Abstract
We have investigated the expression of chemokines and their receptors in leprosy skin lesions using immunohistochemistry. Skin biopsies from 25 leprosy patients across the leprosy spectrum, 11 patients undergoing type I reversal reactions and four normal donors were immunostained by ABC peroxidase method using antibodies against CC and CXC chemokines and their receptors. Using an in situ hybridization technique we have also studied the expression of monocyte chemoattractant protein 1 (MCP-1), RANTES and interleukin (IL)-8 chemokines mRNA in leprosy skin lesions. Chemokines and receptor expression was detected in all leprosy skin biopsies. Expression of CC chemokines MCP-1 (P < 0·01) and RANTES (P < 0·01) were elevated significantly in borderline tuberculoid leprosy in reversal reaction compared to non-reactional borderline tuberculoid leprosy, but there was no difference in the expression of IL-8 chemokine. Surprisingly, there was no significant difference in the expression of CC (CCR2 and CCR5) and CXC (CXCR2) chemokine receptors across the leprosy spectrum. Similarly, there was no significant difference in the expression of mRNA for MCP-1, regulated upon activation normal T cell expressed and secreted (RANTES) and IL-8 chemokines. Here, the presence of a neutrophil chemoattractant IL-8 in leprosy lesions, which do not contain neutrophils, suggests strongly a role of IL-8 as a monocyte and lymphocyte recruiter in leprosy lesions. These results suggest that the chemokines and their receptors, which are known to chemoattract T lymphocytes and macrophages, are involved in assembling the cellular infiltrate found in lesions across the leprosy spectrum.
Keywords: chemokines, chemokine receptors, leprosy, immunohistochemistry, in situ hybridization
INTRODUCTION
Leprosy, caused by Mycobacterium leprae, is a human chronic infectious disease causing damaging inflammatory lesions in the skin and peripheral nerve. There is a clinical spectrum of pathology that is determined by the host immune response. Tuberculoid patients mount a vigorous cell-mediated immune response in skin and nerve and display a delayed-type hypersensitivity response to M. leprae antigens [1]. At the opposite pole, lepromatous patients exhibit specific cellular unresponsiveness to M. leprae antigens associated with high bacterial loads in the skin and nerve. Most leprosy patients have pathology between the polar states and are classified as borderline tuberculoid (BT) or borderline lepromatous (BL). Leprosy reactions are common in these immunologically unstable borderline groups. These involve an up-regulation of the host response to M. leprae antigens. For instance, type I (reversal) reactions involve an increase in delayed-type hypersensitivity and a shift towards the tuberculoid pole, accompanied by lesion inflammation and neuritis.
There is a histopathological difference across the leprosy spectrum. Tuberculoid skin lesions possess a higher ratio of helper CD4+ to CD8+ T lymphocyte cells than lepromatous lesions [2]. In addition, tuberculoid lesions contain organized T cell and epithelioid cell granulomas, which kill mycobacteria and prevent their dissemination. However, lepromatous lesions have a more disorganized histology characterized by inactive macrophages, in which mycobacteria are multiplying.
Chemokines are potent chemoattractors of specific leucocyte subsets and therefore are likely to be involved in directing the cellular infiltration in the various forms of leprosy lesions. The CXC group, which contains interleukin (IL)-8, predominantly attracts neutrophils whereas the CC group, which contains regulated upon activation normal T cell expressed and secreted (RANTES) and monocyte chemoattractant protein 1 (MCP-1), predominantly attracts monocytes and lymphocytes. In the skin, lymphocytes and a wide range of non-immune cells, including keratinocytes and fibroblasts, secrete multiple chemokines upon activation by immune mediators such as tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) [3,4].
