Abstract
Leptin, the 16 kDa product of the ob gene, is a an adipocyte-secreted hormone that centrally regulates weight. However, the physiological role of leptin is not limited to the regulation of food intake and energy expenditure, and leptin has a variety of effects in peripheral tissues, such as a regulatory role modulating the immune system. Thus, leptin receptor is expressed in human peripheral blood mononuclear cells, mediating the leptin stimulation of proliferation and activation, the production of proinflammatory cytokines from cultured monocytes, and the prevention of apoptotic death in serum-deprived monocytes. Because leptin can stimulate monocytes and the production of reactive oxygen species (ROS) are the result of monocyte activation, we investigated the effect of leptin on ROS production by human monocytes in vitro. Oxidative burst was measured by oxidation of the redox-sensitive dye 2′,7′-dichlorofluorescein diacetate, and analysed by flow cytometry. We have found that stimulation with leptin produces oxygen radical formation by monocytes. This effect is dependent on the dose and maximal response is achieved at 10 nm leptin. Because HIV infection induces the production of ROS, we next investigated the effect of leptin on ROS production in monocytes from HIV-positive (HIV+) subjects. We have also found that monocytes from HIV+ subjects spontaneously produced increased amounts of free radicals. In contrast, leptin stimulation of monocytes from these patients partially inhibited the production of ROS. This effect of leptin was also dependent on the dose and maximal effect was achieved at 10 nm. The effect of leptin stimulating the production of ROS is consistent with the proinflammatory role in the immune system. On the other hand, the inhibitory effect on monocytes from HIV+ subjects may be explained by the attenuation of the oxidative burst by a delayed activation of monocytes in a hyperinflammatory state.
Keywords: HIV, leptin, monocytes, oxidative burst, PBMC, ROS
INTRODUCTION
Leptin, the 16-kDa non-glycosylated protein product of the ob gene [1], is a hormone synthesized mainly in adipose cells [2] to regulate weight control in a central manner [3]. Leptin can also be expressed at lower levels in other tissues, such as placenta and stomach [4,5]. Leptin is released into the circulation, and plasma levels correlate with total body fat mass [6]. On the other hand, there is increasing evidence that leptin has systemic effects apart from those related to energy homeostasis, including regulation of neuroendocrine, reproductive, haematopoietic and immune function [7].
Leptin amino acid sequence indicated that it could belong to the long-chain helical cytokine family [8], and the leptin receptor (Ob-R) shows sequence homology to members of the class I cytokine receptor (gp130) superfamily [9]. Moreover, Ob-R has been shown to have signalling capabilities of interleukin (IL)-6-type cytokine receptors [10]. Besides, Ob-R expression is not limited to the hypothalamus, but is widely distributed, including haematopoietic cells [10–12]. In this context, a role for leptin in haematopoiesis and the immune system at the stem cell level has been proposed [13].
Obese leptin-deficient ob/ob mice and db/db mice, in which the leptin receptor is truncated, display immune dysfunction and lymphoid organ atrophy [14–16]. Thus, they have reduced levels of peripheral T and B cells [16] suggesting that leptin may have a role in lymphopoiesis. Also, leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice [16]. Consistent with these findings, human leptin deficiency caused by a missense mutation also produces immune system dysfunction [17]. In this regard, we have found recently that leptin is able to promote activation and proliferation of human monocytes and to enhance activation and proliferation of preactivated T lymphocytes [18,19]. Moreover, leptin can induce the synthesis of proinflammatory cytokines [IL-6 and tumour necrosis factor (TNF)-α] by human monocytes and the production of Th1-type cytokines [IL-2 and interferon (IFN)-γ] by human T lymphocytes cultured in vitro[18,19]. These effects are mediated by the leptin receptor which is present in peripheral blood monocytes and T lymphocytes, and also triggers signal transduction [18–21]. The leptin receptor is up-regulated in blood mononuclear cells stimulated in vitro with lectins, and in vivo in HIV-infected subjects [22]. More recently, we have found that leptin promotes survival of human circulating blood monocytes prone to apoptosis in serum-free culture, suggesting that leptin may have a role as a trophic factor for the survival of blood monocytes [23].
