Abstract
Dendritic cells (DC) are the most professional antigen-presenting cells of the immune system and are capable of initiating immune responses in vitro and in vivo. One of the great challenges in immunotherapy protocols is to introduce relevant antigens into DC for stimulation of major histocompatibility complex (MHC) class I- and class II-restricted anti-tumour or anti-viral immunity. This review will focus on the development of mRNA-loaded DC-based immunotherapy vaccines. First, several published results concerning mRNA transfection efficiency in DC are compared. Next, an overview is given for several published studies describing CD8+ and CD4+ T-cell clone activation using RNA-loaded DC. These data show that RNA-loaded DC efficiently process and present antigenic epitopes. Next, published data from in vitro T-cell activation studies using RNA-loaded DC are summarized and provide evidence that RNA-loaded DC can efficiently stimulate in vitro primary and secondary immune responses. Finally, the summarized data provide evidence that RNA-loaded DC are a promising strategy for the development of future cancer vaccination strategies.
Keywords: dendritic cells, electroporation, immunotherapy, RNA, T cells
INTRODUCTION
Dendritic cells (DC) are part of a specialized network of immune mechanisms that can guard the body against cancer and viruses [1,2]. Within the immune system, DC have the privileged role of helping to decide whether the immune system should be activated and defend the host against unwanted invaders or aberrant cell growth. DC form part of the so-called family of antigen-presenting cells (APC), which also includes monocytes, macrophages and B cells. The in vivo origin of DC is still unclear, but they derive most probably from bone marrow haematopoietic cells, and reside in an immature state in many different parts of the body. The skin is one of the important first defence barriers of the body and comes into frequent contact with pathogens and cancer-inducing agents. A specialized DC system is present in the skin: it consists of two well-studied DC-types, Langerhans cells and dermal dendritic cells. They are believed to help protect the body by giving correct activation or inactivation signals to immune effector cells.
In peripheral tissues, immature DC are able to take up and process self-antigens, viral antigens or tumour-derived antigens for presentation via MHC class I and II proteins. Following migration from the site of antigen uptake towards the lymph nodes, DC are able, dependent on their maturation status, to induce activity in different kinds of T cells. Antigen-specific interferon (IFN)-γ producing type I T cells induced by mature type I DC (DC1) are involved mainly in the maintenance of a cellular cytotoxic immune response [1]. Antigen-specific and non-specific regulatory T cells (Treg), characterized by secretion of interleukin (IL)-10 or transforming growth factor (TGF)-β, are able to inhibit the effector function of type I T cells [3,4]. Antigen-specific IL-4- and IL-5-producing type II T cells induced by mature type II DC (DC2) are involved mainly in stimulation of a humoral immune response [5].
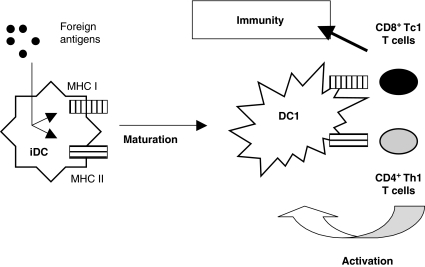
For the purpose of immunotherapy, we are interested mainly in the induction of a strong cellular cytotoxic immune response. During infection in vivo, upon release of danger signals, immature DC become immunity-inducing mature DC1 (Fig. 1). Foreign viral or bacterial antigens are taken up by immature DC in peripheral tissues, followed by presentation of these antigens via MHC class I and II proteins. The aggressive character of these infections is associated with release of viral (e.g. double-stranded RNA) or bacterial (e.g. lipopolysaccharide) products that induce DC maturation (for a review on danger signals, DC maturation and activation, see Van Tendeloo et al. [1]). Upon full maturation, type I DC are able to induce antigen-specific IFN-γ-producing CD4+ type I helper T cells (Th1). These activated T cells can activate mature DC1 via CD40 ligation. Activated mature DC1 will then be able to activate functional CD8+ cytotoxic T cells (Tc1), which will lead to cellular immunity.
Fig. 1.
Proposed model for the induction of immunity by type I dendritic cells (DC1).
