Abstract
Previous studies have shown that human natural killer (NK) cells are lost from the periphery and are functionally suppressed during HIV-1 infection, and that the administration of highly active antiretroviral therapy (HAART) results in a recovery of NK cell numbers in HIV-1-infected individuals. However, despite this recovery, interleukin (IL)-2 + IL-12-driven interferon (IFN)-γ production by NK cells has been shown to remain suppressed after HAART. Here we show that the innate immune factor IL-15 in combination with IL-12 is also unable to recover NK cell IFN-γ production in HAART-treated individuals. Furthermore, we also demonstrate an imbalance in the distribution of CD56loCD16hi and CD56hiCD16– NK subsets after successful HAART, CD56hiCD16– cells being reduced substantially in HIV-1 patients on HAART. Treatment of patients with combined human growth hormone and antiretroviral therapy resulted in further enhancement in the absolute numbers and the proportion of NK cells in some individuals in the absence of parallel effects on CD4+ T cells. Furthermore, in these individuals HAART with growth hormone resulted in an enhancement of cytokine-driven NK cell activation and IFN-γ production compared to the HAART-only baseline.
Keywords: AIDS, cytokines, NK cells
INTRODUCTION
Natural killer (NK) cells play a key role in the production of antiviral factors early in the development of antiviral immune responses [1]. NK cells are among the earliest cell types to respond to viruses by producing key immunomodulators such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α. Such rapid responses are due at least in part to the ability of NK cells to respond to innate early cytokines such as IL-12 [1]. In addition, NK cells are activated by and proliferate in response to T cell-derived factors such as IL-2 [2]. In this way NK cell responses can be influenced by both specific and innate immune responses.
NK cells are likely to play a critical role in immune defence against HIV-1. First, they are capable of killing HIV-1-infected autologous target cells in vitro either by natural or antibody-dependent cellular cytotoxicity (ADCC) [3]. Secondly, they activate inflammatory cells via early cytokine production [4]; thirdly, they produce chemokines which compete with viral receptors on the cell surface [5,6]; and fourthly, they are a source of α-defensins which have been shown recently to have potent antiviral activity in HIV-1 infection [7,8]. Furthermore, particular combinations of killer immunoglobulin-like receptors (KIR) and MHC Class I molecules have also been shown to correlate with slow HIV-1 disease progression [9].
NK cells are, however, generally reduced in number and are functionally impaired during chronic, untreated infection with HIV-1 [10–14]. There are several possible mechanisms to account for this, including the induction of cell death, diminishment of T cell-derived (IL-2) and innate (IL-12) growth factors and modulation of NK receptors for MHC Class I [15–17]. Highly active antiretroviral therapy (HAART) has been shown to permit a recovery of NK cell numbers, a recovery of natural cytotoxicity and a reduction in inhibitory C-type lectin expression [18,19].
T cell (IL-2) and myeloid cell (IL-15)-derived cytokines partially activate NK to express CD69, proliferate and have cytolytic activity against MHC class I negative target cells. Such signals alone are, however, insufficient to induce NK cell cytokine production and have been shown synergize with IL-12 for this function. NK cell interferon gamma production in response to IL-2 in combination with IL-12 remains suppressed in HIV-1+ individuals receiving HAART [20]. Furthermore, evidence suggests that failure of human NK cells from HAART-treated HIV-1+ patients to produce IFN-γ in response to IL-12 + IL-2 may represent a developmental defect in the NK lineage [20,21].
Growth hormone is thought to play a role in the differentiation of bone marrow-derived lymphoid precursors [22,23]. Furthermore, rhGH has been shown to induce a partial recovery of HIV-1-specific IFN-γ-producing CD8+ T cells in HIV-1+ individuals receiving HAART [24]. It was therefore of interest to us to study the effects of rhGH therapy on NK cell activation and IFN-γ production in these individuals.
In this paper we investigate whether defects in NK cell activation and IFN-γ production are specific for IL-2 stimulation or can be recovered in HIV-1+ individuals on HAART using the innate cytokine IL-15 in combination with IL-12 and whether functional subsets of NK cells are affected differentially. We also demonstrate that rhGH enhances preferentially the numbers of NK cells in a small cohort of individuals receiving recombinant human growth hormone with HAART and investigate whether there is any recovery of cytokine-driven NK function in such individuals.
