Abstract
Human neonates are immunologically immature and consequently are highly susceptible to infection. The cellular basis for the dysfunctional immune responses of neonates is not clear, but is likely to reflect the immaturity of both B and T cell populations. Here we have examined the ability of human cord blood B cells to respond to antigen receptor cross-linking and also to CpG containing oligodeoxynucleotides (ODN), and compared their responses with those of adult peripheral blood B cells. Antigen receptor cross-linking with soluble F(ab′)2 anti-IgM antibodies, induced HLA-DR and CD86 up-regulation and proliferation to a similar extent in adult and cord blood B cells. Both interleukin (IL)-2 and IL-4 co-stimulated anti-IgM-induced proliferation, but cord blood B cells were less sensitive than adult B cells to the co-stimulatory effects of IL-2. Antigen receptor cross-linking induced secretion of the chemokines macrophage inflammatory protein-1α (MIP-1α) and MIP-1β in adult and cord blood B cells, and secretion was enhanced by IL-2 or IL-4. CpG-ODN induced up-regulation of HLA-DR and CD86 expression and proliferation of adult and cord blood B cells, and anti-IgM and CPG-ODN synergized in the induction of proliferation. CpG-ODN also induced MIP-1α and MIP-1α secretion in adult and cord blood B cells. In addition to functional studies we examined the expression of CD62L (l-selectin), CCR7 and CXCR5. Our data show that surface expression of CD62L and CCR7 is lower on cord blood B cells than on adult B cells, suggesting that human cord blood B cells may exhibit homing defects.
Keywords: B cell, BCR, CpG, MIP-1α/β, neonatal
INTRODUCTION
The immaturity of the developing immune system is thought to be responsible for susceptibility to infectious diseases in early life. Deficiencies have been demonstrated to be present in both innate and adaptive immune responses in human neonates [1]. Neonatal antibody responses to viral and bacterial infection are quantitatively and qualitatively different from those generated in later life [2]. Although neonates are able to mount IgG antibody responses, the isotype distribution and magnitude of the response does not become adult-like until approximately 3 months of age, and antibody responses in infants under 1 year are short-lived. Following in utero infection or exposure to antigen, IgM antibodies predominate, but vaccines can elicit IgG in both adults and infants, demonstrating that class switching can occur in infants under certain conditions. The diversification of the immunoglobulin repertoire also progresses during early life. Somatic mutation and selection of mutated B cell clones, processes which occur in germinal centres, have been shown to reach adult levels at 8–12 months of age [3]. Importantly, IgG responses to most thymus independent (TI) bacterial capsular polysaccharides (PS) are poor until 2 years of age. Consequently, infants are highly susceptible to infections with encapsulated bacteria. The mechanism(s) responsible for the impaired PS response have been suggested to include the immaturity of the splenic marginal zone in infants [4] and reduced levels of expression of CD21/CR2 on neonatal and infant B lymphocytes [5], which might result in weaker activation signals delivered through the B cell antigen receptor (BCR)/CD21/CD19 signalling complex following binding of PS/C3d. In addition, human neonates are relatively deficient in C3, which only reaches adult levels at around one year of age [6,7] and this may contribute to defective B cell activation.
The exact mechanisms underlying deficiencies in the humoral immune responses of neonates remain to be elucidated. They could reflect intrinsic differences between adult and neonatal B and/or T cells or aspects of the neonatal environment, which are not sufficiently developed to allow a mature, adult-like response to occur. The evidence indicates that human neonatal CD4+ T cells are immunocompetent, and do not differ from adult T cells in their ability to produce Th1-type cytokines in vitro or to develop into Th2 effectors [8], although murine studies demonstrate a bias towards Th-2 type helper responses [9]. Phenotypic and functional differences between adult and neonatal B cells are well documented in the mouse (reviewed in [10]). In particular, while ligation of the B cell antigen receptor (BCR) induces MHC Class II up-regulation and proliferation in mature adult B cells, neonatal B cells fail to hyperexpress MHC class II [11] and are induced to undergo apoptosis by this stimulus [12]. In contrast to these murine studies, some authors have shown that human cord blood B cells can respond positively to cross-linking of the antigen receptor [13–15].
An effective humoral immune response requires a productive interaction between antigen-specific T and B lymphocytes; B cells must efficiently present antigenic peptide, in association with MHC class II, to antigen-specific T cells. Up-regulation of B cell surface co-stimulatory molecules such as CD86 is also necessary for T cell activation via CD28. In order for an antibody response to occur these rare, antigen-specific lymphocytes must come into physical contact. Both the integrin and chemokine family of molecules play important roles in the migration and localization of lymphocytes to and within secondary lymphoid organs, and chemokines mediate the interactions between APC and T helper cells which are necessary for a humoral immune response to occur. Recently it has been shown that antigen receptor cross-linking on murine splenic and blood B cells induces the expression of common chemokines, including MIP-1α (CCL3) and MIP-1β (CCL4), which strongly attract T cells [16] a response also shown previously to occur in human peripheral blood B cells [17].