DENDRITIC CELLS FOR IMMUNOTHERAPY
If DC can induce immune responses to viral proteins, then what is the problem with inducing immune responses to cancer, where ‘foreign’ proteins are often expressed? In the early stages of cancer development, disease development is less aggressive than in viral infections, implying that the immune system does not recognize early cancer development as life-threatening. Indeed, it is unlikely that the immune system would see an increased growth rate of some cells as cancer development because many normal processes, for example wound healing, are also characterized by increased cell growth rate. Although DC will come into contact with cancer cells and take up and process their tumorigenic antigens, an efficient induction of an immune response seems to be lacking. Because of the absence of danger signals, compared to lipopolysaccharide or RNA fragments during, respectively, bacterial and viral infections, DC do not become activated to a fully mature state and fail to induce a strong immune response. Moreover, if it is correct that immature DC in the steady state are able to induce tolerance to self-proteins [6], it is very likely that tolerance can and will be induced to tumour-specific proteins. Thus, in addition to the efforts to stimulate and expand tumour-specific T cells, bypassing possible immune tolerance mechanisms will be a second major concern in the development of immune-based cancer vaccines.
Currently, in vitro strategies have been developed to culture DC for use in cancer vaccines. DC can be cultured in vitro starting from bone marrow haematopoietic progenitor cells [7,8], but preference is likely to be given to the differentiation of DC starting from peripheral blood monocytes [9]. These monocyte-derived DC (Mo-DC) can be obtained easily in relatively large numbers from patients. If in vitro cultured DC are properly loaded with tumour antigens and supplied with the necessary danger or activation signals, they are believed to be able to break T cell tolerance to a tumour and to induce a functional cytotoxic T cell immune response upon readministration to cancer patients. Different strategies have been developed for loading DC with tumour or viral antigens [10]. Direct use of previously characterized antigenic peptides is a very efficient loading strategy [11], but is restricted to a limited amount of human leucocyte antigen (HLA) type-restricted epitopes. Alternatively, DC can be pulsed with recombinant proteins to induce immune responses to all possibly derived immunogenic epitopes [12]. By using these methods, immune response will be generated to selected proteins. To obtain full spectrum tumour antigen-loaded DC, isolated native peptides [13], tumour lysate-pulsed DC [14] or DC-tumour hybrids [15] have been used. However, the latter methods are highly dependent on the amount of tumour tissue available. To overcome this restriction, many non-viral and viral gene transfer technologies have been developed for DC [16]. While viral vectors have high transduction efficiency [17], plasmid DNA transfer into DC has not been very efficient [18]. With regard to clinical applicability, novel antigen-loading strategies have been developed based on transfection of defined and full-spectrum tumour mRNA into DC, in order to obtain immune responses against characterized and non-characterized immunogenic targets. Thus far, our own data and data from an expanding group of RNA-using DC researchers provide functional in vitro and in vivo evidence for future mRNA-loaded DC-based cancer vaccines.
RNA LOADING OF DENDRITIC CELLS
The first reports using mRNA to load DC with tumour antigens were from Gilboa and colleagues. Murine and human DC, passively pulsed or lipofected with defined or total tumour mRNA, were able to induce antigen-specific immune responses [19–21]. Although lipofection of mRNA coding for the enhanced green fluorescent protein (EGFP) resulted in detectable EGFP expression by flow cytometric analysis, passive pulsing did not result in any detectable EGFP protein [22,23]. In general, RNA was described as being very unstable and difficult to handle, and pulsing of mRNA was considered to be insufficient to induce high protein expression levels in transfected cells compared to viral transduction technologies. All these points can now be addressed, because novel technologies have been developed that make mRNA transfection a highly efficient alternative to DNA transfection and very competitive with viral transduction techniques.