MATERIALS AND METHODS
Patients
Thirty-four individuals were used in this study. Of these, 10 were healthy volunteers, 12 were HIV-1+ individuals on successful HAART and 12 were HIV-1+ individuals on successful HAART receiving growth hormone for a minimum of 12 and a maximum of 24 weeks. All HIV-1+ individuals had been on successful HAART for at least 12 months and with the exception of two patients receiving human growth hormone (patient 3: baseline; 1555 copies/ml and week 48; 2788 copies/ml and patient 8: baseline; 17089 copies/ml and week 12–48; <50 copies/ml) had viral loads of less than 50 copies/ml blood (detection limit) with no observed viral recrudescence. CD4+ T cell counts were greater than 250 cells/µl blood for all patients. CD4+ T cell counts throughout rhGH part of the study did not vary significantly from baseline. The mean CD4 T cell counts were as follows: baseline; 478 ± 56, week 12; 452 ± 47; week 24; 550 ± 54·5 and week 48; 472 ± 42.
Blood was taken into lithium heparin and peripheral blood mononuclear cells (PBMC) from all donors were prepared by standard Ficoll–Hypaque gradient centrifugation. Cells were cultured in RPMI 10% pooled human AB serum (Sigma, Poole, UK).
Stimulations
IL-2 (Hoffman La Roche, Basel, Switzerland) and IL-15 (Peprotech EC, London, UK) were pretitrated to assess optimal concentrations for NK cell activation (CD69 expression) within PBMC. In experiments described here IL-12 (Peprotech) was used at a final concentration of 1 ng/ml in combination with IL-2 (10 U/ml) or IL-15 (100 ng/ml) for 16–20 h.
Flow cytometry
Unstimulated PBMC were labelled with the following monoclonal antibodies for determination of NK cell surface phenotype. Anti-CD16 (Serotec, Oxford, UK) was used as a direct PE conjugate. Unconjugated antibodies were detected with PE conjugated goat antimouse immunoglobulin and then blocked with an excess of mouse IgG. Cells were then counterstained with fluorescein-conjugated anti-CD3 (Serotec) in combination with phycoerythrin–cyanin 5·1 (PC5) conjugated anti-CD56 (Beckman Coulter, Oxford, UK) to identify CD3–CD56+ NK cells.
Cell surface CD69 expression was monitored after 16 h in untreated cells or in cytokine-stimulated cells using anti-human CD69PE (Becton Dickinson, Oxford, UK) and compared to isotype control (mIgG1PE, Becton Dickinson).
For intracellular staining of interferon gamma production, cells were pulsed with 10 µg/ml of brefeldin A (Sigma) between 16 and 20 h of culture and then surface-stained with CD3 FITC and CD56 PC5. Cells were then fixed and permeabilized using FACS™ permeabilizing solution (Becton Dickinson) and then labelled with anti-IFN-γ or control antibodies (Becton Dickinson). For surface staining 10 000–20 000 events were collected and for intracellular staining 30 000 events were collected where possible. Cells were analysed on a FACScalibur™ flow cytometer (BD) and data were analysed using WinMDI software.
Statistical analysis
These were performed with StatView™ software using a Mann–Whitney non-parametric test.
RESULTS
Cytokine-activated expression of CD69 is reduced in HAART-treated individuals
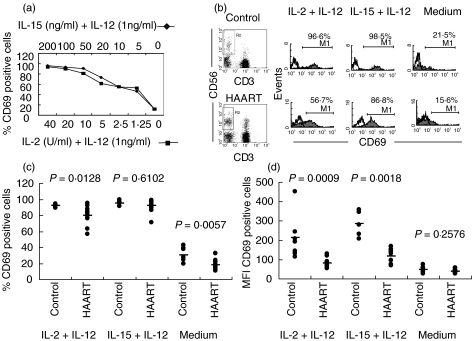
NK cells from HIV-1+ individuals have been shown to recover their cytolytic and proliferative capacities during HAART for HIV-1. CD69 is induced on peripheral blood NK cells in an IL-2-dependent manner and its ligation can lead to proliferation [25,26]. We therefore tested whether CD69 expression was also conserved in cytokine-activated NK cells from HAART-treated individuals. IL-2 or IL-15 combined with IL-12 for the induction of CD69 expression in NK cells, whereas little or no activation was observed in the absence of IL-2 or IL-15 (IL-12 only), as shown for a healthy control individual (Fig. 1a). Concentrations of IL-2 and IL-15 were used subsequently which gave equivalent CD69 expression in control donors. A reduction in the proportion of NK cells expressing CD69, over and above the isotype control, was observed in HAART-treated individuals compared to healthy controls after either leaving cells in medium alone (range for HAART group: 10·9–32·4% and control group: 19·3–39·4%), or after stimulation with IL-2 + IL-12 (range for HAART group: 56·7–95·1% and control group: 89·2–94·2%) (Fig. 1b,c). However, a similar proportion of NK cells expressed CD69 in healthy controls and in patients receiving HAART after IL-15 + IL-12 stimulation (range for HAART group, 71·3–98·8% and control group, 91·8–99·4%) (Fig. 1b,c). In view of the relative preservation in the percentage of CD69 expressing NK cells from HAART-treated individuals, particularly in IL-15 treated cultures compared to IL-2 treated cultures, we also assessed the mean fluorescence intensity (MFI) for this marker in gated CD3–CD56+ cells to see if the effect was enhanced in magnitude. In this case the MFI for CD69 expression on NK cells from HAART-treated individuals was reduced significantly in both IL-2 + IL-12 (range for HAART group: 53·5–129·9 units and control group: 114·4–453·3 units) and IL-15 + IL-12-treated cultures (range for HAART group: 70·2–169·9 units and control group: 231·0–357·8 units) (Fig. 1d), showing that early activation is reduced in NK cells from HAART-treated individuals irrespective of the stimulus used.