We hypothesized that defects of these functions in neonatal human B cells might affect their ability to respond to antigens directly, or indirectly affect T cell responses because they could be unable to present antigen effectively or attract T cells with which they could interact. We therefore investigated the functional maturity of neonatal human B cells by examining the response of B cells from cord blood to antigen receptor mediated signals and innate stimuli. As correlates for functionality we have investigated the ability of neonatal B cells to proliferate in response to surrogate antigen and the effects of cytokines on this response. We also assessed their ability to modulate co-stimulatory molecules, as an indicator of their ability to effectively present antigen to T cells and their capacity to secrete chemokines known to be important in attracting T cells into lymphoid follicles. To examine whether poor neonatal immune responses might be due partly to impaired trafficking or homing of neonatal B cells we analysed their expression of cell surface molecules known to be critical for these functions. We show that, in contrast to murine neonatal B cells, human cord blood B cells are able to proliferate, up-regulate Class II and CD86 and secrete T cell chemoattractants MIP-1α and MIP-1β in response to BCR cross-linking. Furthermore, we present the first evidence that CpG containing oligonucleotides induce proliferation, MHC Class II and CD86 up-regulation, and MIP-1α and MIP-1β secretion in neonatal B cells. In addition, we have examined the expression of surface molecules involved in lymphocyte homing and found cord blood B cells to express low levels of CD62L (l-selectin) and CCR7, suggesting that a significant percentage of cord blood B cells would be unable to efficiently enter peripheral lymphoid organs.
MATERIALS AND METHODS
Samples
Cord blood was obtained following elective caesarian section at the Liverpool Women's Hospital. Informed consent was obtained from the mothers prior to caesarian section and the project was approved by the Liverpool Research Ethics Committee and the Royal Liverpool University Hospitals Ethical Comittee. Adult cells were from peripheral blood from healthy adult volunteers or buffy coats obtained from adult peripheral blood supplied by the National Blood Service, Manchester. Adult peripheral blood and cord blood was collected into sterile heparinized tubes and used within 1 h of collection.
Isolation of cells
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat, peripheral blood or cord blood, by centrifugation with Lymphoprep (Axis-Shield, Oslo, Norway). The PBMC isolated were used for B cell isolation using the Human B Cell Isolation Kit (Miltenyi, Bergisch, Gladbach, Germany). This system results in the isolation of untouched B cells by magnetic depletion of T cells, natural killer (NK) cells, myeloid cells, basophils, platelets and early erythroid cells. Cells isolated by this method were routinely 95–98% CD19+ (adult) and 90–98% CD19+ (cord).
Cell culture and proliferation assays
Purified B cells were cultured in 96-well plates at 2 × 105/well in RPMI-1640, supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine and 1 mm sodium pyruvate for 72 h. Cultures were pulsed with 0·5 µCi [3H] thymidine (Amersham, UK) for the final 16 h of culture, before being harvested and uptake measured by liquid scintillation spectroscopy. For HLA-DR and CD86 up-regulation experiments, cells were cultured in 48- or 24-well plates at 2 × 106/ml for 48 h with the indicated stimuli and expression was analysed by flow cytometry.
Antibodies and reagents
The following antibodies were used in this study: goat F(ab′)2 antihuman IgM used for B cell stimulation was from Jackson Immunoresearch (West Grove, PA, USA). For FACS analysis the following antibodies were used: anti-CD19 (Cy-Chrome), anti-HLA-DR (PE), anti-CD86 (biotin), anti-CD62L (PE) (Becton Dickinson, Oxford, UK), and anti-CXCR5 (biotin) and anti-CCR7 (PE) (R&D Systems, Oxford, UK). Biotin-labelled reagents were detected using streptavidin-RPE (Serotec). Isotype controls were from Becton Dickinson (Oxford, UK). Recombinant human interleukin (IL)-2/proleukin was obtained from Chiron (Emeryville, CA, USA) and recombinant human IL-4 was a generous gift from S. Christmas. Unmodified (phosphodiester), and modified nuclease-resistant (phosphorothioate) oligodeoxynucleotides (ODNs) were purchased from MWG-Biotech (Milton Keynes, UK). The sequences used were as follows: unmodified CpG ODN 2080 (5′-TCG TCG TTC CCC CCC CCC CC-3′) and non-CpG control 2078 (5′-TGC TGC TTC CCC CCC CCC CC-3′), phosphorothioate CpG ODN 2006 (5′-TCG TCG TTT TGT CGT TTT GTC GT-3′) and non-CpG control 2137 (5′-TGC TGC TTT TGT GCT TTT GTG CT-3′).
Cell staining and flow cytometry
Flow cytometry was performed using a FACScan flow cytometer (Becton Dickinson, UK). Dead cells were excluded on the basis of their forward- and side-scatter characteristics: an electronic gate was set to include cells with the characteristics of lymphocytes. Analysis was based on the collection of 25 000–50 000 events.
MIP-1α/MIP-1β ELISA
Purified B cells were cultured at 2 × 106/ml with the indicated stimuli. Cell-free supernatants were harvested after 48 h incubation and the concentration of MIP-1α and MIP-1β in culture supernatants was measured using DuoSet ELISA development kits (R&D Systems) according to the manufacturer's instructions.