Chemokine expression has been associated with chronic inflammatory diseases such as tuberculosis [5], sarcoidosis [6], cutaneous leishmaniasis [7] and psoriasis [8]. MCP-1 and macrophage inflammatory protein-1 alpha (MIP-1α) mRNA is expressed by monocytes and T lymphocytes in the granulomatous skin lesions of human cutaneous leishmaniasis [7]. Expression of the chemokines MCP-1 and MIP-1α is up-regulated in pleural endothelial cells, monocytes and macrophages from patients with active tuberculosis but not RANTES [9,10]. However, IL-8 is only up-regulated in macrophages [10]. RANTES, MCP-1 and IL-8 expression is also elevated in bronchoalveolar lavage fluid from patients with active pulmonary tuberculosis but not MIP-1α[10]. In addition, MCP-1 and MIP-1α plasma levels are elevated in patients with sarcoidosis, a disorder characterized by granulomatous lesions [11]. Interferon gamma-inducible protein 10 (IP-10) and RANTES levels have been correlated with the level of T cell recruitment in sarcoidosis patients [6,12]. To date, IP-10 is the only chemokine to have been investigated in leprosy skin lesions. IP-10 expression was found in the epidermis and dermal granuloma of tuberculoid lesions but not in lepromatous lesions [13].
Cells respond to chemokines through a family of seven transmembrane G protein-coupled receptors that are also subdivided into CCR and CXCR chemokine receptors. Each chemokine receptor has a distinct but overlapping chemokine specificity [14].
Leprosy provides an ideal opportunity to investigate the mechanisms and role of chemokines and their receptors involved in leprosy skin lesions. We hypothesized that different expression of chemokines and receptors contribute to the differences in histo-pathology seen in leprosy skin lesions. Skin biopsies from patients representative of the immunopathological spectrum of leprosy were studied using monoclonal and polyclonal antibodies against a number of chemokines and their receptors by immunohistochemistry. We have also investigated the expression of chemokine mRNA in leprosy skin lesions using in situ hybridization with antisense probes of MCP-1, RANTES and IL-8.
MATERIALS AND METHODS
Patients
Skin biopsies were taken for diagnosis from new, untreated leprosy patients (n = 22) and patients undergoing type I reversal reactions (n = 13) attending the Blue Peter Research Centre. Patients were graded clinically and histologically on the leprosy spectrum, according to the Ridley Jopling classification [15], as tuberculoid (TT), borderline tuberculoid (BT), borderline lepromatous (BL) and lepromatous (LL).
A reversal reaction was diagnosed when a patient presented with reactional skin changes (erythema and/or oedema of existing leprosy lesions, new skin lesions that were not relapsing leprosy or erythema nodosum leprosum, ENL) or acute neuritis (peripheral nerve tenderness, new sensory symptoms or signs or new motor symptoms or signs). Reversal reactions were confirmed histologically and classified as borderline tuberculoid in reaction (BT-RR) or borderline lepromatous in reaction (BL-RR).
Punch biopsies 6 mm in diameter were taken under local anaesthetic using 1% ligocaine from the active edge of the lesion. The biopsy was halved and the tissue intended for this study was snap frozen in liquid nitrogen and stored at −70°C. Normal skin biopsies from healthy volunteers (n = 4) were taken by the same procedure. Cryostat sections (6 µm) were stained with haematoxylin and eosin (H&E) for general morphological examination and a modified Fite–Faraco procedure for staining M. leprae. The bact-erial load in each site was counted and expressed on a logarithmic scale as the bacillary index, B.I. [16].
Permission was obtained for this study from the local ethics committee for Blue Peter Research Centre, Hyderabad and the London School of Hygiene and Tropical Medicine ethics committee. Informed consent was obtained from all the subjects involved in this study.
Antibodies
Monoclonal antibodies against RANTES, MCP-1 and IL-8 chemokines were purchased from R&D Systems (Abingdon, UK). Chemokines receptor monoclonal antibodies against CCR5 and CXCR2 were obtained from R&D Systems. CCR2 rabbit polyclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Macrophages were stained using monoclonal anti-CD68 antibody (Dako Ltd, Ely, UK) and T lymphocytes using anti-CD4 and anti-CD8 antibodies (Becton Dickinson, Oxford, UK). Isotype control antibodies mouse IgG1, IgG2 or rabbit Ig fraction (Dako) were used as negative controls.
Immunostaining
Cryostat frozen sections (6 µm) were collected on silanated microscope slides (BDH, Lutterworth, UK) and dried for 2–3 h at room temperature (RT) before fixing in acetone at 4°C for 10 min and air-drying. For chemokine and chemokine receptor staining, sections were incubated with normal serum as part of the avidin–biotin complex (ABC) Vectastain Elite kit protocol (Vector Laboratories, Peterborough, UK) for 30 min at RT.