Fig. 1.
CD69 expression on NK cells. Flow cytometric analysis of gated CD3-CD56+ NK cells. (a) Titration of IL-2 (▪) or IL-15 (♦) in combination with IL-12 to stimulate CD69 expression in NK cells from a healthy control donor. (b) Flow cytometric profiles are shown of CD69 expression compared to isotype control on gated (left panels) NK cells from a healthy control donor and an HIV-1+ patient receiving HAART only with or without cytokine stimulation. (c) Basal and IL-2 + IL-12-induced NK cell CD69 expression are both reduced significantly in a group of 12 patients on HAART compared to healthy controls. (d) Reduction in MFI for IL-2 + IL-12 and IL-15 + IL-12 stimulation.
Interferon gamma production remains suppressed in IL-15 + IL-12 stimulated NK cells
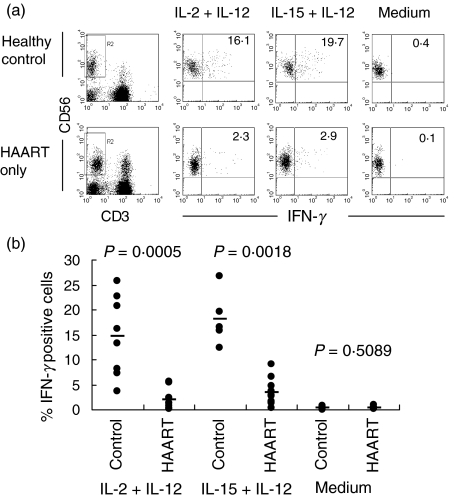
In view of our observation that the intensity of IL-15 + IL-12 induced CD69 expression is reduced, we compared the ability of IL-2 and IL-15 as co-stimuli with IL-12 to stimulate IFN-γ production during HAART. As expected, only few cells from HAART-treated individuals produced IFN-γ in response to IL-2 + IL-12 compared to healthy controls (range for HAART group: 0·1–5·6%versus control group: 3·7–25·9%) (Fig. 2a,b). Notably, IL-15 was unable to overcome the suppression of IFN-γ production (range for HAART group: 0·3–9·1%; control group: 12·3–26·7%) (Fig. 2a,b).
Fig. 2.
IFN-γ production by NK cells. (a) Flow cytometric profiles for intracellular IFN-γ expression in gated (left panel) NK cells from a healthy control donor and an HIV-1+ patient receiving HAART only with or without cytokine stimulation. (b) Both IL-2 + IL-12 and IL-15 + IL-12-induced NK cell IFN-γ expression are reduced significantly in 12 patients on HAART compared to healthy controls.