Statistical analysis
The statistical significance of differences in the co-stimulatory response of neonatal and adult B cells to anti-IgM with IL-2 or IL-4 were determined by one-way anova with Tukey's post-test. Student's t-test was used to compare the expression of CD62L and CCR7 by adult and neonatal CD19+ cells.
RESULTS
Neonatal B-lymphocytes are less responsive to IL-2
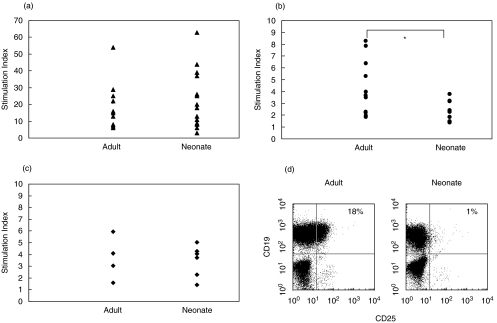
T cell-derived cytokines, including IL-2 and IL-4, support B cell proliferation and can induce differentiation. To examine the effects of these cytokines on anti-IgM induced proliferation, purified B lymphocytes from adult and cord blood were induced to proliferate in response to F(ab′)2 anti-IgM antibodies (Fig. 1a). Unlike the situation with murine neonatal B cells, cord blood B cells proliferated as strongly as adult B cells to antigen receptor cross-linking. Both IL-2 (Fig. 1b) and IL-4 (Fig. 1c) enhanced the anti-IgM-induced proliferation in adult and neonatal B cells, but the co-stimulation induced by IL-2 on anti-IgM stimulated cord blood B cells was significantly less than that induced in adult B cells. In contrast, the co-stimulation induced by IL-4 was not significantly different for adult and neonatal B cells. A possible explanation for the relatively low sensitivity of cord blood B cells to IL-2 is low expression of components of the high-affinity IL-2R. When B cells were analysed for expression of CD25, a significant proportion (30·1 ± 9·8%, n = 5) of adult B cells were positive. In contrast, almost no neonatal B cells (2·2 ± 1·6%, n = 5) expressed this component of the IL-2 receptor (Fig. 1d). The presence of this population of B cells in adult blood expressing the high affinity IL-2R may account for the higher co-stimulation index observed in the presence of IL-2.
Fig. 1.
Proliferative responses to anti-IgM in the presence/absence of T cell cytokines. Purified adult and cord blood B cells (2 × 105/well) were stimulated for 3 days with medium alone, F(ab′)2 anti-IgM alone (10 µg/ml); (a) [n = 10 adult, 14 neonatal]; anti-IgM plus rIL-2 (100 U/ml); (b) [n = 12 adult, 10 neonatal], or anti-IgM plus rIL-4 (100 U/ml); (c) [n = four adult, six neonatal]. The cultures were pulsed with [3H]-thymidine for final 16 h of a 72-h culture. Anti-IgM alone stimulated cultures were compared with cultures in medium alone and anti-IgM plus cytokine cultures were compared with anti-IgM alone cultures and the proliferation induced was expressed as a stimulation index. Control, unstimulated adult B cells incorporated 494 ± 126 cpm and neonatal B cell controls 806 ± 129 cpm. There was no significant difference between adult and cord blood B cell responses to anti-IgM alone. Using one-way anova the co-stimulation indices for anti-IgM + IL-2 were significantly different (P < 0·05), those for anti-IgM + IL-4 were not significantly different. Adult and cord blood mononuclear cells were stained for expression of CD19 and CD25 (d); the percentage of CD19+ cells also expressing CD25 is shown. The FACS data are representative of five independent experiments.
Neonatal B cells secrete MIP-1α and MIP-1β in response to BCR cross-linking
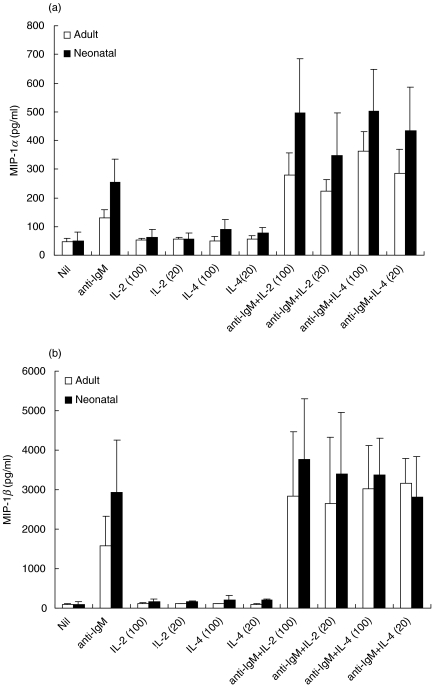
The ability of cord blood B cells and adult B cells to secrete the T cell chemoattractants MIP-1α and MIP-1β in response to antigen receptor cross-linking was analysed. Cord blood and adult B cells were induced to secrete both chemokines when stimulated with F(ab′)2 anti-IgM (Fig. 2). IL-2 or IL-4 alone had no significant effect on MIP-1α and β secretion. However, anti-IgM-induced secretion of MIP-1α, by both adult and cord blood B cells, was enhanced strongly by co-incubation with either IL-2 or IL-4. IL-2 and IL-4 also enhanced secretion of MIP-1β by anti-IgM stimulated B cells. These data are representative of three separate experiments, and demonstrate clearly that IL-2 is co-stimulatory for cord blood B cells in the induction of MIP-1α and MIP-1β secretion. A more extensive survey of responsiveness would be required before conclusions can be drawn about the relative sensitivity of cord blood B cells to IL-2, as we have shown for the proliferative response.