Sections were then incubated with the primary antibody dilute in phosphate buffered saline (PBS) for 1 h at RT. Staining was completed using the ABC peroxidase method (Vector Laboratories) and the enzymatic reaction was developed using 3,3′-diaminobenzidine chromogen (Sigma, Poole, UK) with haematoxylin counterstaining.
For cell phenotype staining, sections were blocked with normal rabbit serum (Dako) for 30 min at RT before incubation with the primary antibody for 1 h at RT. Sections were stained using the peroxidase–antiperoxidase (PAP) method using a rabbit antimouse secondary antibody (Dako) and a mouse PAP complex (Dako). Staining was developed using DAB (Sigma). All slides were counterstained with Harris haematoxylin (BDH) and mounted in DPX (BDH). Controls for specificity of staining included the use of appropriate isotype antibodies and nonimmune serum and omission of the prim-ary antibody.
Chemokine and receptor staining was assessed by grading the sections on a scale of 0: negative, 1: few scattered positive cells, 2: 10–30% cells positively stained, 3: 30–50% cells positively stained and 4: 50–80% cells positively stained (Table 1). We have used this scale in previous work [17]. In addition, cell and chemokine staining was evaluated and confirmed by an independent observer who was blind to the leprosy type of each section using the agreed-upon scale (Table 1).
Table 1.
Scoring of chemokine and chemokine receptor expression in leprosy skin
| Average intensity scoringa of chemokines and chemokine receptors staining | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokines and receptors | C-X-C chemokines and receptors | |||||||||
| MCP-1 | CCR2 | RANTES | CCR5 | IL-8 | CXCR2 | |||||
| Classificationb | No. of patients | mRNA | IHC | IHC | mRNA | IHC | IHC | mRNA | IHC | IHC |
| TT | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 2 | 3 |
| BT | 7 | 4 | 2 | 2 | 2 | 1 | 3 | 3 | 1 | 2 |
| BT-RR | 7 | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 2 | 2 |
| BL | 7 | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 2 | 3 |
| BL-RR | 6 | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 1 | 3 |
| LL | 4 | 3 | 3 | 1 | 1 | 1 | 4 | 2 | 1 | 3 |
Average scoring of staining was as follows: 0, negative; 1, <10% of cells staining positive; 2, 10–30% of cells staining positive; 3, 30–50% of cells staining positive; 4, 50–80% of cells staining positive; 5, 80–100% of cells staining positive.
TT, tuberculoid leprosy; BT, borderline tuberculoid leprosy; BT–RR, borderline tuberculoid leprosy in reversal reaction; BL, borderline lepromatous leprosy; BL-RR, borderline lepromatous leprosy in reversal reaction; LL, lepromatous leprosy.
In situ hybridization
In situ hybridization (ISH) was carried out as described by other authors [18,19]. Briefly, 6 µm skin biopsy cryostat sections were collected on RNase-free polysine glass slides (BDH, UK). The sections were brought to RT, fixed in 4% paraformaldehyde, acetylated using 0·25% acetic anhydride in 0·1 m triethanolamine, before dehydration in ethanol and overnight hybridization at 37°C with digoxigenin-labelled oligonucleotide probe cocktail for the chemokines MCP-1, RANTES and IL-8 (R&D Systems), diluted 1 : 400 in hybridization buffer. Sections were washed in 2 × SSC for 1 h at RT, 1 × SSC for 1 h at RT and 1 × SSC for 30 min at 37°C. The slides were then processed for immunological detection by first blocking with normal sheep serum followed by alkaline phosphatase conjugated sheep antidigoxigenin antibody (Boehringer-Mannheim, UK) with the substrate Sigma fastTM BCIP/NBT. The sections were mounted in PBS/glycerol.
Incubation with hybridization buffer alone was used as negative control and demonstrated the specificity of the hybridization products with the chemokine probes.
Statistical analysis
Statistical analysis of data was by the two-sample Wilcoxon rank-sum (Mann–Whitney) test. All data are expressed as median unless otherwise indicated. P-values = 0·05 were considered significant.