Leptin enhances cytokine production granulocyte-macro-phage colony stimulating factor (GM-CSF and G-CSF) in murine peritoneal macrophages [24], and phenotypic abnormalities have been found in macrophages from leptin-deficient, obese mice [25]. Furthermore, leptin up-regulates both phagocytosis and the production of proinflammatory cytokines by murine macrophages [26].
Phagocytic cells, such as monocytes and macrophages play a key role in the innate immune response [27]. Immunological activation of macrophages is achieved by Th1 cytokines and lipopolysaccharide (LPS) [28]. Cell activation results in the release of proinflammatory cytokines and oxygen species [28]. The generation of reactive oxygen species (ROS) during the respiratory burst is mediated by membrane-bound NADPH oxidase [29].
As leptin can activate monocytes, promoting proliferation and the production of proinflammatory cytokines, we wanted to invest-igate the effect of leptin on ROS production by human circulating monocytes. On the other hand, monocytes and macrophages from HIV+ patients have often increased ROS production [30–32]. Also, because HIV-infected subjects have increased expression of leptin receptor, we also sought to assess the effect of leptin on ROS production by human circulating monocytes from HIV-positive (HIV+) subjects.
MATERIALS AND METHODS
Materials
Human recombinant leptin was from R&D Systems (Minneapolis, MN, USA). 2′,7′-dichlorofluorescin (DCF) diacetate was obtained from Sigma-Aldrich (Alcobendas, Madrid, Spain). Phycoerythrin (PE)-conjugated monoclonal mouse anti-CD14 (anti-CD14-PE) was from Becton Dickinson, Immunocytometry Systems (San Jose, CA, USA)
Patients
HIV-infected patients were from the infectious diseases unit (Virgen Macarena Hospital) and were selected by their similar clinical characteristics, low viral load and low–intermediate number of CD4+ T cells (Table 1). Most patients included were on highly active antiretroviral therapy, and three had lypodystrophy. Informed consent was obtained from the 13 patients and the studies had the approval from the ethical committee of the Virgen Macarena University Hospital.
Table 1.
Features of HIV-infected patients (n = 12)
| Median age in years (range) | 45 (33–46) |
| Male gender | 12 |
| Median evolution of HIV infection in years (range) | 6 (1–15) |
| Risk behaviour | |
| Parenteral drug use | 8 (51·5) |
| Homosexual/bisexual | 3 (23·1) |
| Heterosexual | 2 (15·4) |
| Co-infection | |
| HCV (%) | 10 (76·9) |
| HBV (%) | 1 (7·7) |
| Previous AIDS diagnosis (%) | 3 (23·1) |
| Highly active antiretroviral therapy (%) | 11 (84·6) |
| Undetectable viral load (<50 copies/ml) (%) | 8 (61·5) |
| Viral load (log) in patients with ≥50 copies/ml(range) | 4·21 (3·2–>5·87) |
| Median CD4 cell count per mm3 (range) | 143 (77–515) |
| Lipodystrophy (%) | 3 (25) |
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC), obtained from normal donors (six healthy subjects, three men and three women, age 26–33 years) and HIV-infected patients were isolated from heparinized venous blood by density-gradient sedimentation over Ficoll-Hypaque (Seromed Biochrom KG, Berlin, Germany) as described previously [33,34]. Cells were then washed twice in phosphate buffered saline (PBS) and resuspended in medium appropriately for cell culture [35], RPMI-1640 supplemented with 25 mm HEPES, 2 mm l-glutamine, 100 µU/ml penicillin, 100 µg/ml streptomycin and amphotericin B (2·5 µg/ml) (all from Biological Industries, Beit Haemek, Israel). PBMC were cultured at 37°C and 5% CO2. An aliquot of cells is used for CD14 staining to help in forward-scattering gating. Cells were incubated for 20 min with the stimulus. Then, we added DCF diacetate (2 µm final concentration), which enter the cells and hydrolyses into DCF, which is fluorescent when oxydized. PBMC were washed with cold PBS twice and analysed immediately by flow cytometry.