Fig. 2.
MIP-1α and MIP-1β secretion induced by F(ab′)2 anti-IgM in the presence/absence of T cell cytokines. Purified adult and cord blood B cells (2 × 106/ml) were cultured for 48 h in medium alone, F(ab′)2 anti-IgM (10 µg/ml), rIL-2 (20 or 100 U/ml), rIL-4 (20 or 100 U/ml) or anti-IgM plus cytokine, as indicated. Supernatants were harvested and MIP-1α (a) and MIP-1β (b) secretion assessed by ELISA, each sample being analysed in duplicate. The data shown are the combined results (mean ± s.d.) from three separate experiments.
Anti-IgM and CpG DNA induce HLA-DR and CD86 up-regulation in cord blood B cells
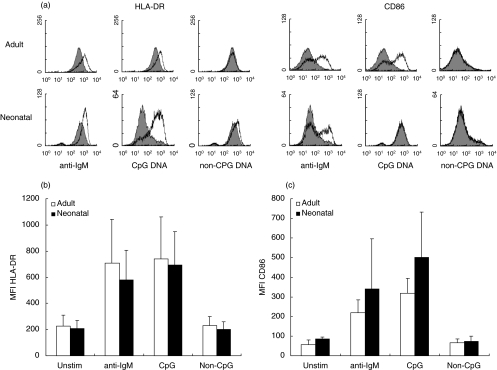
The ability of anti-IgM and CpG containing oligonucleotides to up-regulate the surface expression of MHC Class II (HLA-DR) and CD86 on cord blood and adult B cells is shown in Fig. 3. After 48 h in culture, in the absence of stimulus, adult and neonatal B cells expressed low levels of CD86 and high levels of HLA-DR. Stimulation with F(ab′)2 anti-IgM induced a substantial increase in both HLA-DR and CD86 expression on adult and cord blood B cells. Stimulation of adult and cord blood B cells with the unmodified phosphodiester CpG ODN 2080, but not the non-CpG ODN 2078, induced up-regulation of HLA-DR expression. Stimulation with CpG ODN also induced a marked up-regulation of CD86 in both adult and cord blood B cells. Signals generated through either the antigen receptor or TLR-9 are thus able to induce, in cord blood B cells, the up-regulation of surface molecules important in T–B cell interactions.
Fig. 3.
Modulation of HLA-DR and CD86 expression by anti-IgM or CpG-ODN. Purified adult and cord blood B cells were cultured with medium alone (filled plots) or stimulated for 48 h with F(ab′)2 anti-IgM (10 µg/ml), CpG-ODN 2080 (30 µg/ml) or non-CpG ODN 2078 (30 µg/ml) (open plots). Anti-IgM was added to cultures at 0 h and ODN were added at 0 h, 2 h and 23 h. Cells were dual-stained for expression of CD19 and HLA-DR or CD19 and CD86 and histograms of marker expression on CD19 positive cells are shown (a). Collated data from four separate experiments shows the mean fluorescence indices (mean ± s.d.) for HLA-DR (b) and CD86 expression (c) on CD19+ cells.
CpG-ODN induce proliferation of cord blood B cells
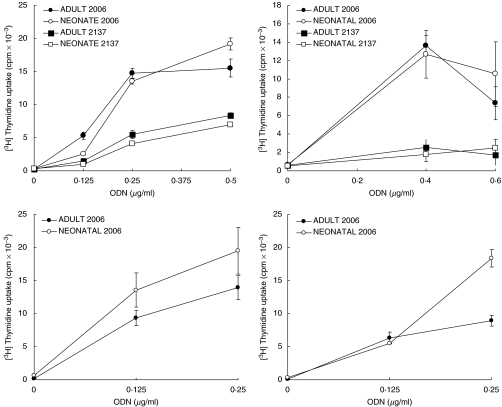
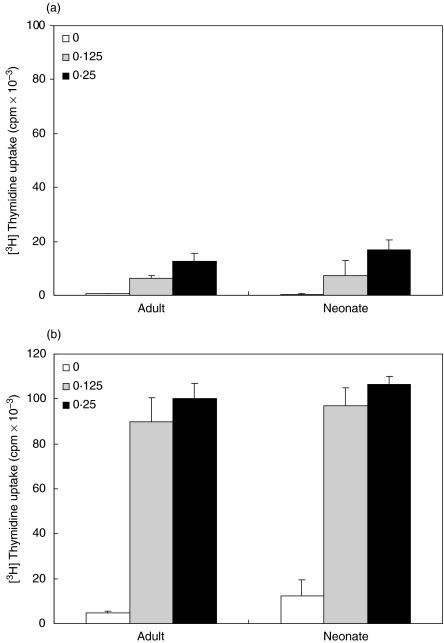
Because CpG-containing ODNs have been shown to be strongly mitogenic for adult human B cells [18], we investigated the effect of CpG ODN on cord blood B cells. Purified adult or cord blood B cells were incubated with the nuclease-resistant phosphorothioate oligonucleotide containing four CpG motifs (2006) [19] which induced equivalent proliferation of cord blood and adult B cells as shown by [3H]thymidine incorporation (Fig. 4). Non-CpG phosphorothioate ODN have also been reported to induce low levels of proliferation in adult B cells. Our data confirm this finding and show that a similar level of proliferation was induced in purified adult and neonatal B cells. In addition to CpG ODN being mitogenic, a strong synergy between CpG-containing DNA and signals through the BCR has previously been demonstrated in adult peripheral B cells, and our data (Fig. 5) show that this strong synergy also occurs in cord blood B cells.