RESULTS
The results of immunostaining skin biopsies from 35 leprosy patients with antibodies against CC and CXC chemokines and their receptors are summarized in terms of semiquantitative grading in Table 1. Chemokines and receptor positively stained cells were located in the dermal granuloma and were determined by the presence of a brown intracellular and/or extracellular staining with PAP antibody or avidin–biotin complex-peroxidase (ABC-HRP) using rabbit-antimouse immunoglobulin or biotin conjugated rabbit-antimouse immunoglobulin, respectively. Chemokines and receptor expression was associated predomin-antly with macrophages and a small proportion of T lymphocytes. Control subjects had negative results for all the chemokines investigated.
Expression of CC and CXC chemokines mRNA in leprosy skin lesions
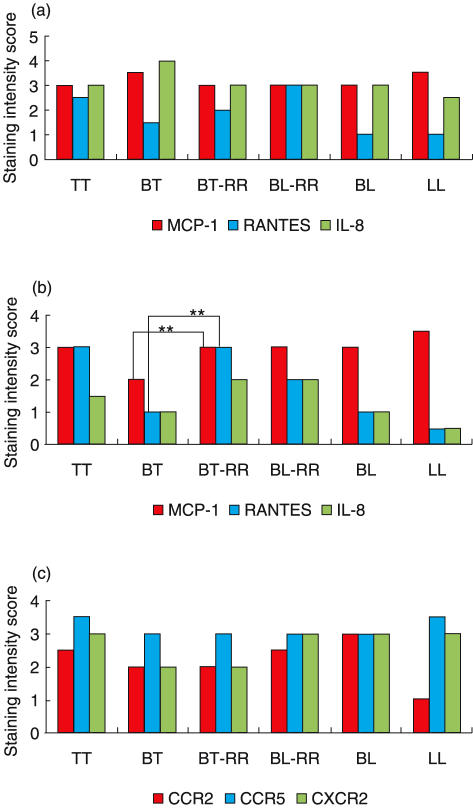
The cellular localization of chemokine mRNA was determined by in situ hybridization using a digoxigenin-labelled antisense probe. Chemokine mRNA production was visible as darkly staining areas within the cytoplasm of positive cells (Fig. 1b). Intense staining was observed and co-localized with areas of granuloma formation. Statistically no significant difference in mRNA express-ion for MCP-1, RANTES and IL-8 chemokines (Fig. 2a) was observed. In addition, a non-significant elevation in the express-ion of MCP-1 and IL-8 mRNA across the leprosy spectrum was also noticeable compared to RANTES mRNA (Fig. 2a,b).
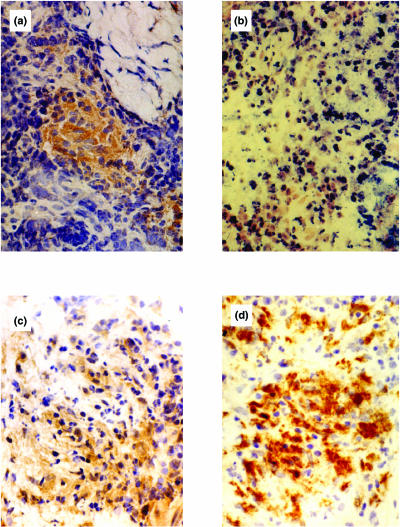
Fig. 1.
Immunohistochemical detection of chemokine and receptor protein in borderline tuberculoid leprosy in reaction (BT-RR) leprosy skin lesions. (a) MCP-1 chemokine-protein-positive cells within a granuloma. (b) MCP-1 chemokine-mRNA positive staining by in situ hybridization. (c) A section of the same lesion stained with the polyclonal antibody CCR2 (a chemokine receptor for MCP-1). (d) Immunostaining of the same skin lesion with the monoclonal antibody CD68 (macrophage marker) indicating a large proportion of positive cells.
Fig. 2.
