dietary antigens may be more effective than those of low avidity in different immune reactions. In coeliac disease the humoral response is commonly thought to be less important than the cell-mediated one. However, to speculate, high-avidity IgG antibodies against gliadin may contribute to the disease process by participating in harmful antibody-mediated immune reactions. Accordingly, T cells might not only cause damage to the mucosa by, e.g. activating tissue-destructive macrophages, but also by helping B cells to produce high-avidity antibodies with pathogenic potential.
Acknowledgments
The skilful technical assistance of Ingela Kinell is very much appreciated. We thank Anders Odén for expert statistical guidance and Bibbi Persson for her help in preparing the manuscript. The study was supported by grants from The Swedish Medical Research Council, The Swedish Association against Asthma and Allergy, The Ellen, Lennart and Walter Hesselman Foundation, Peter Silverskiöld Foundation, The Swedish Orphanage Foundation and The Göteborg Society of Physicians.
REFERENCES
- 1.Husby S, Foged N, Höst A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut. 1987;28:1062–72. doi: 10.1136/gut.28.9.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kletter B, Gery I, Freier S, Davies AM. Immune responses of normal infants to cow milk. I. Antibody type and kinetics of production. Int Arch Allergy Appl Immunol. 1971;40:656–66. doi: 10.1159/000230447. [DOI] [PubMed] [Google Scholar]
- 3.Fällström SP, Ahlstedt S, Carlsson B, Lönnerdal B, Hanson LÅ. Serum antibodies against native, processed and digested cow's milk proteins in children with cow's milk protein intolerance. Clin Allergy. 1986;16:417–23. doi: 10.1111/j.1365-2222.1986.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 4.Fälth-Magnusson K, Jansson G, Stenhammar L, Magnusson KE. Serum food antibodies analyzed by enzyme-linked immunosorbent assay (ELISA) and diffusion-in-gel (DIG)-ELISA methods in children with and without celiac disease. J Pediatr Gastroenterol Nutr. 1994;18:56–62. doi: 10.1097/00005176-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Labrooy JT, Hohmann AW, Davidson GP, Hetzel PA, Johnson RB, Shearman DJ. Intestinal and serum antibody in coeliac disease: a comparison using ELISA. Clin Exp Immunol. 1986;66:661–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Hvatum M, Scott H, Brandtzaeg P. Serum IgG subclass antibodies to a variety of food antigens in patients with coeliac disease. Gut. 1992;33:632–8. doi: 10.1136/gut.33.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fällström SP, Ahlstedt S, Hanson LÅ. Specific antibodies in infants with gastrointestinal intolerance to cow's milk protein. Int Arch Allergy Appl Immunol. 1978;56:97–105. doi: 10.1159/000232011. [DOI] [PubMed] [Google Scholar]
- 8.Little CH, Georgiou GM, Shelton MJ, Cone RE. Production of serum immunoglobulins and T cell antigen binding molecules specific for cow's milk antigens in adults intolerant to cow's milk. Clin Immunol Immunopathol. 1998;89:160–70. doi: 10.1006/clin.1998.4594. [DOI] [PubMed] [Google Scholar]
- 9.Lerner A, Rossi TM, Park B, Albini B, Lebenthal E. Serum antibodies to cow's milk proteins in pediatric inflammatory bowel disease. Crohn's disease versus ulcerative colitis. Acta Paediatr Scand. 1989;78:384–9. doi: 10.1111/j.1651-2227.1989.tb11097.x. [DOI] [PubMed] [Google Scholar]
- 10.Knoflach P, Park BH, Cunningham R, Weiser MM, Albini B. Serum antibodies to cow's milk proteins in ulcerative colitis and Crohn's disease. Gastroenterology. 1987;92:479–85. doi: 10.1016/0016-5085(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 11.Przemioslo RT, Lundin KE, Sollid LM, Nelufer J, Ciclitira PJ. Histological changes in small bowel mucosa induced by gliadin-sensitive T lymphcytes can be blocked by anti-interferon-gamma antibody. Gut. 1995;36:874–9. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster AD, Slavin G, Shiner M, Platts-Mills TA, Asherson GL. Coeliac disease with severe hypogammaglobulinaemia. Gut. 1981;22:153–7. doi: 10.1136/gut.22.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halstensen TS, Hvatum M, Scott H, Fausa O, Brandtzaeg P. Association of subepithelial deposition of activated complement and immunoglobulin G and M response to gluten in celiac disease. Gastroenterology. 1992;102:751–9. doi: 10.1016/0016-5085(92)90155-r. [DOI] [PubMed] [Google Scholar]