Fig. 4.
Proliferation induced by CpG-ODN. Purified adult and cord blood B cells (2 × 105/well) were cultured with medium alone or with various concentrations of phosphorothioate CpG-ODN (2006) or non-CpG control ODN (2137), as indicated. Cultures were pulsed with [3H]-thymidine for the final 16 h of a 72-h culture. Data from four separate experiments (mean ± s.d.) are shown, based on triplicate cultures for each point in each experiment.
Fig. 5.
Anti-IgM and CpG-ODN co-stimulation of proliferation. Purified adult and cord blood B cells were cultured with medium alone or CpG ODN 2006 at 0·125 or 0·25 µg/ml, in the absence (a) or presence (b) of F(ab′)2 anti-IgM (10 µg/ml). Cultures were pulsed with [3H]-thymidine for the final 16 h of a 72-h culture. The data shown are the combined results (mean ± s.d.) of three experiments in each of which all points were tested in triplicate.
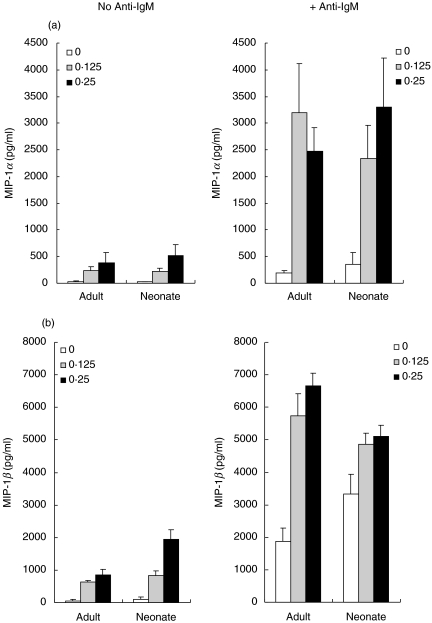
CpG-ODN induce MIP-1α and MIP-1β secretion by adult and cord blood B cells
Because we have shown that signals generated through the BCR induced MIP-1α and MIP-1β secretion in both adult and neonatal B cells (Fig. 2), we examined the ability of TLR-9 signals to induce the secretion of these T cell chemoattractants. Incubation with CpG ODN 2006 for 48 h induced the secretion of significant amounts of both MIP-1α and MIP-1β from both adult and cord blood B cells (Fig. 6). Co-incubation with F(ab′)2 anti-IgM induced a synergistic increase in MIP-1α secretion from both adult and neonatal B cells. Synergy was also observed for MIP-1β secretion in adult B cells, but the high level of MIP-1β secretion induced by anti-IgM alone in cord blood B cells was not increased significantly in the presence of CpG ODN.
Fig. 6.
Anti-IgM and CpG-ODN co-stimulation of MIP-1α and MIP-1β secretion. Purified adult and cord blood B cells were cultured for 48 h with medium alone or CpG ODN 2006 at 0·125 or 0·25 µg/ml, in the absence or presence of F(ab′)2 anti-IgM (10 µg/ml), as indicated. Supernatants were harvested and MIP-1α (a) and MIP-1β (b) secretion assessed by ELISA. Results (pg/ml) are the means of duplicate determinations. The data shown are the combined results (mean ± s.d.) of four experiments in each of which all points were tested in duplicate.
