Abstract
CXC chemokines modulate host immunity, neovascularization, growth and invasive behaviour of tumours. Despite their relevance in tumour biology, chemokine expression in intestinal- and diffuse-type gastric carcinoma, which exhibit a completely different growth pattern, has not been investigated in detail. In this study, expression of the CXC chemokines CXCL8 [interleukin (IL)-8], CXCL1 [growth-related oncogene alpha (Groα)], CXCL9 [monokine induced by interferon (IFN)-γ] and CXCL10 [IFN-γ-inducible protein-10 (IP-10)] and the corresponding chemokine receptors CXCR1–3 was investigated by immunohistochemistry in intestinal- and diffuse-type gastric carcinoma. Tumour cells of all patients expressed CXCL8. CXCL8 expression was significantly stronger in tumour cells of diffuse- rather than intestinal-type gastric carcinoma (P < 0·01) as determined by a semiquantitative score. CXCL1 was expressed almost exclusively by diffuse- but not intestinal-type carcinoma cells. The corresponding chemokine receptors, CXCR1 and CXCR2, were found on carcinoma cells. Furthermore, CXCL8 expression correlated with number of tumour vessels (P < 0·01), suggesting an angiogenetic function in gastric carcinoma not only in vitro but also in vivo. CXCL10 and CXCL9, attractants for T cells, were expressed by peritumorous macrophages in close proximity to IFN-γ-producing CXCR3-positive T cells in both tumour types. These chemokines may attract gastric carcinoma-infiltrating T cells via an IFN-γ-mediated pathway and enhance host immunity against the tumour. In gastric carcinoma a complex interplay between CXC-chemokine signals derived from both tumour cells and tumour-infiltrating immune cells may exhibit pleiotropic effects in tumour biology that go far beyond their originally described functions as leucocyte chemoattractants. Because CXCL8 and CXCL1, which are known to increase growth and invasive behaviour of malignant tumours, are significantly stronger expressed in diffuse- than intestinal-type gastric carcinoma, one may speculate that these chemokines influence the different growth pattern of gastric carcinoma types.
Keywords: chemokine receptors, CXC chemokines, gastric carcinoma
INTRODUCTION
Chemokines play a divergent role in controlling the growth of malignant tumours. Certain chemokines enhance non-specific or specific host immunity against tumour implantation, while others may favour tumour growth and metastasis by promoting tumour cell proliferation or neovascularization in tumour tissue [1–5].
Gastric carcinoma, globally the second most common tumour [6], is classified histopathologically according to Lauren into intestinal and diffuse types with each type exhibiting different patterns of growth and invasive behaviour [7]. Distinct glandular formation is typically of the intestinal type of gastric carcinoma, while the diffuse type is characterized by isolated cancer cells with an infiltrative growth pattern. Characteristically, the tumour cells are surrounded by a prominent, highly vascularized, desmoplastic stroma and a marked infiltration by T cells, neutrophils and macrophages.
The traditional function of chemokines is the cell-specific recruitment of leucocyte subsets to inflammatory sites by a receptor-mediated mechanism [8]. It is therefore tempting to speculate that chemokines are involved critically in the trafficking of specific and non-specific cells of the immune system into the tumour stroma. The CXC chemokines interleukin-8 (CXCL8, IL-8) and growth-related oncogene α (CXCL1, Groα) attract neutrophils and T lymphocytes via the chemokine receptors CXCR1 (receptor for CXCL8) and CXCR2 (receptor for both chemokines CXCL8 and CXCL1 [9,10]). Interferon (IFN)-γ-inducible protein-10 (CXCL10, IP-10) and a monokine induced by interferon-γ (CXCL9, MIG) selectively attract T lymphocytes via the corresponding chemokine receptor CXCR3 [11–13].
In addition, these four CXC chemokines regulate tumour angiogenesis. CXCL8 and CXCL1 have a potent stimulatory effect on tumourangiogenesis, while CXCL10 and CXCL9 inhibit neovascularization [1–4].
As recent data suggest that chemokines regulate growth and invasive behaviour of malignant tumours [5], the question arises as to whether the two types of gastric carcinoma, which show a completely different growth and invasive behaviour, differ in their chemokine expression pattern.
Despite the clear relevance of chemokines in the biology of malignant tumours, their role in gastric carcinoma has not been investigated in detail. This study focuses on the expression pattern and microanatomical localization of the CXC chemokines CXCL8/CXCL1 and CXCL10/CXCL9 and their corresponding chemokine receptors CXCR1, CXCR2 and CXCR3 in both diffuse and intestinal types of gastric carcinoma.