Analysis of immunohistochemical staining of chemokines and chemokine receptor expression across the leprosy spectrum. (a) Detection of chemokine mRNA expression by in situ hybridization; (b) immunohistochemical staining of MCP-1, RANTES and IL-8 chemokines protein; (c) immunohistochemical detection of chemokine receptor protein (CCR2, CCR5 and CXCR2). Statistical analysis was by the two-sample Wilcoxon rank-sum (Mann–Whitney) test, **P < 0·01. bTT, tuberculoid leprosy; BT, borderline tuberculoid leprosy; BT-RR, borderline tuberculoid leprosy in reversal reaction; BL-RR, borderline lepromatous leprosy in reversal reaction; BL, borderline lepromatous leprosy; LL, lepromatous leprosy.
Expression of CC and CXC chemokines and their receptors in leprosy skin lesions
In order to investigate CC and CXC chemokines and receptor protein expression in relation to mRNA expression, we compared the number of cells expressing mRNA detected by in situ hybridization to the number of cells expressing chemokine and receptor protein detected by immunohistochemistry. The expression of CC chemokines MCP-1 (P < 0·01) and RANTES (P < 0·01) was significantly higher in borderline tuberculoid leprosy in reversal reaction compared to non-reactional borderline tuberculoid leprosy (Fig. 2b). In addition, the RANTES chemokine protein expression was elevated in reactional lesions compared to BT and BL (Figs 2b, 3a,b). Similarly, IL-8 expression was higher in react-ional lesions compared to non-reactional BT and BL (Fig. 2b). Surprisingly, there was no significant difference in the levels of chemokine receptor expression across the leprosy spectrum despite the general trend of CCR2 expression being low in BT and LL patients (Fig. 2c). MCP-1 chemokine protein-positive cells were detected within the granuloma (Fig. 1a), as well as its CCR2 receptor (Fig. 1c). The expression of both MCP-1 chemokine and its receptor appeared predominantly to be associated with macrophages (Fig. 1d). In addition, expression of CCR5 (RANTES receptor) was elevated in BL-RR compared to BL (Fig. 1c,d). However, this elevation was not statistically significant.
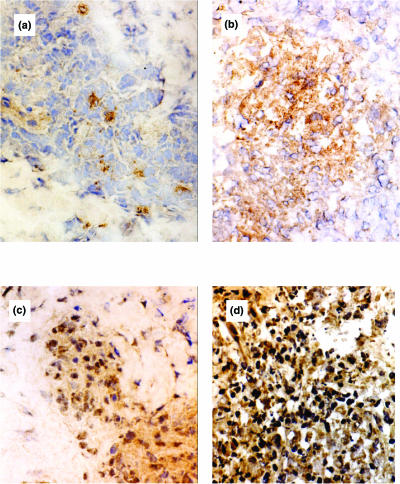
Fig. 3.
Immunohistochemical staining for RANTES and CCR5 chemokine receptor. (a) Detection of RANTES chemokine protein in borderline lepromatous (BL) leprosy skin lesion. (b) Immunostaining for RANTES chemokine protein in borderline lepromatous leprosy in reaction (BL-RR), showing more cells positive. (c,d) Immunostaining for CCR5 protein in BL and BL-RR, respectively.
Immunohistochemical analysis of cell phenotypes
Immunohistochemical investigation of cell markers on leprosy skin (n = 28) was carried out using monoclonal antibodies against T lymphocyte markers CD4 and CD8 and the macrophage marker CD68 (Table 2). Double-staining experiments using MCP-1 and CD68 antibodies showed a large proportion of macro-phage MCP-1 positive cells compared to a small proportion of T lymphocytes (data not shown). The percentage of T cells CD4+ was in the range 41·5 ± 6·7% to 57·7 ± 3·4% and the range of T cells CD8+ was in the range 19·6 ± 14·6% to 34·1 ± 6%. The CD4 : CD8 ratio was higher than 1 in all leprosy lesions. The CD4 : CD8 ratio was highest in the TT and BT groups (2·9 and 2·4 for TT and BT, respectively) but lower in the reactional and lepromatous groups. The percentage of cells CD68+ in leprosy lesions ranged from 36·1 ± 11·3% to 53·9 ± 11·9% and was highest in the lepromatous and reactional groups and lower in the TT and BT groups.
Table 2.