CD56hiCD16– NK cells are lost in HIV-1+ individuals receiving HAART
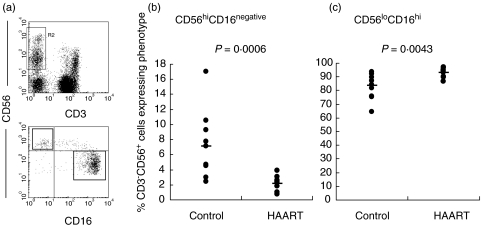
CD56hiCD16– NK cells have been shown to be the major producers of IFN-γ in response to IL-12 + IL-15 [5]. The relative proportions of CD56hiCD16– NK cells were therefore measured in HIV1+ individuals receiving HAART and compared to healthy controls. Remarkably, the proportions of CD56hiCD16loCD3– cells were diminished significantly in individuals receiving HAART (range 0·7–3·9% compared to control group range 3·0–17·0%) (Fig. 3a). A parallel increase was observed in the reciprocal CD56loCD16hi subset (range for HAART group: 89·7–96·9%; control: 64·4–93·8%) (Fig. 3b). This was reflected directly in the ratio of the CD56hiCD16– to the CD56loCD16hi subset, which was significantly lower in HAART-treated individuals (mean 0·09 ± 0·06, range 0·03–0·26) compared to healthy controls (mean 0·02 ± 0·01, range 0·01–0·04).
Fig. 3.
Distribution of CD56hiCD16– and CD56loCD16+ subsets. Gated NK cells (a, upper panel) were analysed for CD56 and CD16 expression and using the regions shown in (a), lower panel. The proportion of CD56hiCD16– cells is reduced significantly in 12 patients receiving HAART (b) and the proportion of CD56loCD16hi cells increases (c).
Enhanced NK numbers in HAART-treated individuals after growth hormone treatment
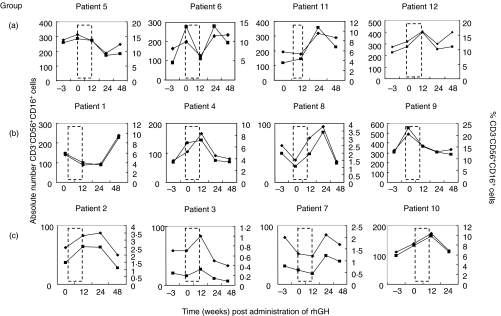
The absolute number and percentage of CD3–CD56+ cells in the peripheral blood were monitored at baseline HAART and after growth hormone treatment. All patients received GH for at least 12 weeks, after which the cohort was randomized into discontinuing rhGH (group A), or receiving this on alternate days (group B) or twice weekly (group C) for a further 12 weeks. No difference was observed in the mean percentage or absolute number of NK cells in any of the treatment groups (data not shown). Fluctuations, and in particular increases, in NK cell number and percentage were, however, observed after rhGH treatment in eight of 12 individual patients either some time during or after rhGH treatment (patients 1, 2, 3, 4, 8, 10, 11, 12) (Fig. 4).
Fig. 4.
Increase in NK cells after long-term rhGH therapy. Twelve individuals receiving HAART for HIV-1 were treated with rhGH daily for 12 weeks (group a), 12 weeks daily and then every 2 days for a further 12 weeks (group b) or 12 weeks daily and then twice weekly for a further 12 weeks (group c). Dashed lines represent 12-week period where all subjects received rhGH. Total CD3–CD56+CD16+ NK cell numbers (▪) and percentages (⋄) were monitored at the time-points shown.
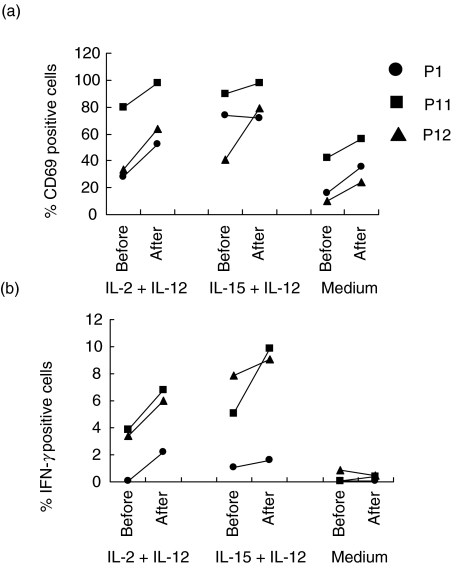
Enhanced NK cell CD69 expression and interferon gamma production after growth hormone treatment
rhGH or its intermediates have been shown to have positive effects on NK cell activation and cytolytic activity [27,28]. We therefore tested whether the activation potential of NK cells from our patients could be enhanced by rhGH treatment. PBMC taken from individuals before and after rhGH treatment were left in medium alone or stimulated with IL-12 in combination with either IL-2 or IL-15, and activation assessed according to the expression of CD69 on gated CD3–CD56+ NK cells. Results from three different individual patients are shown in Fig. 5a. Basal CD69 expression (cells left in medium alone) was enhanced in all three patients tested after rhGH treatment compared to baseline (Fig. 5a; donors P1, P11, P12). This was also the case after stimulation, where the proportion of CD3–CD56+ cells expressing CD69 increased substantially, particularly for IL-12 + IL-2 stimulation (Fig. 5a; donors P1, P11, P12), and also in one case for IL-15 stimulation (Fig. 5a; donor, P12). The stimulated increases did not, however, approach the levels observed in healthy controls (range for rhGH + HAART group: IL-2 + IL-12, 52·6–98·2%; IL-15 + IL-12, 72–97·8%, range control group: IL-2 + IL-12, 89·2–94·2%; IL-15 + IL-12, 91·8–99·4%). Only moderate increases in the calculated mean fluorescence intensities for CD69 expression were observed in some cases, but again these values did not approach the levels in healthy controls (range rhGH + HAART group: IL-2 + IL-12, 32·9–66·6 units; IL-15 + IL-12, 41·4–72·8 units, range control group: IL-2 + IL-12, 114·4–453·3 units; IL-15 + IL-12, 231·0–357·8 units) (data not shown).