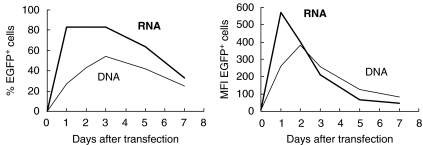
Initially, we optimized electroporation of mRNA on K562 haematopoietic cells [23]. Comparison of EGFP RNA and EGFP DNA electroporation in K562 cells showed, surprisingly, that mRNA electroporation was more efficient than DNA electroporation as measured by the number of transfected cells. Moreover, the level and duration of EGFP expression after mRNA electroporation was comparable with the level and duration of EGFP expression after DNA electroporation, as measured by flow cytometric analysis (Fig. 2). The same mRNA electroporation technique, after full optimization, resulted in high level of protein (EGFP) expression in progenitor- and monocyte-derived DC [23,24]. In general, more than 80% of viable monocyte-derived DC showed high-level EGFP expression after electroporation, while electroporation-related cell mortality on total DC cultures was lower than 10%. These results in gene transfer efficiency and viability after electroporation of mRNA were strikingly different compared to DNA electroporation in DC [18], where transfection efficiency was only 2% with electroporation-related mortality up to 60%, and can be explained by the electrical settings used for electroporation. Plasmid DNA has to reach the nucleus in order to be expressed properly. Because DC are non-dividing cells, DNA has to be brought directly into the nucleus, which implies that a strong electrical current is needed in order to allow the DNA to pass two membranes. However, RNA has only to reach the cytoplasm, which implies that only the cell membrane has to be passed. RNA electroporation thus results in high efficiency transfection, less cytotoxicity and rapid expression of protein.
Fig. 2.
Comparison of EGFP mRNA electroporation and DNA electroporation in K562 cells. K562 cells were electroporated at 300 V and 150 µF in a 4-mm standard electroporation cuvette with 20 µg EGFP mRNA or 20 µg EGFP DNA. In a time-course, the percentage of EGFP-positive cells was followed flowcytometrically by percentage (left side), as well as mean fluorescence intensity (MFI) of EGFP positive cells (right side).
While we have been using an exponential decay pulse during electroporation, Sæbøe-Larssen et al. describe an optimized mRNA electroporation technique using square-wave electroporation with similar results based on EGFP expression after transfection [25]. Kalady et al. also compared passive pulsing, lipofection and electroporation of EGFP mRNA, and concluded that only mRNA electroporation resulted in high-level protein expression in DC [26]. Lundqvist et al. performed an extensive side-by-side comparison of non-viral and viral gene transfer in DC [27]. Although viral gene-transfer resulted in slightly more transfected cells with higher levels of protein expression, results obtained by mRNA electroporation were shown to be a valuable alternative. Previously, all experiments using mRNA electroporation were performed on human DC. Van Meirvenne et al. used our previously described mRNA electroporation technique to transfect murine bone marrow-derived DC [28]. Their data showed high transfection efficiency and high-level EGFP expression in murine DC, similar to our data on human DC.
In summary, Table 1 provides an overview of the results presented in different transfection studies. Taking all these data together, we can conclude that mRNA electroporation is a valuable alternative to viral transduction of DC. Moreover, compared to viral transduction, which implies a more complex and labourious manipulation associated with safety issues, mRNA electroporation is an easy, low-cost and clinically applicable technology.
Table 1.
Efficiency of EGFP mRNA lipofection and electroporation in dendritic cells: comparison of different studies
| Lipofection | Electroporation | ||||
|---|---|---|---|---|---|
| Study | DC type | A | B | A | B |
| Strobel et al. [19] | iMo-DC | 16%n.s. | 7·6%n.s. | ||
| Van Tendeloo et al. [20] | iMo-DC | 7·5% | 9% | 63% | 78% |
| mMo-DC | 4% | 5% | 33% | 41% | |
| 34-LC | 0% | 0% | 50% | 62% | |
| 34-DC | 0% | 0% | 73% | 85% | |
| Saebφe-Larssen et al. [22] | iMo-DC | – | n.d. | 95% | |
| Kalady et al. [23] | iMo-DC | n.d. | 12% | n.d. | 50% |
| Lundqvist et al. [24] | iMo-DC | 4%n.s. | 60%n.s. | ||
| 34-DC | – | 40%n.s. | |||
| Ponsaerts et al. [21] | iMo-DC | – | 73% | 85% | |
| Van Tendeloo et al. [25] | BM-DC | – | 70%n.s. | ||
Efficiencies are given as the number of EGFP-positive cells measured by flow cytometry. DC type: iMo-DC, human immature monocytes-derived dendritic cells; mMo-DC, human mature monocytes-derived dendritic cells; 34-DC, human CD34 progenitor-derived Langerhans cells; 34-DC, human CD34 progenitor-derived dendritic cells; BM-DC, murine bone-marrow derived dendritic cells. A: Transfection efficiency on total DC population (including dead cells). B: Transfection efficiency on viable DC population. n.d.: no data. n.s.: not specified A or B.