Cell phenotype staining in leprosy skin
| Classificationb | No. of patients | CD68 (% positive cells ± s.d.) | CD4 (% positive cells ± s.d.) | CD8 (% positive cells ± s.d.) | CD4 : CD8 ratio |
|---|---|---|---|---|---|
| TT | 3 | 38·5 ± 3·3 | 57·7 ± 3·4 | 19·8 ± 2·7 | 2·9 |
| BT | 5 | 36·1 ± 11·3 | 46·7 ± 12·9 | 19·6 ± 14·6 | 2·4 |
| BT-RR | 4 | 47·8 ± 11·1 | 55·2 ± 14·7 | 28·5 ± 8·6 | 1·9 |
| BL | 7 | 50·1 ± 10·4 | 41·5 ± 6·7 | 33·3 ± 6·6 | 1·2 |
| BL-RR | 5 | 53·9 ± 11·9 | 55·2 ± 13·7 | 34·1 ± 6 | 1·6 |
| LL | 4 | 43·8 ± 4·5 | 51·3 ± 5·6 | 30·1 ± 14·3 | 1·7 |
Percentage of positive cells was estimated by microscopic evaluation of several fields corresponding to >500 cells per section.
TT, tuberculoid leprosy; BT, borderline tuberculoid leprosy; BT-RR, borderline tuberculoid leprosy in reversal reaction; BL, borderline lepromatous leprosy; BL–RR, borderline lepromatous leprosy in reversal reaction; LL, lepromatous leprosy.
DISCUSSION
Although chemokines and their receptors have been implicated in several pathological states, this is the first study to investigate the expression of CC and CXC chemokines and their receptors in leprosy skin lesions. We found expression of the CXC chemokine IL-8 and the CC chemokines MCP-1 and RANTES in skin biopsies from lesions across the leprosy spectrum. Surprisingly, despite the differences in histopathology between tuberculoid and lepromatous skin lesions, there was no significant difference observed in the expression of the chemokine mRNA and receptors across the leprosy spectrum. Previous studies have demonstrated a lack of correlation between the expression of chemokine mRNA and protein [20,21]. Therefore, we did not attempt to correlate the expression of the chemokine mRNA to protein or receptor. Nevertheless, this study has shown a significant elevation in the expression of the CC chemokine protein MCP-1 and RANTES in borderline tuberculoid in reversal reaction compared to non-reactional borderline tuberculoid leprosy. In accord with previous reports, overexpression of chemokines has been associated with a number of diseases such as arthritis, multiple sclerosis and respiratory disorders [22–25]. It was noted initially that large quantities of IL-8, activated neutrophils and T lymphocytes were present in the epidermis of patients with psoriasis vulgaris [26]. Furthermore, CXCR2 mRNA levels were shown to be higher in lesional psoriatic epidermis than in normal epidermis. This has led to the suggestion that the overexpression of IL-8 receptor is responsible for the leucocyte infiltration seen in psoriasis. Indeed, antipsoriatic drugs have been shown to be potent inhibitors of IL-8 receptor expression on keratinocytes [26,27]. IL-8 predominantly recruits neutrophils, but also monocytes and T lymphocytes, which express the IL-8 receptors CXCR1 and CXCR2 [28]. Thus, the presence of IL-8 in leprosy lesions, which do not contain neutrophils, in this study strongly suggests a role of this chemokine as a monocyte and lymphocyte recruiter. Monocytes infected with M. tuberculosis have been shown to produce IL-8 in vitro[5,10]; however, macrophages are a probable source of IL-8 in leprosy lesions. In addition, activated T lymphocytes could also express IL-8 as they have been shown to do so in vitro[29]. It is feasible, therefore, to suggest that IL-8 could play a role in the dynamic directing of immune cell influx in leprosy lesions.