Flow cytometry data acquisition and analysis
Data were acquired on a FACScalibur flow cytometer using CELLQuest software (BDIS). A total of 10 000 cells were acquired routinely for analysis of monocyte cell population gated in forward-scattering and as positive for CD14-PE (FL-2). During monocyte oxidative burst, non-fluorescent DCF is oxidized producing green fluorescence (FL-1). ROS production was assessed as FL-1, and the mean fluorescence intensity was used to quantify the responses from gated monocytes.
RESULTS
Leptin effect on ROS production by monocytes from control donors
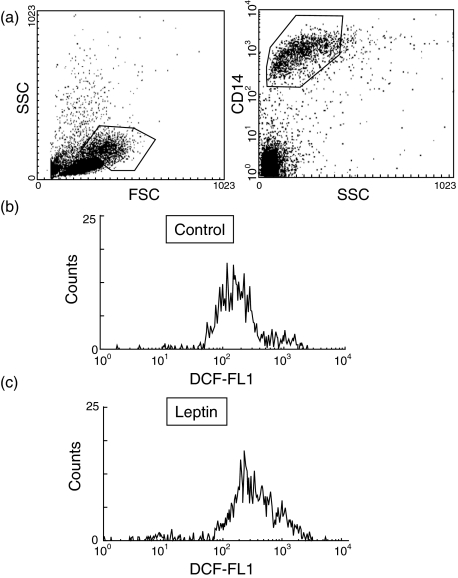
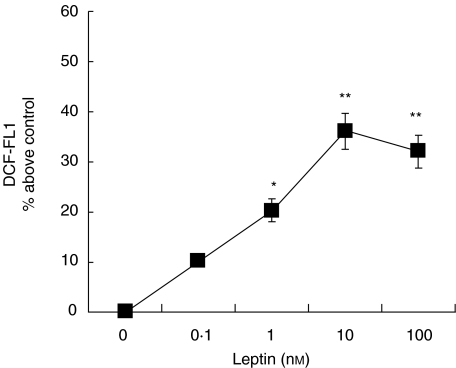
Oxygen burst was determined by measuring the ROS production using the fluorescence marker DCF. PBMC were incubated in the presence or absence of 10 nm leptin for 20 min, followed by 10 min of incubation in the presence of DCF. As shown in Fig. 1 leptin stimulated ROS production by human monocytes. The effect was dependent on the dose and maximal effect was achieved at 10 nm leptin (Fig. 2), which increased the basal DCF fluorescence about 40%. A significant effect was obtained when monocytes were exposed to 1 nm leptin, which increased DCF fluorescence about 20% from control monocytes.
Fig. 1.
Reactive oxygen species (ROS) production in monocytes from healthy subjects. Monocytes were immunostained with anti-CD14 to gate the monocyte population in forward-side scattering (a). PBMC from healthy donors were incubated in the absence (b) or presence (c) of 10 nm leptin for 20 min, followed by 10 min in the presence of the redox-sensitive dye DCF. ROS production was analysed by flow cytometry. Data are the results of a representative experiment.
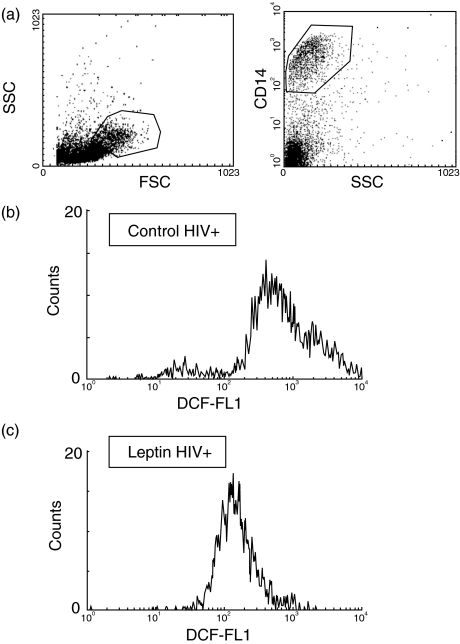
Fig. 2.
Reactive oxygen species (ROS) production in monocytes from HIV-infected patients. Monocytes from were immunostained with anti-CD14 to gate monocyte population in side-forward scattering (a). PBMC from HIV-infected patients were incubated in the absence (b) or presence (c) of 10 nm leptin for 20 min, followed by 10 min in the presence of DCF. ROS production was analysed by flow cytometry. Data are representative of a representative experiment.