MATERIALS AND METHODS
Tissues studied
Gastric carcinoma tissue specimens from 22 surgical patients were investigated by immunohistochemistry. The gastric carcinomas were classified histopathologically according to the Lauren classification [7] as either intestinal (12/22) or diffuse type (10/22). Immunohistochemistry for CXCL8, CXCL1, CXCL10, CXCL9, IFN-γ, CD3, CD68 and CD34 was performed on cryostat tumour tissue, which had been snap-frozen immediately after surgery and stored at −70°C until use. For immunohistochemical localization of the chemokine receptors CXCR1, CXCR2 and CXCR3, formalin-fixed tumour tissue was used. For better morphological recognition of tumour cells, especially in diffuse-type gastric carcinoma, serial sections were stained with haematoxylin–eosin and, if necessary, with PAS.
Antibodies and staining technique
For immunohistological analyses the following monoclonal and polyclonal antibodies were used at the dilutions indicated: mouse monoclonal anti-CD3 (1 : 500, Dako, Hamburg, Germany), mouse monoclonal anti-CD68 (1 : 8000, clone KIM6, gift from the Institute of Pathology, Kiel, Germany), mouse monoclonal anti-CD34 (1 : 20, Immunotech, Marseille, France), mouse monoclonal anti-CXCL8 (1 : 50, Bender Medical Systems, Vienna, Austria, clone NAPII); mouse monoclonal anti-CXCL1 (1 : 25, R&D Systems, Wiesbaden, Germany, clone 20326·1); rabbit polyclonal anti-CXCL10 (1 : 500, Pepro Tech EC Ltd, London, UK); rabbit polyclonal anti-CXCL9 (1 : 800, Pepro Tech); mouse monoclonal anti-IFN-γ (1 : 50, R&D Systems, clone 42705·111); mouse monoclonal anti-CXCR1 (1 : 100, R&D Systems, clone 42705·111); mouse monoclonal anti-CXCR2 (1 : 100, R&D Systems, clone 48311·211); mouse monoclonal anti-CXCR3 (1 : 4000, R&D Systems, clone 49801·111).
For CXCL8, CXCL1, CXCL10, CXCL9, IFN-γ, CD3, CD68 and CD34 staining consecutive 4 µ cryostat sections of tumour tissue were incubated for 30 min at room temperature (RT) in methanol/H2O2 (40 : 1). Sections for CXCL10 and CXCL9 immunohistochemistry were treated additionally with proteinase K (1 µg/ml diluted in PBS 1 : 66, 10 min at 37°C; Sigma, Deisenhofen, Germany). Formalin-fixed tissue sections for chemokine receptor CXCR1 staining were deparaffinized and treated with proteinase K, as described above, while tissue sections for chemokine receptors CXCR2 and CXCR3 were treated with 0·01 m citrate-buffer followed by exposure to microwaves (3 × 5 min). Non-specific binding sites were blocked with buffered casein solution (Power Block Universal Blocking Reagent, Bio Genex, San Ramon, USA) by incubation for 10 min at RT. Prepared sections were incubated overnight at 4°C with the first-step antibody of choice. A biotin–streptavidin–peroxidase antibody detection system (Super Sensitive Multilink HRP Detection System, Bio Genex) was used according to the manufacturer's instructions with 3,3′-diaminobenzidine tetra-hydrochloride (DAB) solution as substrate. To exclude non-specific staining, the first antibody was replaced by an isotype control antibody.
Quantification of CXCL8 and CXCL1 expression
The percentage of CXCL8 and CXCL1 positive gastric carcinoma cells was scored in three grades (grade 0 = none, grade 1 = 1–40%, grade 2 = 41–75%, grade 3 = 75–100% chemokine expressing tumour cells). In addition, the intensity of chemokine expression by the tumour cells was determined (grade 0 = none, grade 1 = low, grade 2 = moderate, grade 3 = strong chemokine expression). The multiplication of these two grading scores calculates the immune-reactive score (IRS) for CXCL8 and CXCL1 expression in stained tissues (% chemokine positive tumour cells × staining intensity = IRS). A similar procedure is used routinely in surgical pathology for the quantification of hormone receptor expression in mammary carcinoma [14].