Recently CC and CXC chemokines have been suggested to play an important role in the recruitment of macrophages and T lymphocytes, granuloma formation and in the controlling of mycobacterial infection in mice [30]. CC chemokines such as MCP-1 and RANTES induce the recruitment of monocytes and T lymphocytes and are thus associated with chronic inflammation. The elevation in RANTES protein observed in this study therefore suggests the role of this chemokine in the induction of cell-ular infiltration associated with leprosy lesions. In agreement, Konig and others have shown an expression of RANTES mRNA predominantly by T lymphocytes associated with synovial lining and cellular infiltrates in arthritis [31]. Furthermore, CC chemokines have been shown to have a role as co-stimulators in T lymphocyte activation [32]. For instance, MCP-1 has been shown as an activator of macrophage killing [33], while studies on RANTES have established that its chemoattractant function is mediated by a separate receptor than that used in T lymphocyte activation [34]. It is possible, therefore, that chemokines could function on two levels in leprosy as chemoattractors and leucocyte activators. The greater level of T cell activation reported in tuberculoid and reactional lesions [35] could, at least in part, be attributed to the chemokine expression, thus providing a plausible explanation for the apparent elevation in their expression observed in this study. However, chemokines may not have a direct role in development of protective immunity. Work on the M. tuberculosis-induced granuloma has interpreted the role of chemokines as leucocyte chemoattractors, which direct granuloma formation but do not direct protective immunity [36]. Never-theless, the leprosy spectrum is often presented as a progression from effective Th1 type cell-mediated immunity, characterized by cytokines such as IFN-γ, at the tuberculoid pole to Th2 responses, characterized by cytokines such as IL-4, at the lepromatous pole [37].
In conclusion, this study has demonstrated the expression of both CC and CXC chemokine in leprosy skin lesions and is, thus, notable because a number of reports have suggested that chemokines are involved in Th1/Th2 lymphocyte differentiation [38–40]. Whether the expression of different chemokines may influence clinical manifestation of leprosy other than leucocyte recruitment to inflammatory sites requires further investigation. Nevertheless, our study provides some evidence that overexpression of the chemokines investigated may drive the disease towards a delayed-type hypersensitivity response to M.leprae antigen. Further investigations will aid the elucidation of the precise mechanisms and role of chemokines in leprosy pathology.
Acknowledgments
We would like to thank all the staff and patients at Blue Peter Research Centre (Hyderabad, India). Blue Peter Research Centre is managed by the UK MRC through Lepra-India. This work and A.A.K. are supported by a grant from the Hospitals and Homes of St Giles, UK. A.C.M. is supported by the Heiser Trust Postdoctoral research fellowship, New York, USA.
REFERENCES
- 1.Dockrell HM, Young SK, Brennan PJ, et al. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect Immunol. 1996;64:4385–9. doi: 10.1128/iai.64.10.4385-4389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra V, Modlin RL. T-lymphocytes in leprosy lesions. Curr Top Microbiol Immunol. 1990;155:97–109. doi: 10.1007/978-3-642-74983-4_7. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Ireland GW, Farthing PM, et al. Epidermal and oral keratinocytes are induced to produce RANTES and IL-8 by cytokine stimulation. J Invest Dermatol. 1996;106:661–6. doi: 10.1111/1523-1747.ep12345482. [DOI] [PubMed] [Google Scholar]
- 4.Rathanaswani P, Hachicha M, Sadick M, et al. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- 5.Kasahara K, Tobe T, Tomita M, et al. Selective expression of monocyte chemotactic and activating factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–47. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 6.Agostini C, Cassatella M, Zambello R, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–20. [PubMed] [Google Scholar]
- 7.Ritter U, Moll H, Laskay T, et al. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J Infect Dis. 1996;173:699–709. doi: 10.1093/infdis/173.3.699. [DOI] [PubMed] [Google Scholar]
- 8.Gillitzer R, Ritter U, Spandau U, et al. Differential expression of GRO-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J Invest Dermatol. 1996;107:778–82. doi: 10.1111/1523-1747.ep12371803. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed KA, Nasreen N, Ward MJ, et al. Mycobacterium-mediated chemokine expression in pleural mesothelial cells: role of C-C chemokines in tuberculosis pleurisy. J Infect Dis. 1998;178:1450–6. doi: 10.1086/314442. [DOI] [PubMed] [Google Scholar]