ANTIGEN PRESENTATION BY RNA-LOADED DENDRITIC CELLS
Although flow cytometric analysis of EGFP expression after mRNA electroporation showed high levels of transgene expression, it was unclear whether RNA-loaded DC are able to process and present antigenic peptides in association with major histocompatibility complex (MHC) class I and class II proteins.
Proteasomal degradation of cellular proteins and antigen presentation in association with MHC class I proteins is a process which takes place in virtually all nucleated cells, including cancer cells. In this way, cells present a repertoire of peptides derived from all intracellular proteins. By showing a whole spectrum of different antigenic peptides, tumour cells can be distinguished from normal cells by the immune system because of the presentation of antigenic epitopes derived from tumour-specific proteins. To activate cytotoxic T cells using DC it is important that antigenic epitopes, derived from the introduced proteins, are processed and presented in association with MHC class I proteins. The use of established CD8+ CTL-clones, which produce IFN-γ upon recognition of a correctly presented MHC-matched epitope, is an elegant method to show functional antigen presentation following antigen loading in DC. Strobel et al. showed that DC, loaded with influenza matrix protein mRNA by means of lipofection, were able to activate specifically an influenza matrix protein M1 peptide-specific CD8+ T cell clone [22]. Moreover, mRNA-loaded DC were more efficient than DNA-loaded DC to process and present this antigenic epitope correctly.
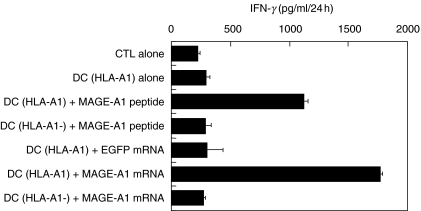
Our own data on presentation of a melanoma Melan-A CD8+ CTL epitope following Melan-A mRNA-loading of DC showed that antigen presentation was more efficient after mRNA electroporation compared to mRNA lipofection, correlating with the respective transfection efficiencies of the transfection techniques used [23]. Passive pulsing of Melan-A mRNA did not result in activation of the CTL-clone. These Melan-A data have been confirmed recently in similar experiments by Kalady et al. [26]. Antigenic epitope presentation after electroporation of DC with melanoma MAGE-A1 mRNA was also very efficient (Fig. 3). Our data show that a HLA-A1-restricted MAGE-A1-specific CD8+ CTL clone produced IFN-γ only upon stimulation with HLA-matched MAGE-A1 peptide-pulsed or MAGE-A1 mRNA-electroporated DC. No aspecific stimulation was seen after co-culture with control EGFP mRNA-electroporated DC. Antigen-presentation was also restricted to HLA-A1, as HLA-A1 negative mRNA-electroporated DC did not activate the T cell clone.
Fig. 3.
Antigen presentation by MAGE-A1 mRNA-electroporated dendritic cells. HLA-A1-positive immature Mo-DC were cultured with GM-CSF and IL-4 and transfected at day 6 of culture by electroporation with MAGE-A1 mRNA or with EGFP mRNA. HLA-A1-positive Mo-DC pulsed with a MAGE-A1 peptide, and HLA-A1-negative Mo-DC electroporated with MAGE-A1 mRNA or pulsed with MAGE-A1 peptide served as controls. Antigen-presenting cells (indicated on the left of the graph) were co-incubated with a HLA-A1-restricted MAGE-A1 specific CD8+ CTL clone to determine antigen loading eficiency, as reflected by IFN-γ production of the CTL clone. Results are shown as mean ± s.d.