Fig. 5.
CD69 Expression and IFN-γ production at baseline and after rhGH therapy. CD69 expression (a) and IFN-γ production (b) were measured in CD3–CD56+ NK cells at baseline and after rhGH therapy. The following comparisons were made. Patient 1 (•): baseline versus week 48; patient 11 (▪): baseline versus week 24 and patient 12 (▴): baseline versus week 12. Experiments were performed on a further occasion using samples from patient 12 giving similar results.
As expected, little or no IFN-γ was detected in cells left in medium alone or after stimulation of cells from patients receiving HAART only prior to rhGH treatment (Fig. 5b). However, rhGH + HAART permitted a recovery of IL-2 + IL-12-driven IFN-γ production in CD3–CD56+ cells from all three patients tested. Furthermore, IL-12 + IL-15-stimulated IFN-γ, which was generally higher than that driven by IL-12 + IL-2, was also enhanced substantially in one of three patients (P11) and was only weakly affected in the other two patients (P1 and P12) (Fig. 5b). Again, the recovered NK-stimulated IFN-γ production was only partial and did not approach the levels observed in healthy controls (range rhGH + HAART group: IL-2 + IL-12, 2·2–6·8%; IL-15 + IL-12 1·6–9·9%, range control group: IL-2 + IL-12, 3·7–25·9%; IL-15 + IL-12, 12·3–26·7%).
DISCUSSION
Our results indicate that innate natural killer cell immune function remains compromised even in HAART-treated HIV-1+ individuals where overall NK cell numbers are recovered. In particular, NK cell CD69 expression and IFN-γ production in response to both IL-2 and IL-15 is compromised in individuals receiving HAART alone. Our observation that CD56hiCD16– cells are substantially depleted in HIV-1+ patients receiving HAART with no detectable viral RNA when compared to healthy controls is consistent with this finding. These cells both contain a higher proportion of IFN-γ producers and secrete larger amounts of IFN-γ on a per cell basis in response to IL-15 + IL-12 [5].
The inability of HAART to induce a recovery in NK cell IFN-γ production in infected individuals may relate to the effects of HIV-1 on the development, homing patterns and/or the available cytokine-mediated signalling pathways in different NK cell subsets.