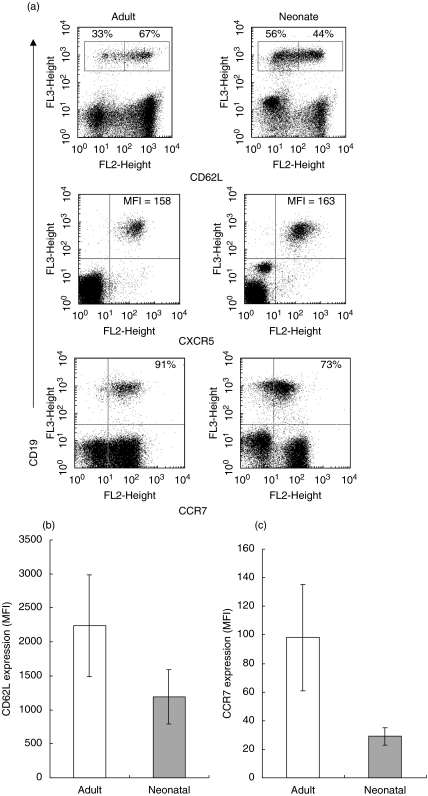
Low expression of CD62L and CCR7 on cord blood B cells
In addition to investigating possible functional differences between isolated cord blood and adult peripheral blood B cells we also analysed the expression of surface molecules required for lymphocyte migration and localization (Fig. 7a). Most adult peripheral blood B cells (68 ± 8%, n = 5) expressed high levels of CD62L (l-selectin), a molecule essential for entry of naive B cells into lymph nodes. In contrast, most cord blood B cells (58 ± 5%, n = 5) expressed low levels of this molecule and the number of B cells with low CD62L expression was significantly greater in neonatal than in adult samples (P = 0·003). As can be seen from Fig. 7b, the overall expression of CD62L was significantly (P = 0·007) lower on cord blood than adult CD19+ B cells, the MFIs being 1390 ± 500 and 2310 ± 836, respectively (n = 6 for both adult and neonatal samples). We also examined expression of the chemokine receptors CXCR5 and CCR7. CXCR5 is expressed on mature B cells, and confers responsiveness to CXCL13 made in follicles of secondary lymphoid organs. Our data show that CXCR5 was expressed at equivalent levels on adult and cord blood B cells. CCR7 is a receptor for the T zone chemokines CCL19 (ELC) and CCL21 (SLC). Over 90% of adult peripheral blood B cells were CCR7 positive, whereas far fewer (44 ± 18%) of cord blood B cells expressed this molecule. The MFI for CCR7 expression on CD19+ adult and cord blood B cells were significantly different (P = 0·026), with the MFI being 98 ± 37 for adult cells and that for neonatal cells 29 ± 6 (Fig. 7c).
Fig. 7.
CD62L, CXCR5 and CCR7 expression. (a) Adult and cord blood mononuclear cells were dual-stained for expression of CD19 and CD62L, CXCR5 or CCR7. Mean fluorescence intensity (MFI) of CXCR5 expression is based on analysis of CD19+ cells only, and percentages indicate cells in regions or quadrants as appropriate. Plots represent the fluorescence of 50 000 events and the data are representative of six independent experiments. (b) Comparison of mean fluorescence indices of staining for CD62L on adult and neonatal CD19+ B cells: expression levels were significantly different (P = 0·007, n = 6). (c) Comparison of mean fluorescence indices of staining for CCR7 on adult and neonatal CD19+ B cells: expression levels were significantly different (P = 0·026, n = 6).
DISCUSSION
Although able to mount immune responses, human and murine neonates are relatively immunodeficient compared to adults. Studies of murine neonatal B cell responses have shown that cross-linking of the BCR transduces negative signals that inhibit subsequent responses to antigen or mitogen and make B cells prone to apoptosis. The splenic B cells of neonatal mice also show a selective inability to up-regulate expression of MHC Class II following BCR ligation, although they are able to respond to other stimuli including IL-4, CD40 ligation, LPS and CpG containing DNA ([11] and our unpublished observations).
The data presented in this report demonstrate that cross-linking of the BCR with soluble anti-IgM antibodies on highly purified (>90% CD19+) human cord blood B cells resulted in proliferation and up-regulation of both HLA-DR and CD86 molecules and the response was similar to that of adult peripheral blood B cells. This is consistent with the findings of others [13], who have demonstrated the proliferation of monocyte-depleted neonatal cord blood cells to both soluble F(ab′)2 anti-µ and also to multivalent antigen receptor cross-linking by anti-IgD conjugated to dextran. However, in this particular report, the B cell population analysed contained only approximately 12% CD19+ cells for both adult and neonatal preparations, and the authors found that the magnitude of the proliferative response was greater in adult than in neonate samples. These authors also reported that anti-δ-dextran enhanced HLA-DR expression on a more enriched neonatal B cell population (70–80% CD19+), but did not examine this response after ligation with soluble anti-Ig antibodies. In a different study [14] cord non-T cells (42% Ig+) and adult B-enriched blood cells (44% Ig+) proliferated equally well in response to anti-µ-coated acrylamide beads. It was also reported that adult B cells had relatively greater stimulation indices with anti-µ and IL-2 compared with cord blood B cells. This is similar to our finding that IL-2 had a significantly lesser co-stimulatory effect on anti-IgM-stimulated cord blood B cells than on adult B cells (Fig. 1c). Previously, it has been reported that the IL-2Rγ chain is expressed at lower levels on cord blood lymphocytes compared with adult cells [20,21], and that IL-2Rα is present at lower levels on cord blood B and T cells [22]. Although we found expression of the IL-2Rγ chain to be similar on adult and neonatal B cells (data not shown), 99% of cord blood B cells were negative for the IL-2Rα chain (CD25). In contrast, a significant percentage (30%) of adult, peripheral blood B cells were CD25+. The IL-2Rα chain is expressed on activated B cells and is essential for the formation of the high affinity IL-2R in conjunction with the IL-2Rβ. The adult B cell preparations may therefore be more sensitive to IL-2 because of the presence of a preactivated population. In contrast, neonatal B cells, coming from a sterile environment, lack this population of preactivated cells.