For efficient activation and maintenance of a primary CD8+ T cell response, several reports show that it is also needed to activate an antigen-specific CD4+ helper T cell response [29,30]. Activation of antigen-specific CD4+ helper T cells can be achieved only by antigen-presenting cells. These cells are able to process and present antigenic peptides derived from exogenous proteins in association with MHC class II proteins, in order to stimulate CD4+ T cells. Van Meirvenne et al. have performed an interesting study on processing and presentation of ovalbumin CD8+ and CD4+ T cell epitopes in murine DC electroporated with mRNA [28]. Their data provide evidence that the use of chimeric RNA constructs, which encode an antigen plus an endosomal targeting sequence derived from the invariant chain protein (Ii), result in efficient processing and presentation of CD8+ and CD4+ T cell epitopes after mRNA electroporation of DC. Su et al. have reported similar data using a chimeric RNA construct encoding telomerase and the endosomal/lysosomal sorting signal of the lysosome-associated membrane protein (LAMP-1) [31]. Their data show that human DC transfected with this RNA construct stimulated CD4+ T cells more efficiently compared to RNA constructs without a targeting signal.
Summarizing all these reports (Table 2) leads to the conclusion that DC, loaded with specially designed RNA constructs, are able to present antigenic epitopes in the context of class I and class II MHC proteins for the efficient stimulation of both a CD4+ and a CD8+ T cell response.
Table 2.
Efficiency of T cell clone activation using mRNA-transfected dendritic cells: comparison of different studies
| Transfection | |||||||
|---|---|---|---|---|---|---|---|
| Study | DC type | Antigen | Clone | Analysis | PP | LIPO | EP |
| Strobel et al. [19] | iMo-DC | IMP | CD8+ | IFN-γ | n.d. | + | n.d. |
| Van Tendeloo et al. [20] | iMoDC | Melan-A | CD8+ | IFN-γ | – | + | + + + |
| mMo-DC | Melan-A | CD8+ | IFN-γ | – | + | + + | |
| 34-LC | Melan-A | CD8+ | IFN-γ | – | – | + | |
| 34-DC | Melan-A | CD8+ | IFN-γ | – | – | + | |
| Ponsaerts et al. this article | iMo-DC | MAGE-A1 | CD8+ | IFN-γ | n.d. | n.d. | + |
| Kalady et al. [23] | iMo-DC | Melan-A | CD8+ | IFN-γ | – | – | + |
| Su et al.[28] | iMo-DC | hTERT | CD8+ | Lysis | n.d. | + | n.d. |
| iMo-DC | hTERT | CD4+ | IFN-γ | + | + + | n.d. | |
| Van Meirvenne et al. [25] | BM-DC | OVA | CD8+ | CPM | – | + | + + + |
| BM-DC | OVA | CD4+ | CPM | – | + | + + + | |
Efficiencies are given as – (no activation) or + (activation). Within experiments, the level of activation is shown by multiple + signs. DC type: iMo-DC, human immature monocytes-derived dendritic cells; mMo-DC, human mature monocytes-derived dendritic cells; 34-DC, human CD34 progenitor-derived Langerhans cells; 34-DC, human CD34 progenitor-derived dendritic cells; BM-DC, murine bone-marrow derived dendritic cells. Antigen: IMP, influenza matrix protein; Melan-A, melanoma antigen; MAGE-A1, melanoma antigen; hTERT, telomerase; OVA, ovalbumin. Analysis: IFN-γ, IFN-γ secretion measured by ELISA or ELISPOT; lysis, lysis assay; CPM, cell proliferation. Transfection: PP, passive pulsing; LIPO, lipofection, EP, electroporation. n.d.: no data.
IN VITRO HUMAN T CELL ACTIVATION USING RNA-LOADED DENDRITIC CELLS
The use of mRNA for antigen loading of human DC has already led to several successes in in vitro T cell activation experiments (Table 3). Induction of carcinoembryonic antigen (CEA)-specific T cells using CEA mRNA-pulsed or -lipofected DC was shown by Nair et al. [21]. In this study, using a chimeric CEA/LAMP-1 RNA construct, both CD4+ and CD8+ CEA-specific T cells were activated. Lipofection of DC with mRNA encoding the human papillomavirus E6 and E7 oncoproteins has been shown by Thornburg et al. to be an efficient strategy for induction of a cellular immune response against E6 and E7 oncogenic antigens [32]. Heiser et al. showed that tolerance to certain tumour antigens, which are also self-proteins, is not absolute and can be reversed by effective stimulation. Stimulation of peripheral blood mononuclear cells (PBMC) with DC passively pulsed with mRNA encoding prostate-specific antigen (PSA) has been reported as an efficient strategy for immunotherapy against prostate cancer [33]. As described above, Su et al. showed the in vitro induction of telomerase-specific T cells [31]. While the antigens described above are cancer-associated targets, Weissman et al. have reported the use of gag mRNA-lipofected DC for induction of a specific immunotherapy against HIV [34]. In conclusion, these data support the use of mRNA-loaded DC for immunotherapy.