- 14.Saalman R, Wold AE, Dahlgren UI, Fällström SP, Hanson LÅ, Ahlstedt S. Antibody-dependent cell-mediated cytotoxicity to gliadin-coated cells with sera from children with coeliac disease. Scand J Immunol. 1998;47:37–42. doi: 10.1046/j.1365-3083.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 15.Saalman R, Carlsson B, Fällström SP, Hanson LÅ, Ahlstedt S. Antibody-dependent cell-mediated cytotoxicity to beta-lactoglobulin-coated cells with sera from children with intolerance of cow's milk protein. Clin Exp Immunol. 1991;85:446–52. doi: 10.1111/j.1365-2249.1991.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karush F. Affinity and the immune response. Ann NY Acad Sci. 1970;169:56–64. doi: 10.1111/j.1749-6632.1970.tb55970.x. [DOI] [PubMed] [Google Scholar]
- 17.Steward MW, Lew AM. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Meth. 1985;78:173–90. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- 18.Siskind GW, Benacerraf B, Goidl EA, Paul WE, Siskind GW, Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. Adv Immunol. 1969;10:1–50. [PubMed] [Google Scholar]
- 19.Griffiths GM, Berek C, Kaartinen M, Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984;312:271–5. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- 20.Fish S, Zenowich E, Fleming M, Manser T. Molecular analysis of original antigenic sin. I. Clonal selection, somatic mutation, and isotype switching during a memory B cell response. J Exp Med. 1989;170:1191–209. doi: 10.1084/jem.170.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heino L, Lönnrot M, Knip M, et al. No evidence of abnormal regulation of antibody response to coxackievirus B4 antigen in prediabetic children. Clin Exp Immunol. 2001;126:432–6. doi: 10.1111/j.1365-2249.2001.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roost HP, Bachmann MF, Haag A, et al. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc Natl Acad Sci USA. 1995;92:1257–61. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macario AJ, De Macario EC. Low and high affinity antibodies can alternate during the immune response. Nature. 1973;245:263–4. doi: 10.1038/245263a0. [DOI] [PubMed] [Google Scholar]
- 24.Devey ME, Lee SR, Richards D, Kemeny DM. Serial studies on the functional affinity and heterogeneity of antibodies of different IgG subclasses to phospholipase A2 produced in response to bee-venom immunotherapy. J Allergy Clin Immunol. 1989;84:326–30. doi: 10.1016/0091-6749(89)90416-8. [DOI] [PubMed] [Google Scholar]
- 25.Steward MW, Steensgaard J. Boca Raton, FL: CRC Press, Inc.; 1983. Antibody affinity: thermodynamic aspects and biological significance. [Google Scholar]
- 26.Vermont CL, van Dijken HH, van Limpt CJP, de Groot R, van Alpen L, van den Dobbelsteen GPJM. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infection Immunity. 2002;70:584–90. doi: 10.1128/IAI.70.2.584-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nossent JC, Huysen V, Smeenk RJ, Swaak AJ. Low avidity antibodies to dsDNA as a diagnostic tool. Ann Rheum Dis. 1989;48:748–52. doi: 10.1136/ard.48.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devey ME, Bleasdale-Barr KM, McLachlan SM, Bradbury J, Clark F, Young ET. Serial studies on the affinity and heterogeneity of human autoantibodies to thyroglobulin. Clin Exp Immunol. 1989;77:191–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Meeuwisse G. Diagnostic criteria in coeliac disease. Acta Paediatr Scand. 1970;59:461–2. [Google Scholar]