Because leptin was obtained from recombinant sources, we wanted to rule out a possible contamination of leptin with endotoxin (LPS) that could account, at least in part, for the leptin mediated ROS production. Thus, we blocked the possible action of LPS by adding to the PBMC culture 1 µm polymyxin B, a well-known inhibitor of LPS binding to CD14 at low concentrations (1–5 µm). As previously found in monocyte activation experiments [18], polymyxin B did not change the effect of leptin (data not shown).
Leptin effect on ROS production by monocytes from HIV-infected subjects
When we examined the ROS production by monocytes from HIV-infected subjects we found increased DCF fluorescence compared to healthy controls (750 ± 80 in HIV+ versus 220 ± 20 in the control group).
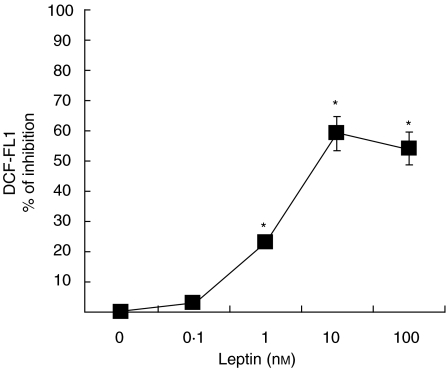
PBMC from HIV-infected subjects were incubated in the same conditions as described for control cells. Thus, cells were challenged with 10 nm leptin for 20 min and the incubation was continued by 10 min in the presence of DCF. As shown in Fig. 3, we noticed that 10 nm leptin consistently reduced the oxidative burst in monocytes from HIV+ subjects. Reduction of ROS formation was almost complete. This effect of leptin was also dependent on the dose, and maximal effect was obtained at 10 nm leptin, decreasing about 60% DCF fluorescence of non-stimulated HIV+ monocytes. Significant inhibition was observed at 1 nm leptin, producing about 25% inhibiton of ROS production (Fig. 4).
Fig. 3.
Dose-dependency of the effect of leptin on ROS production by monocytes from healthy controls. PBMC from healthy controls were incubated in the absence or presence of increasing concentrations of leptin. ROS production by monocytes was analysed by flow cytometry. Data are means ± s.e.m. of the mean DCF fluorescence of monocyte population. *P < 0·05; **P < 0·001 versus control.
Fig. 4.
Dose-dependency of the effect of leptin on ROS production by monocytes from HIV-infected patients. PBMC from HIV-infected patients were incubated in the absence or presence of increasing concentrations of leptin. ROS production by monocytes was analysed by flow cytometry. Data are means ± s.e.m. of the mean DCF fluorescence of monocyte population. *P <0·05; **P <0·001 versus control.
DISCUSSION
Leptin has been demonstrated to modulate monocyte-macrophage function and to regulate the proinflammatory response [25,26,36,37]. Moreover, leptin has been shown previously to enhance cytokine production (GM-CSF and G-CSF) by murine peritoneal macrophages [24] and human circulating monocytes (TNF-α and IL-6) [18], where the effect of leptin is comparable to that produced by LPS or PMA. As LPS administration in mice increases leptin expression and circulating leptin levels, these effects of leptin may be physiologically important, functioning as an amplification signal for monocyte activation [36]. Moreover, leptin has been found to have a trophic effect on human monocytes, preventing apoptosis induced by serum deprivation [23]. Therefore, leptin seems to be a potent stimulatory hormone on human peripheral blood monocytes [38].
In this context, a role of ROS production in cellular responses to proinflammatory cytokines, such as TNF-α has been described [39]. Therefore, we aimed to investigate the possible effect of leptin on ROS production in human circulating monocytes. As expected for a proinflammatory cytokine, leptin significantly increases ROS production by human monocytes. A similar dose–response to that observed for activation, proliferation and cytokine production [18] was obtained. Therefore, ROS may be another second messenger molecule to be considered for leptin receptor signalling in monocytes [20,21]. In fact, ROS potentiates the production by monocytes of proinflammatory cytokines [40] Alternatively, or in addition, ROS production may mediate the role of monocytes in the defence against microorganisms, as they are phagocytic cells [41]. Consistent with these results, leptin-deficient mice exhibit defective macrophage function, including phagocytosis [25,26]. More recently, it has been shown that leptin-deficient mice have impaired alveolar macrophage phagocytosis of Klebsiella pneumoniae in vitro, which can be restored by theexogenous addition of leptin [42].