CD56hiCD16– NK cells express CCR7 exclusively and migrate to MIP-3β and SLC, whereas CD56loCD16+ NK cells express CXCR1 and CX3CR1 exclusively and migrate predominantly to fractalkine and IL-8 [29]. This pattern of chemokine responsiveness and, additionally, the expression of l-selectin on CD56hiCD16– cells, is consistent with a preferential migration of these cells to secondary lymphoid organs [29]. Indeed, Fehniger and co-workers have shown recently that the CD56hiCD16– migrate preferentially to the T cell areas of peripheral lymph nodes and require T cell derived factors for their activation [30]. Persistent infection with HIV-1 occurs in secondary lymphoid tissues, even during the latent phase of the infection [31–33]. It is therefore likely that HIV-1 would have selective detrimental effects on the migrated CD56hiCD16– subset either prior to or even following the initiation of HAART. Furthermore, CD56hiCD16– cells preferentially express both CCR5 and CXCR4 and, although there is little evidence for productive infection of this subset, expression of such molecules may lead to at least some interaction of HIV-1 at the cell surface [29]. Such interactions could conceivably play a role in the retention of the CD56hi subset in lymphoid tissue and render these cells susceptible to bystander apoptosis as has been shown for uninfected CD4+ T cells. Recently Valentin et al. have demonstrated HIV-1 infection of a rare CD4+CD56+CD3– cell population in human peripheral blood [34]. HIV-1 infection could lead ultimately to the depletion of such a cell population. However, the relationship between this population and CD56hiCD16– or CD56loCD16+ subsets or IFN-γ production by NK cells has not yet been established. Indeed, in a range of healthy donors this population accounted for an estimated 0·3 and 6·5% of peripheral blood NK cells [34]. This would therefore not seem to correlate either with the proportions of CD56hiCD16negativeCD3– cells (range 3·0–17%) or IFN-γ producing NK cells (ranges IL-2 + IL-12: 3·7–25·9%; IL-15 + IL-12: 12·3–26·7%) observed here in healthy controls and is therefore unlikely to account for the loss of these populations in HAART-treated individuals.
Evidence is emerging that IL-15 and IL-12 are important at different stages of NK cell development. IL-15 drives the maturation of NK precursors into mature NK cells and IL-12 promotes mature NK cell differentiation into IFN-γ-producing cells. Both CD56hi and CD56lo cells express IL-15Rβ/γc complexes and the expression of IL-12Rα is induced on human NK cells [35]. CD56hi cells additionally express the IL-2Rα chain [35]. We have shown that NK cell IFN-γ production is affected similarly, whether IL-2 or IL-15 are used as a co-stimulus along with IL-12. Furthermore, our results demonstrating reduction of NK cell CD69 induction, which can occur in the absence of IL-12, would imply that the IL-15/IL-2 components of the stimulus may be as affected as the IL-12 component, which is required for NK cell differentiation into IFN-γ-producing cells [21,36]. Indeed, the IL-2, IL-15 and IL-12 pathways of events can all be compromised severely during HIV-1 infection, including impaired IL-2, IL-12 and IL-15 production [17,37–39] and a failure of PBMC to respond to IL-12 [40]. Studies on cytokine receptor expression and signalling in NK cells and their subsets during HAART will shed more light on the importance of different pathways.
Recent work has, however, additionally shown a reduced in-vivo activation of CD4+ T cells in aviraemic individuals receiving PI-based HAART compared to PI-sparing regimens [41]. It will also therefore be important to consider whether NK cells are similarly affected, either directly or indirectly, and the effects of different antiretroviral drugs on cytokine signalling pathways investigated.
In the majority of patients receiving rhGH and HAART we observe a further increase in the proportion and absolute number of CD3–CD56+ NK cells in the peripheral blood compared to baseline HAART only. However, we have observed that the distribution of KIR/NKG2A receptors and the relative proportions of the CD56hiCD16– and CD56loCD16hi cells remained unaffected by rhGH treatment (data not shown), implying that the observed recovery in NK cell activation does not relate to an altered distribution of NK subsets compared to HAART alone.
We observed that the low-level unstimulated CD69 expression seen in control subjects is diminished in HIV-1+ patients receiving HAART. Basal CD69 expression was found exclusively on CD56lowCD16+ cells in all individuals tested. Interestingly, a substantial recovery of basal CD69 expression on CD56lowCD16+ cells was observed in NK cells of individuals receiving rhGH + HAART, suggesting that hGH affects this population preferentially. Cross-linking of CD16 (FcγRIII) provides partial signals for NK cell activation and cytokine/chemokine secretion [6]. We have observed these effects both in cells cultured in human serum and in fetal calf serum (FCS), implying that if the observed activation is Fc-mediated it may involve prebound serum Ig (data not shown). It is therefore possible that rhGH augments the generation of NK cells susceptible to Fc-mediated activation.
A partial recovery in NK cell IFN-γ secretion was observed in individuals receiving rhGH and where NK cell numbers had increased over baseline. It is conceivable that GH could effect the differentiation and proliferation of NK cell progenitors or precursors in the bone marrow or thymus. hGH-deficient individuals have significantly diminished numbers of NK cells in the peripheral blood, although short-term rhGH therapy alone did not appear to be effective at driving NK cell repopulation and functional recovery in these individuals [42,43]. There is evidence that supplementary rhGH can enhance NK activity in healthy adults with normal GH secretion [28]. Furthermore, the GH-intermediate IGF-1 enhances the in vitro function of natural killer cells from GH-deficient patients [27]. In a previous study no overall differences were observed in NK activity (K562 cell killing) before and after growth hormone treatment, despite some enhancement of HIV-1 envelope protein specific IL-2 production by T cells [44]. It is conceivable that rhGH has preferential effects on cytokine signalling pathways in HIV-1+ individuals. Indeed, GH and its intermediates utilize signalling elements and transcription factors common to cytokine-mediated pathways and in this way could, potentially, affect NK cell differentiation into cytokine producing cells [22,23].