In order for a humoral immune response to proceed, antigen-binding B cells must be able to enter and recruit antigen-specific T cells to precise locations within lymphoid organs. It is well established that chemokines play important roles in directing cell movements required during immune responses [23]. It has been shown that activation of B cells and professional antigen-presenting cells induces the expression of common chemokines [16]. Cells producing MIP-1α/β mRNA have been detected in extrafollicular T cell zones and GC of lymph nodes [24], and these chemokines have been shown to regulate T lymphocyte trafficking into lymph nodes during an immune response [25]. Multivalent cross-linking of the antigen receptor on human adult B cells with Staphylococcus aureus Cowan I (SAC) or polyclonal anti-IgM coupled to beads has been shown to induce the up-regulation of MIP-1α and MIP-1β mRNA levels and secretion of both chemokines [17] and the secretion induced by anti-IgM was enhanced significantly by co-stimulation with IL-4. The data presented here show that neonatal B cells are able to secrete both MIP-1α and MIP-1β in response to soluble F(ab′)2 anti-IgM and that the secretion was enhanced by either IL-2 or IL-4 and the responses were equivalent to those of adult peripheral blood B cells. These data demonstrate that following BCR ligation, neonatal B cells are capable of secreting at least some chemokines important in attracting T cells.
Responses of neonatal mice to conventional vaccines are frequently biased towards a Th2 pattern; this results in a deficiency in CTL responses required for protection against intracellular pathogens. The mechanism of the Th2 bias is thought to lie in suboptimal antigen presentation to T cells by immature APC [1]. CpG DNA, in the form of DNA vaccination or co-administration of CpG-ODN, has been shown to drive adult murine immune responses to vaccines and infectious diseases in a Th1 direction. This is likely to be due to potent stimulatory effects on dendritic cells and B cells. This effect of Th1 polarization of an immune response to antigens has also been demonstrated in neonatal mice [26]; co-injection of CpG-ODN with a weak tetanus toxoid antigen resulted in enhanced antibody responses, and the isotypes distribution was altered from an IgG1 to IgG1 and IgG2a. In addition, splenocytes from neonatal mice immunized previously with measles virus antigen secreted higher levels of IL-5 and lower levels of interferon (IFN)-γ when restimulated in culture, compared to splenocytes from adult mice. However when neonatal mice were immunized with the antigen in the presence of CpG-ODN, IFN-γsecretion was enhanced to adult levels and IL-5 production was inhibited. This effect was attributed to the ability of CpG-ODN to activate neonatal APC to produce IL-12. CpG-ODN were also shown to induce proliferation in spleen B cells from 1-week-old and 4-week-old mice. In summary, CpG-ODN activate and induce the proliferation of murine neonatal B cells and are able to circumvent the Th2 polarization of neonatal vaccine responses. A bias of human neonatal responses towards a Th2 pattern is unproven, but analysis of the ability of neonatal cells to respond to CpG-containing DNA is still important. Following the identification of CpG motifs active on human cells [19], it has been demonstrated that CpG DNA directly activates human plasmacytoid dendritic cells and B cells (reviewed in [27]). Adult human primary B cells are induced to proliferate and express increased levels of CD86, CD40, CD54 and MHC-II. Low concentrations of CpG DNA strongly synergized with signals generated through BCR, to enhance proliferation, IL-6 and antigen-specific Ig secretion [18]. In these reports, only adult B cell responses to CpG DNA were analysed. In this study we have demonstrated for the first time that human neonatal B cells respond as well as adult B cells to CpG-ODN in terms of proliferation, co-stimulation with BCR signals, up-regulation of CD86 and HLA-DR and chemokine secretion. This indicates that CpG DNA could possibly direct human neonatal vaccine responses in a Th1 direction. However, CpG responses of neonatal APC other than B cells have yet to be analysed. Our data, demonstrating CpG-induced proliferation of cord blood B cells which do not express CD27 ([28] and our unpublished observations), is in contrast to the recent report that naive, CD27 negative adult peripheral blood B cells do not divide in response to the same CpG-ODN (2006) [29]. However, it may be noteworthy that the authors also reported that B cells failed to divide in response to antigen receptor ligation with F(ab′)2 anti-Ig.
The selective entry of B and T lymphocytes into LN is a tightly controlled process dependent upon both lymphocyte adhesion molecules and chemokines and their receptors. Naive B and T cells migrate directly from the blood into secondary lymphoid organs by extravasation through high endothelial venules (HEVs) and in addition to CD62L (l-selectin) the chemokine receptor CCR7 plays a role in the efficient migration of B lymphocytes across HEV and in localization of lymphocytes into specialized microenvironments within peripheral lymphoid organs [30]. The low expression of CD62L and CCR7 shown by our data indicate that a significant percentage of cord blood B cells may be unable to gain entry into peripheral lymphoid organs.