Table 3.
T cell activation using RNA-transfected dendritic cells
| Study | Type of RNA | Transfection | Activation |
|---|---|---|---|
| Nair et al. [18] | CEA RNA | PP/LIPO | CD8+/CD4+ |
| Thornburg et al. [29] | HPV E6, E7 RNA | LIPO | CD8+ |
| Heiser et al. [30] | PSA RNA | PP | CD8+ |
| Weissman et al.[31] | HIV gag RNA | LIPO | CD8+/CD4+ |
| Su et al. [28] | TERT RNA | LIPO | CD8+/CD4+ |
| Strobel et al. [19] | IMP RNA | LIPO | CD8+ |
| Ponsaerts et al. [21] | IMP RNA | EP | CD8+ |
| Ponsaerts et al. [32] | IMP RNA | EP | CD8+ |
| Saebφe-Larssen et al. [22] | TERT RNA | EP | CD8+ |
| Kalady et al. [23] | Melan-A RNA | EP | CD8+ |
| Heiser et al.[33] | Total renal tumour RNA | PP | CD8+ |
| Heiser et al. [35] | Prostate tumour RNA (Ampl.) | PP | CD8+ |
| Milazzo et al. [36] | Total myeloma RNA | EP | CD8+ |
Type of RNA: CEA, carcino-embryonic antigen; HPV, human papillomavirus; PSA, prostate specific antigen; HIV, human immunodeficiency virus; TERT, telomerase; IMP, influenza matrix protein; Melan-A, melanoma antigen; Ampl., amplified. Transfection: PP, passive pulsing; LIPO, lipofection, EP, electroporation.
Although electroporation of DC with mRNA encoding defined antigens has been introduced only recently [23], several data have already shown their T cell stimulatory capacity. We have compared influenza matrix protein mRNA-electroporated DC, either cultured conventionally for 6–7 days using granulocyte macrophage colony stimulating factor (GM-CSF) + interleukin (IL)-4 in serum containing medium [24] or cultured serum-free for 2 days with GM-CSF only [35], and concluded that both types of mRNA-electroporated DC showed similar autologous stimulatory capacity. Currently we are exploring immunotherapeutic strategies using DC electroporated with defined mRNA against leukaemia, cervical cancer and HIV. Stimulation of cytotoxic T cell responses using mRNA-electroporated DC have also been shown to telomerase by Sæbøe-Larssen et al. [25], and to Melan-A by Kalady et al. [26].
As explained previously, one of the goals for the use of RNA-loaded DC is to use total tumour-derived mRNA. By so doing, the induction of immune responses will not be limited to one single antigen, but a whole spectrum of tumour- and patient-specific antigens will be available. Heiser et al. have shown that human DC, passively pulsed with mRNA derived from renal tumours, were able to activate renal tumour-specific cytotoxic T cells [36]. However, RNA isolated from tumour cells is not unlimited. Therefore, molecular biology technologies for amplification of tumour cell mRNA have been developed by Boczkowski et al. [37]. Using this technology, Heiser et al. showed that DC transfected with amplified tumour RNA from prostate cancer cells were able to induce a polyclonal prostate cancer-specific T cell response [38]. Also, a recent report from Milazzo et al. showed the induction of myeloma specific cytotoxic T cells using DC electroporated with tumour-derived mRNA [39].