On the other hand, we have found that leptin reduces ROS production in monocytes from HIV-infected subjects, which spontaneously produces increased ROS levels. Thus, in the present work, we have confirmed the previously reported increased redox status of monocytes from HIV-infected patients [32,43]. However, when PBMC from HIV+ subjects were stimulated in vitro with leptin, we observed the opposite effect of that observed in control cells, i.e. a reduction in ROS production by monocytes. This inhibitory effect showed the same dose-dependency observed for the stimulatory effect in monocytes from healthy controls. A possible explanation for this discrepancy may be the monocyte desensitization in HIV+ subjects, in a similar way to that observed in other hyper-inflammatory states such as sepsis, in which the function of monocytes is shifted to a hypoinflammatory state characterized by anergy to stimulation with LPS [44], or even attenuation of the oxidative burst in response to LPS [45]. We know that HIV-infected patients have increased expression of leptin receptor in blood mononuclear cells, demonstrating further the hyper-activation state of monocytes in these subjects [22]. Because ROS production is one of the mechanisms that participate in T-lymphocyte depletion by triggering programmed cell death (apoptosis) [46], and monocytes can also induce their own apoptosis by producing ROS [47], the low leptin levels that have been found in these patients [48] may contribute to the immune deficiency of HIV+ patients. In fact, leptin levels have been found to correlate with CD4 numbers in HIV infection and to increase along with CD4 T lymphocytes during highly active antiretroviral therapy [49]. Moreover, this effect of leptin reducing oxidative burst is consistent with the anti-apoptotic action of leptin on human monocytes cultured in the absence of serum [23]. Therefore, leptin may be considered as an important factor in the development and evolution of the disease in HIV+ patients.
Redox status of monocytes from HIV-infected patients seems to correlate with viral load [32]. However, even though we have found increased ROS production consistently in every sample studied, we have not found significant differences between HIV+ samples. It should be noted, however, that we have studied monocytes from patients with low viral load. In a similar way, we have consistently found a decrease in ROS production by incubation with leptin.
On the other hand, we do not know whether HCV co-infection may contribute to the increased ROS production observed in circulating monocytes, as most of the HIV-infected patients of this study had HCV co-infection. Nevertheless, the patients negative for HCV also have increased ROS production, suggesting that HIV infection may be sufficient for this effect. On the other hand, we do not know whether HCV infection in HIV seronegatives may also have increased ROS production, because non-structural 3 protein of HCV has been found to trigger an oxidative burst in human monocytes via activation of NADPH oxidase [50]. Even though we have not studied the effect of leptin on ROS production in HIV-negative patients infected with HCV, we think it may be worth investigating a possible role of leptin in the regulation of oxidative burst in hepatitis C as well as other inflammatory-infectious diseases.
In conclusion, we have found that leptin activation of human monocytes stimulates ROS production, consistent with the reported stimulatory effect on monocyte/macrophage cells. On the other hand, we have found that leptin reduces the increased oxidative status of monocytes from HIV+ patients, suggesting that leptin may be an important factor for the redox status of monocytes, which may be relevant for the immunological alterations in HIV infection.