- 10.Sadek MI, Sada E, Toosi Z, et al. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–21. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto S, Nakayama T, Gon T, et al. Correlation of plasma monocyte chemoattractant protein-1 (MCP-1) and monocyte inflammatory protein-1 alpha (MIP-1 alpha) levels with disease activity and clinical course of sarcoidosis. Clin Exp Immunol. 1998;111:604–10. doi: 10.1046/j.1365-2249.1998.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrek M, Pantelidis P, Southcott AM, et al. The source and role of RANTES in interstitial lung disease. Eur Respir J. 1997;10:1207–16. doi: 10.1183/09031936.97.10061207. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan G, Luster AD, Hancock G, et al. The expression of a gamma interferon-induced protein-10 (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward SG, Bacon K, Westwick J. Chemokines and T-lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 15.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 16.Ridley DS. Basle: Ciba-Geigy Ltd; 1985. Skin Biopsy in Leprosy; pp. 59–60. [Google Scholar]
- 17.Little D, Khanolkar-Young S, Coulthart A, et al. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect Immunol. 2001;69:3413–7. doi: 10.1128/IAI.69.5.3413-3417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson JE, Newcombe J, Cuzner ML, et al. Expression of monocyte chemoattractant protein-1 and other β-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–49. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–8. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 20.Fantuzzi L, Canini I, Belardelli F, et al. IFN-beta stimulates the production of beta-chemokines in human peripheral blood monocytes. Importance of macrophage differentiation. Eur Cytokine Netw. 2001;12:597–603. [PubMed] [Google Scholar]
- 21.Nanki T, Lipsky PE. Lack of correlation between chemokine receptor and Th1/Th2 cytokine expression by individual memory T cells. Int Immunol. 2000;12:1659–67. doi: 10.1093/intimm/12.12.1659. [DOI] [PubMed] [Google Scholar]
- 22.Schroder JM, Noso N, Sticherling M, et al. Role of eosinophil–chemotactic C-C chemokines in cutaneous inflammation. J Leukoc Biol. 1996;59:1–5. [PubMed] [Google Scholar]
- 23.Standiford TJ, Kunkel SL, Greenberger MJ, et al. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–8. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel SL, Lukacs N, Kasama T, et al. The role of chemokines in inflammatory joint disease. J Leukoc Biol. 1996;59:6–12. doi: 10.1002/jlb.59.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Lukacs NW, Kunkel SL. Chemokines and their role in disease. Int J Clin Laboratory Res. 1998;28:91–5. doi: 10.1007/s005990050025. [DOI] [PubMed] [Google Scholar]
- 26.Schulz BS, Michel G, Wagner S, et al. Increased expression of epidermal IL-8 receptor in psoriasis: down-regulation by FK-506 in vitro. J Immunol. 1993;151:4399–406. [PubMed] [Google Scholar]
- 27.Lemster BH, Carroll PB, Rilo HR, et al. IL-8/IL-8 receptor expression in psoriasis and response to systemic tacrolimus (FK506) therapy. Clin Exp Immunol. 1995;99:148–54. doi: 10.1111/j.1365-2249.1995.tb05525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 29.Wechler AS, Gordon MC, Dendorfer U, et al. Induction of IL-8 expresssion in T cells uses the CD28 costimulatory pathway. J Immunol. 1994;153:2515–23. [PubMed] [Google Scholar]
- 30.Roach DR, Bean AGD, Demangel C. TNF regulates chemokine induction essential for cell recruitment, granuloma formation and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 31.Konig A, Krenn V, Toksoy A, et al. Mig, GRO alpha and RANTES messenger RNA expression in lining layer, infiltrates and different leukocyte populations of synovial tissue from patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Virchows Arch. 2000;436:449–58. doi: 10.1007/s004280050472. [DOI] [PubMed] [Google Scholar]
- 32.Taub DD, Turcovski-Corrales SM, Key ML, et al. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 33.Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–6. [PubMed] [Google Scholar]
- 34.Bacon KB, Premack BA, Gardner P, et al. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–30. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 35.Volc Platzer B, Stemberger H, Luger T, et al. Defective intralesional interferon-gamma activity in patients with lepromatous leprosy. Clin Exp Immunol. 1988;71:235–40. [PMC free article] [PubMed] [Google Scholar]
- 36.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 37.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [published erratum appears in Science 1992 January 255 (5040): 12]. [DOI] [PubMed] [Google Scholar]
- 38.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–4. [PubMed] [Google Scholar]