In summary, our data demonstrate that neonatal cord blood B cells are able to respond as well as adult B cells to BCR signals and innate stimuli. The ability of human neonatal B cells to proliferate and up-regulate co-stimulatory molecules in response to BCR ligation contrast with those of murine neonatal B cells. Cord blood B cells were also shown to secrete T cell attractant chemokines. Taken together, these observations lead us to conclude that human neonatal B cells should be as capable as adult B cells of attracting T cells to their vicinity and presenting antigen to them. However, for these functions to be fully expressed both T and B cells need proper access to the appropriate microenvironment. We have found that neonatal B cells differ from adult B cells in their expression of molecules required for homing to peripheral lymphoid organs. We conclude that this difference could result in restricted access of neonatal B cells to the appropriate location; this could limit their full functionality and contribute from to the poor immune responses of human neonates.
Acknowledgments
The authors thank the midwives and theatre staff at the Liverpool Women's Hospital, Liverpool for their co-operation and assistance with cord blood collection. We also thank Art Krieg for helpful advice for use of CpG-ODN. This work was supported by funding from the European Commission for the NEOVAC project QLK2-CT-1999–00429.
REFERENCES
- 1.Kovaric J, Siegrist CA. Immunity in early life. Immunol Today. 1998;19:150–2. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 3.Ridings J, Dinan L, Williams R, Roberton D, Zola H. Somatic mutation of immunoglobulin V(H)6 genes in human infants. Clin Exp Immunol. 1998;114:33–9. doi: 10.1046/j.1365-2249.1998.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zandvoort A, Lodewijc ME, de Boer NK, Dammers PM, Kroese FGM, Timens W. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens. 2001;58:234–42. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- 5.Griffioen AW, Franklin SW, Zegers BJ, Rijkers GT. Expression and functional characteristics of the complement receptor type 2 on adult and neonatal B lymphocytes. Clin Immunol Immunopathol. 1993;69:1–8. doi: 10.1006/clin.1993.1142. [DOI] [PubMed] [Google Scholar]
- 6.Davis CA, Vallota EH, Forristal J. Serum complement levels in infancy: age related changes. Pediatr Res. 1979;13:1043–6. doi: 10.1203/00006450-197909000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Norman ME, Gall EP, Taylor A, Laster L, Nilsson UR. Serum complement profiles in infants and children. J Pediatr. 1975;87:912–6. doi: 10.1016/s0022-3476(75)80904-8. [DOI] [PubMed] [Google Scholar]
- 8.Delespesse G, Yang LP, Ohshima Y, et al. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine. 1998;16:1415–9. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 9.Adkins B. T cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 10.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up'. Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 11.Tasker L, Marshall-Clarke S. Immature B cells from neonatal mice show a selective inability to upregulate MHC Class II expression in response to antigen receptor ligation. Int Immunol. 1997;9:475–84. doi: 10.1093/intimm/9.4.475. [DOI] [PubMed] [Google Scholar]
- 12.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404–13. [PubMed] [Google Scholar]
- 13.Halista SM, Johnson-Robbins LA, El-Mohandes AE, Lees A, Mond JJ, Kotana IM. Characterization of early activation events in cord blood B cells after stimulation with T cell-independent activators. Pediatr Res. 1998;43:496–503. doi: 10.1203/00006450-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Watson W, Oen K, Ramdahin R, Harman C. Immunoglobulin and cytokine production by neonatal lymphocytes. Clin Exp Immunol. 1991;83:169–74. doi: 10.1111/j.1365-2249.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macardle PJ, Weedon H, Fusco M, et al. The antigen receptor complex on cord B lymphocytes. Immunology. 1997;90:376–82. doi: 10.1111/j.1365-2567.1997.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz A. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 17.Krzysiek R, Lefèvre EA, Zou W, et al. Antigen receptor engagement selectively induces macrophage inflammatory protein-1α (MIP-1α) and MIP-1β chemokine production in human B cells. J Immunol. 1999;162:4455–63. [PubMed] [Google Scholar]
- 18.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–52. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 20.Zola H, Fusco M, Weedon H, Macardle PJ, Ridings J, Roberton DM. Reduced expression of the interleukin-2-receptor gamma chain on cord blood lymphocytes: relationship to functional immaturity of the neonatal immune response. Immunology. 1996;87:86–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Saito S, Morii T, Umekage H, et al. Expression of the interleukin-2 receptor γ chain on cord blood mononuclear cells. Blood. 1996;87:3344–50. [PubMed] [Google Scholar]
- 22.Zola H, Fusco M, Macardle PJ, Flego L, Roberton D. Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr Res. 1995;38:397–403. doi: 10.1203/00006450-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 24.Trumpfheller C, Tenner-Racz K, Racz P, Fleischer B, Frosch S. Expression of macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES genes in lymph nodes from HIV+ individuals: correlation with a Th1-type response. Clin Exp Immunol. 1998;112:92–9. doi: 10.1046/j.1365-2249.1998.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedla N, Wang H-W, McNeil HP, et al. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1α and MIP-1β. J Immunol. 1998;161:5663–72. [PubMed] [Google Scholar]
- 26.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligdeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–7. [PubMed] [Google Scholar]
- 27.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 28.Agematsu K, Nagumo H, Yang F-C, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997;27:2073–9. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 30.Förster R, Schubel A, Breitfield D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]