Acknowledgments
This work was supported by the University Hospital Virgen Macarena, Sevilla, Servicio Andaluz de Salud, Andalucía, Spain and Plan Andaluz de Investigación, Junta de Andalucía, Spain.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Fei H, Lee GH, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamism and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–60. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995;80:15–8. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 4.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–33. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 5.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 6.Frederich RC, Löllmann B, Hamann A, et al. Expression of Ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 8.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13–8. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 9.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 10.Baumann H, Morella KK, White DW, et al. The full leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 12.Cioffi J, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms. Possible role of leptin in hematopoiesis and reproduction. Nature Med. 1996;2:585–8. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 13.Bennet BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Mathews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 14.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 15.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity. Role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farooqi S, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell responsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 21.Martín-Romero C, Sánchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells. Possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Margalet V, Martín-Romero C, González-Yanes C, Goberna R, Rodríguez-Baños J, Muniain MA. Leptin receptor expression is induced in activated mononuclear cells in vitro and in vivo in HIV-infected patients. Clin Exp Immunol. 2002;129:119–24. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najib S, Sanchez-Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002;220:143–9. doi: 10.1016/s0008-8749(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 24.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–8. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee F-YJ, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Pysiol. 1999;276:C386–C394. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 26.Loffreda S, Rai R, Yang SQ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 27.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 28.Vadiveloo PK. Macrophages − proliferation, activation, and cell cycle proteins. J Leukoc Biol. 1999;66:579–82. doi: 10.1002/jlb.66.4.579. [DOI] [PubMed] [Google Scholar]
- 29.Rossi F. The O2-forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and nutrition. Biochim Biophys Acta. 1986;853:65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Kameoka M, Ikuta K. Amplification of superoxide anion generation in phagocytic cells by HIV-1 infection. FEBS Lett. 1993;326:232–6. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- 31.Trial J, Birdsall HH, Hallum JA, et al. Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection. Effects of soluble immune complexes, cytokines, subcellular particulates from apoptotic cells, and HIV-1-encoded proteins on monocytes phagocytic function, oxidative burst, transendothelial migration, and cell surface phenotype. J Clin Invest. 1995;95:1690–701. doi: 10.1172/JCI117845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbin C, Pillet S, Prevost MH, et al. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. J Virol. 1999;73:4561–6. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 34.Lucas M, Sánchez-Margalet V, Sanz A, Solano F. Protein kinase C activation promotes cell survival in mature lymphocytes prone to apoptosis. Biochem Pharmacol. 1994;47:1994. doi: 10.1016/0006-2952(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 35.García-Mauriño S, González-Haba MG, Calvo JR, et al. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: a possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol. 1997;159:574–81. [PubMed] [Google Scholar]
- 36.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 38.Sánchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S. Role of leptin in the immunomodulation of mononuclear cells. Mechanisms of action. Clin Exp Immunol. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor α: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–89. [PubMed] [Google Scholar]
- 40.Chaudri G, Clark IA. Reactive oxygen species facilitate the in vitro and in vivo lipopolysaccharide-induced release of tumor necrosis factor. J Immunol. 1989;143:1290–7. [PubMed] [Google Scholar]
- 41.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immnunol. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 43.Bandres JC, Trial J, Musher DM, Rossen RD. Increased phagocytosis and generation of reactive oxygen products by neutrophils and monocytes of men with stage 1 human immunodeficiency virus infection. J Infect Dis. 1993;168:75–83. doi: 10.1093/infdis/168.1.75. [DOI] [PubMed] [Google Scholar]
- 44.Karp CL, Wysocka M, Ma X, et al. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128–36. doi: 10.1002/(SICI)1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.von Knethen A, Brüne B. Delayed activation of PPARγ by LPS and IFN-γ attenuates the oxidative burst in macrophages. FASEB J. 2001;15:535–44. doi: 10.1096/fj.00-0187com. [DOI] [PubMed] [Google Scholar]
- 46.Buttke T, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 47.Um HD, Orenstein Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–77. [PubMed] [Google Scholar]
- 48.Estrada V, Serrano-Rios M, Martinez Larrad MT, et al. Leptin and adipose tissue maldistribution in HIV-infected male patients with predominant fat loss treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:32–0. doi: 10.1097/00126334-200201010-00004. [DOI] [PubMed] [Google Scholar]
- 49.Matarese G, Castelli-Gattinara G, Cancrini C, et al. Serum leptin and CD4+ T lymphocytes in HIV+ children during highly active antiretroviral therapy. Clin Endocrinol. 2002;57:643–6. doi: 10.1046/j.1365-2265.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 50.Bureau C, Bernard J, Chaouche N, et al. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem. 2001;276:23077–83. doi: 10.1074/jbc.M100698200. [DOI] [PubMed] [Google Scholar]