IN VIVO HUMAN T CELL ACTIVATION USING RNA-LOADED DENDRITIC CELLS
The use of mRNA-loaded DC has been very successful in in vitro experiments. This strategy has now been translated into several clinical trials. Published results from Heiser et al. have shown that vaccination with autologous DC passively pulsed with PSA mRNA could stimulate CTL responses against metastatic prostate tumours in patients with prostate cancer [40]. Nair et al. showed that vaccination with DC transfected with total tumour-derived mRNA could stimulate a tumour-specific immune response in a patient with a CEA-expressing adenocarcinoma [41]. Several other clinical trials using RNA-loaded DC are still ongoing, and will hopefully pave the way for improved DC therapy of cancer in the future.
CONCLUSIONS
The results from the clinical trials presented support the use of mRNA-loaded DC as a promising strategy for the development of future cancer vaccination strategies. Novel technologies, which have been developed for the amplification, transfection and processing of mRNA, can now be considered for implementation in future clinical trials.
Acknowledgments
This work was supported by grant no. G.0313·01 of the Fund for Scientific Research, Flanders, Belgium (FWO-Vlaanderen) and by grants of the Scientific Committee of the Fortis Bank (FB) Verzekeringen-financed Cancer Research. P. P. holds a PhD fellowship from the Flemish Institute for Science and Technology (IWT). V. F. I. V. T. is a postdoctoral fellow of the FWO-Vlaanderen. We thank Dr Pierre Van der Bruggen (Ludwig Institute for Cancer Research, Brussels, UCL, Belgium) for help with MAGE-A1 experiments.
REFERENCES
- 1.Van Tendeloo VFI, Van Broeckhoven C, Berneman ZN. Gene-based cancer vaccines: an ex vivo approach. Leukemia. 2001;15:545–58. doi: 10.1038/sj.leu.2402069. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8 (+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 4.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25 (+) regulatory T cells convey suppressor activity to conventional CD4 (+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czerniecki BJ, Cohen PA, Faries M, Xu S, Roros JG, Bedrosian I. Diverse functional activity of CD83+ monocyte-derived dendritic cells and implications for cancer vaccines. Crit Rev Immunol. 2001;21:157–78. [PubMed] [Google Scholar]
- 6.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lardon F, Snoeck HW, Berneman ZN, et al. Generation of dendritic cells from bone marrow progenitors using GM-CSF, TNF-α, and additional cytokines: antagonistic effects of IL-4 and IFN-γ and selective involvement of TNF-α receptor-1. Immunology. 1997;91:553–9. doi: 10.1046/j.1365-2567.1997.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst B, Köhler G, Mackensen A, et al. In vitro differentiation of CD34+ hematopoietic progenitor cells toward distinct dendritic cell subsets of the Birbeck granule and MIIC-positive Langerhans cell and the interdigitating dendritic cell type. Blood. 1996;88:2541–8. [PubMed] [Google Scholar]
- 9.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for induction of antitumor immunity. J Immunother. 2002;25:289–303. doi: 10.1097/00002371-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Thurner B, Haendle I, Röder C, et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;11:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santin AD, Hermonat PL, Ravaggi A, et al. Induction of human papillomavirus-specific CD4 (+) and CD8 (+) lymphocytes by E7-pulsed autologous dendritic cells in patients with human papillomavirus type 16-and 18-positive cervical cancer. J Virol. 1999;3:5402–10. doi: 10.1128/jvi.73.7.5402-5410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr W, Ranieri E, Olson W, Zarour H, Gesualdo L, Storkus WJ. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4 (+) and CD8 (+) T lymphocyte responses. Blood. 2000;96:1857–64. [PubMed] [Google Scholar]
- 14.Schott M, Feldkamp J, Schattenberg D, et al. Induction of cellular immunity in a parathyroid carcinoma treated with tumor lysate-pulsed dendritic cells. Eur J Endocrin. 2000;142:300–6. doi: 10.1530/eje.0.1420300. [DOI] [PubMed] [Google Scholar]
- 15.Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–6. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 16.Kirk CJ, Mulé JJ. Gene-modified dendritic cells for use in tumor vaccines. Hum Gene Ther. 2000;11:797–806. doi: 10.1089/10430340050015419. [DOI] [PubMed] [Google Scholar]