Further studies on NK cell redistribution and the potential deficiencies in NK cytokine pathways, particularly those driven by IL-12, will help to identify the causes of persistent defects in HAART-treated HIV-1+ individuals. Extended investigations are being carried out into the effects of immune-based therapies on NK cells in HIV-1 infection, which will support the development of additional therapies to target NK cell defects.
Acknowledgments
This work was supported by the Wellcome Trust: awards 058700 and 059437.
REFERENCES
- 1.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148:2429–36. [PubMed] [Google Scholar]
- 3.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 5.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–20. [PubMed] [Google Scholar]
- 6.Oliva A, Kinter AL, Vaccarezza M, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–31. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obata-Onai A, Hashimoto S, Onai N, et al. Comprehensive gene expression analysis of human NK cells and CD8 (+) T lymphocytes. Int Immunol. 2002;14:1085–98. doi: 10.1093/intimm/dxf086. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Yu W, He T, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 9.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 10.Hu PF, Hultin LE, Hultin P, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56- cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–40. [PubMed] [Google Scholar]
- 11.Lucia B, Jennings C, Cauda R, Ortona L, Landay AL. Evidence of a selective depletion of a CD16+ CD56+ CD8+ natural killer cell subset during HIV infection. Cytometry. 1995;22:10–5. doi: 10.1002/cyto.990220103. [DOI] [PubMed] [Google Scholar]
- 12.Plaeger-Marshall S, Spina CA, Giorgi JV, et al. Alterations in cytotoxic and phenotypic subsets of natural killer cells in acquired immune deficiency syndrome (AIDS) J Clin Immunol. 1987;7:16–23. doi: 10.1007/BF00915420. [DOI] [PubMed] [Google Scholar]
- 13.Mansour I, Doinel C, Rouger P. CD16+ NK cells decrease in all stages of HIV infection through a selective depletion of the CD16+CD8+CD3- subset. AIDS Res Hum Retroviruses. 1990;6:1451–7. doi: 10.1089/aid.1990.6.1451. [DOI] [PubMed] [Google Scholar]
- 14.Sirianni MC, Tagliaferri F, Aiuti F. Pathogenesis of the natural killer cell deficiency in AIDS. Immunol Today. 1990;11:81–2. doi: 10.1016/0167-5699(90)90032-5. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad R, Sindhu ST, Tran P, et al. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J Med Virol. 2001;65:431–40. [PubMed] [Google Scholar]
- 16.Andre P, Brunet C, Guia S, et al. Differential regulation of killer cell Ig-like receptors and CD94 lectin-like dimers on NK and T lymphocytes from HIV-1-infected individuals. Eur J Immunol. 1999;29:1076–85. doi: 10.1002/(SICI)1521-4141(199904)29:04<1076::AID-IMMU1076>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Chehimi J, Starr SE, Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber K, Meyer D, Grosse V, Stoll M, Schmidt RE, Heiken H. Reconstitution of NK cell activity in HIV-1 infected individuals receiving antiretroviral therapy. Immunobiology. 2000;202:172–8. doi: 10.1016/S0171-2985(00)80063-7. [DOI] [PubMed] [Google Scholar]
- 19.Sirianni MC, Ensoli F, Alario C, et al. Distribution of the natural killer-related receptor for HLA-C during highly active antiretroviral therapy for human immunodeficiency virus infection. Hum Immunol. 2001;62:1328–34. doi: 10.1016/s0198-8859(01)00355-x. [DOI] [PubMed] [Google Scholar]
- 20.Azzoni L, Papasavvas E, Chehimi J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–70. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 21.Loza MJ, Perussia B. Final steps of natural killer cell maturation: a model for type 1–type 2 differentiation? Nat Immunol. 2001;2:917–24. doi: 10.1038/ni1001-917. [DOI] [PubMed] [Google Scholar]
- 22.Murphy WJ, Rui H, Longo DL. Effects of growth hormone and prolactin immune development and function. Life Sci. 1995;57:1–14. doi: 10.1016/0024-3205(95)00237-z. [DOI] [PubMed] [Google Scholar]
- 23.Murphy WJ, Longo DL. Growth hormone as an immunomodulating therapeutic agent. Immunol Today. 2000 2002;21:211–3. doi: 10.1016/s0167-5699(00)01594-2. [DOI] [PubMed] [Google Scholar]
- 24.Pires T. Growth hormone enhances thymocyte development and induces differentiation into functional peripheral T cells in HAART treated HIV-1+ patients. 9th Conference on Retroviruses and Opportunistic Infections. Abstract 513-M.