- 30.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Meth. 1986;86:83–7. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 31.Roberton DM, Carlsson B, Coffman K, et al. Avidity of IgA antibody to Escherichia coli polysaccharide and diphtheria toxin in breast milk from Swedish and Pakistani mothers. Scand J Immunol. 1988;28:783–9. doi: 10.1111/j.1365-3083.1988.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 32.Luxton RW, Thompson EJ. Affinity distributions of antigen-specific IgG in patients with multiple sclerosis and in patients with viral encephalitis. J Immunol Meth. 1990;131:277–82. doi: 10.1016/0022-1759(90)90199-6. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale F, Friman V, Carlsson B, Björkander J, Armenio L, Hanson LÅ. Aberrations in titre and avidity of serum IgM and IgG antibodies to microbial and food antigens in IgA deficiency. Scand J Immunol. 1992;36:279–83. doi: 10.1111/j.1365-3083.1992.tb03100.x. [DOI] [PubMed] [Google Scholar]
- 34.Bradley J. Distribution-free statistical tests. London: Prentice Hall; 1968. Distribution-free statistical tests; pp. 68–86. [Google Scholar]
- 35.Mantel N. Chi-square tests with one degree of freedom; extentions of the Mantel–Haenszel procedure. J Am Statist Assoc. 1963;58:690–700. [Google Scholar]
- 36.Devey ME, Beckman S, Kemeny DM. The functional affinities of antibodies of different IgG subclasses to dietary antigens in mothers and their babies. Clin Exp Immunol. 1993;94:117–21. doi: 10.1111/j.1365-2249.1993.tb05987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–70. [PubMed] [Google Scholar]
- 38.Lew AM, Anders RF, Edwards SJ, Langford CJ. Comparison of antibody avidity and titre elicited by peptide as a protein conjugate or as expressed in vaccinia. Immunology. 1988;65:311–4. [PMC free article] [PubMed] [Google Scholar]
- 39.Steward MW, Petty RE. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972;22:747–56. [PMC free article] [PubMed] [Google Scholar]
- 40.Husby S, Höst A, Teisner B, Svehag SE. Infants and children with cow milk allergy/intolerance. Investigation of the uptake of cow milk protein and activation of the complement system. Allergy. 1990;45:547–51. doi: 10.1111/j.1398-9995.1990.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 41.Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991;352:530–2. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- 42.Troncone R, Gianfrani C, Mazzarella G, et al. Majority of gliadin-specific T-cell clones from celiac small intestinal mucosa produce interferon-gamma and interleukin-4. Dig Dis Sci. 1998;43:156–61. doi: 10.1023/a:1018896625699. [DOI] [PubMed] [Google Scholar]
- 43.Holland GP, Holland N, Steward MW. Interferon-gamma potentiates antibody affinity in mice with a genetically controlled defect in affinity maturation. Clin Exp Immunol. 1990;82:221–6. doi: 10.1111/j.1365-2249.1990.tb05430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devey ME, Bleasdale B-KM, Bird P, Amlot PL. Antibodies of different human IgG subclasses show distinct patterns of affinity maturation after immunization with keyhole limpet haemocyanin. Immunology. 1990;70:168–74. [published erratum appears in Immunology 1990 September; 71: 152]. [PMC free article] [PubMed] [Google Scholar]
- 45.Persson MA, Brown SE, Steward MW, et al. IgG subclass-associated affinity differences of specific antibodies in humans. J Immunol. 1988;140:3875–9. [PubMed] [Google Scholar]
- 46.Husby S, Foged N, Oxelius V, Svehag S. Serum IgG subclass antibodies to gliadin and other dietary antigens in children with coeliac disease. Clin Exp Immunol. 1986;64:526–35. [PMC free article] [PubMed] [Google Scholar]
- 47.Saalman R, Dahlgren UI, Fällström SP, Hanson LÅ, Wold AE, Ahlstedt S. ADCC-mediating capacity in children with cow's milk protein intolerance in relation to subclass profile of serum antibodies to beta-lactoglobulin. Scand J Immunol. 1995;42:140–6. doi: 10.1111/j.1365-3083.1995.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 48.Steward MW, Reinhardt MC, Staines NA. The genetic control of antibody affinity. Evidence from breeding studies with mice selectively bred for either high or low affinity antibody production. Immunology. 1979;37:697–703. [PMC free article] [PubMed] [Google Scholar]