- 17.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102–7. doi: 10.1016/s1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 18.Van Tendeloo VFI, Snoeck HW, Lardon F, et al. Nonviral transfection of distinct types of human dendritic cells: high-efficiency gene transfer by electroporation into hematopoietic progenitor- but not monocyte-derived dendritic cells. Gene Ther. 1998;5:700–7. doi: 10.1038/sj.gt.3300626. [DOI] [PubMed] [Google Scholar]
- 19.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–82. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 22.Strobel I, Berchtold S, Gotze A, Schulze U, Schuler G, Steinkasserer A. Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther. 2000;7:2028–35. doi: 10.1038/sj.gt.3301326. [DOI] [PubMed] [Google Scholar]
- 23.Van Tendeloo V, Ponsaerts P, Lardon F, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells. Superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Ponsaerts P, Van Tendeloo VFI, Cools N, et al. mRNA-electroporated mature dendritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreservation. Leukemia. 2002;16:1324–30. doi: 10.1038/sj.leu.2402511. [DOI] [PubMed] [Google Scholar]
- 25.Sæbøe-Larssen S, Fossberg E, Gaudernack G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT) J Immunol Meth. 2002;259:191–203. doi: 10.1016/s0022-1759(01)00506-3. [DOI] [PubMed] [Google Scholar]
- 26.Kalady MF, Onaitis MW, Padilla KM, Emani S, Tylor DS, Pruitt SK. Enhanced dendritic cell antigen presentation in RNA-based immunotherapy. J Surg Res. 2002;105:17–24. doi: 10.1006/jsre.2002.6435. [DOI] [PubMed] [Google Scholar]
- 27.Lundqvist A, Noffz G, Pavlenko M, et al. Nonviral and viral gene transfer into different subsets of human dendritic cells yield comparable efficiency of transfection. J Immunother. 2002;25:445–54. doi: 10.1097/00002371-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Van Meirvenne S, Straetman L, Heirman C, et al. Efficient genetic modifications of murine dendritic cells by electroporation with mRNA. Cancer Gene Ther. 2002;9:787–97. doi: 10.1038/sj.cgt.7700499. [DOI] [PubMed] [Google Scholar]
- 29.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T helper and a T killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 30.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 31.Su Z, Vieweg J, Weizer AZ, et al. Enhanced induction of telomerase-specific CD4+ T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–8. [PubMed] [Google Scholar]
- 32.Thornburg C, Boczkowski D, Gilboa E, Nair SK. Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus E6 and E7 RNA: implications for cervival cancer immunotherapy. J Immunother. 2000;23:412–8. doi: 10.1097/00002371-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Heiser H, Dahm P, Yancey DR, et al. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol. 2000;164:5508–14. doi: 10.4049/jimmunol.164.10.5508. [DOI] [PubMed] [Google Scholar]
- 34.Weissman D, Ni HP, Scales D, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol. 2000;165:4710–7. doi: 10.4049/jimmunol.165.8.4710. [DOI] [PubMed] [Google Scholar]
- 35.Ponsaerts P, Van den Bosch G, Cools N, et al. Messenger RNA electroporation of human monocytes, followed by rapid in vitro differentiation, leads to highly stimulatory antigen-loaded mature dendritic cells. J Immunol. 2002;169:1669–75. doi: 10.4049/jimmunol.169.4.1669. [DOI] [PubMed] [Google Scholar]
- 36.Heiser A, Maurice MA, Yancey DR, Coleman DM, Dahm P, Vieweg J. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T cell responses against antigens expressed by primary and metastatic tumors. Cancer Res. 2001;61:3388–93. [PubMed] [Google Scholar]
- 37.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–34. [PubMed] [Google Scholar]
- 38.Heiser A, Maurice MA, Yancey DR, et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J Immunol. 2001;166:2953–60. doi: 10.4049/jimmunol.166.5.2953. [DOI] [PubMed] [Google Scholar]
- 39.Milazzo C, Reichardt VL, Müller MR, Grünebach F, Brossart P. Induction of myeloma specific cytotoxic T cells using dendritic cells transfected with tumor-derived RNA. Blood. 2002;101:977–82. doi: 10.1182/blood-2002-04-1273. [DOI] [PubMed] [Google Scholar]
- 40.Heiser A, Coleman DM, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2001;109:409–17. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair SK, Morse M, Boczkowski D, et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–9. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]