- 25.Werfel T, Boeker M, Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–9. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 26.Borrego F, Robertson MJ, Ritz J, Pena J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97:159–65. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auernhammer CJ, Feldmeier H, Nass R, Pachmann K, Strasburger CJ. Insulin-like growth factor I is an independent coregulatory modulator of natural killer (NK) cell activity. Endocrinology. 1996;137:5332–6. doi: 10.1210/endo.137.12.8940354. [DOI] [PubMed] [Google Scholar]
- 28.Crist DM, Kraner JC. Supplemental growth hormone increases the tumor cytotoxic activity of natural killer cells in healthy adults with normal growth hormone secretion. Metabolism. 1990;39:1320–4. doi: 10.1016/0026-0495(90)90191-e. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JJ, Qin S, Unutmaz D, et al. Unique subpopulations of CD56+ NK and NK–T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 30.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 31.Embretson J, Zupancic M, Ribas JL, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 32.Nuovo GJ, Becker J, Burk MW, Margiotta M, Fuhrer J, Steigbigel RT. In situ detection of PCR-amplified HIV-1 nucleic acids in lymph nodes and peripheral blood in patients with asymptomatic HIV-1 infection and advanced-stage AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1994;7:916–23. [PubMed] [Google Scholar]
- 33.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 34.Valentin A, Rosati M, Patenaude DJ, et al. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2002;99:7015–20. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. [Google Scholar]
- 36.Loza MJ, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (interferon gamma) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood. 2002;99:1273–81. doi: 10.1182/blood.v99.4.1273. [DOI] [PubMed] [Google Scholar]
- 37.Kinter A, Fauci AS. Interleukin-2 and human immunodeficiency virus infection: pathogenic mechanisms and potential for immunologic enhancement. Immunol Res. 1996;15:1–15. doi: 10.1007/BF02918280. [DOI] [PubMed] [Google Scholar]
- 38.Zerhouni B, Sanhadji K, Kehrli L, Livrozet JM, Touraine JL. Interleukin (IL)-2 deficiency aggravates the defect of natural killer cell activity in AIDS patients. Thymus. 1997;24:147–56. [PubMed] [Google Scholar]
- 39.Ahmad R, Sindhu ST, Toma E, Morisset R, Ahmad A. Studies on the production of IL-15 in HIV-infected/AIDS patients. J Clin Immunol. 2003;23:81–90. doi: 10.1023/a:1022568626500. [DOI] [PubMed] [Google Scholar]
- 40.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–11. [PubMed] [Google Scholar]
- 41.Hunt P, J Martin D, Bangsberg A, et al. Protease inhibitor-containing HAART is associated with decreased in vivo T-cell activation independent of its effects on viral replication. Abstracts from the 10th Conference on Retroviruses and Opportunistic Infections, Boston. 2003:181. [Google Scholar]
- 42.Span JP, Pieters GF, Smals AG, Koopmans PP, Kloppenborg PW. Number and percentage of NK-cells are decreased in growth hormone-deficient adults. Clin Immunol Immunopathol. 1996;78:90–2. doi: 10.1006/clin.1996.0014. [DOI] [PubMed] [Google Scholar]
- 43.Kiess W, Malozowski S, Gelato M, et al. Lymphocyte subset distribution and natural killer activity in growth hormone deficiency before and during short-term treatment with growth hormone releasing hormone. Clin Immunol Immunopathol. 1988;48:85–94. doi: 10.1016/0090-1229(88)90159-6. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen BY, Clerici M, Venzon DJ, et al. Pilot study of the immunologic effects of recombinant human growth hormone and recombinant insulin-like growth factor in HIV-infected patients. AIDS. 1998;12:895–904. doi: 10.1097/00002030-199808000-00012. [DOI] [PubMed] [Google Scholar]