Quantification of microvessels in gastric carcinoma
Blood vessels in the tumour were highlighted by staining endothelial cells for CD34 as described above. Vessel counts were assessed by two observers in six areas of gastric carcinoma with highest neovascularization. These areas were identified by scanning at low power (×40) with light microscopy. Vessel counting was performed at ×400 magnification in the designated areas and the average count was calculated. A vessel lumen was not required for identification of a microvessel and single cells, or cell clusters, were counted. Large vessels with thick muscular walls, or with lumina greater 50 µm, were excluded from the count. This method is performed according to procedures established in the literature [15,16].
Statistical analysis
Differences in CXCL8 protein expression (expressed as IRS values, see above) between intestinal- and diffuse-type gastric carcinoma were analysed by Student's t-test. Relationships between microvessel counts and CXCL8 protein expression was examined by linear regression. Both test results were considered as significant at the level of P = 0·05.
RESULTS
CXCL8 and CXCL1 expression by carcinoma cells is significantly stronger in diffuse-type gastric carcinoma than in intestinal-type gastric carcinoma
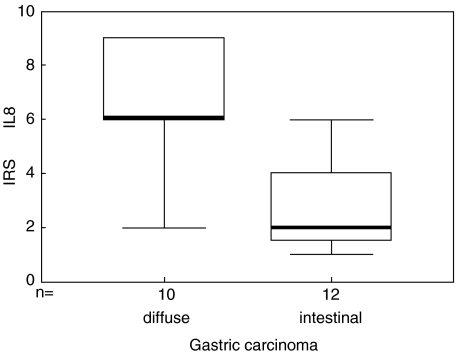
Carcinoma cells in all patients with diffuse-type (10/10; Fig. 1a) and in all patients with intestinal-type of gastric carcinoma (12/12; Fig. 1b) expressed CXCL8. In contrast, CXCL1 was expressed in 70% of specimens from patients with diffuse-type carcinoma cells (7/10; Fig. 1d), while only 8% of patients with intestinal-type carcinoma cells were positive for CXCL1 expression (1/12; Fig. 1e). The number and intensity of CXCL8 and CXCL1 expression by tumour cells was significantly stronger in diffuse-type gastric carcinoma (Figs 1a,d) compared to intestinal-type gastric carcinoma (Figs 1b,e). This resulted in significantly higher immunereactive scores (IRSs) for CXCL8 (P < 0·01; Fig. 2) and CXCL1 in diffuse-type gastric carcinoma compared with the IRS values calculated for intestinal-type gastric carcinomas.
Fig. 1.
CXCL8 and CXCL1 expression by gastric carcinoma cells, endothelial cells and neutrophils by immunohistochemistry. (a–c) CXCL8 was expressed by carcinoma cells (arrowheads) in all patients with gastric carcinoma. Both the number of CXCL8-expressing tumour cells as well as the intensity of CXCL8 expression by tumour cells was significantly stronger in diffuse- (a) compared to intestinal (b) type-gastric carcinoma. The inset in (a) shows expression of CXCL8 by endothelial cells in the tumour tissue. The isotype control for CXCL8 is completely negative (c). (d–f) CXCL1 was expressed by carcinoma cells (arrowheads) of diffuse-type gastric carcinoma (d), whereas expression of CXCL1 was not detected in most cases of intestinal-type gastric carcinoma cells (arrowheads). CXCL1 expressing tumour-infiltrating neutrophils (arrows) serve as an internal positive control (e). Inset in (e) shows negative carcinoma cells (arrowheads) and positive neutrophils (arrows) in more detail.The isotype control for CXCL1 is completely negative (f). Magnification (a–d,f) ×500; (e) ×1000.
Fig. 2.
Box–Whisker plot of CXCL8 expression in gastric carcinoma. CXCL8 expression was quantified by an immunoreactive score (IRS =% CXCL8 positive tumour cells × staining intensity of CXCL8). Boxes show the ranges of 1st and 3rd quartiles, with the horizontal bars representing median values. Gastric carcinomas of the diffuse type express significantly stronger levels of CXCL8 than gastric carcinomas of the intestinal type (P < 0·01).
In addition to tumour cells, tumour-infiltrating neutrophils (Fig. 1e) and endothelial cells of tumour capillaries (Fig. 1a, inset) expressed both chemokines.
In many patients with gastric carcinoma, in which the tumour cells expressed high levels of CXCL8 and CXCL1, the tumour stroma was infiltrated by only a few neutrophils. In contrast, many gastric carcinoma-expressing low levels of CXCL8 and CXCL1 were infiltrated by a high number of neutrophils.
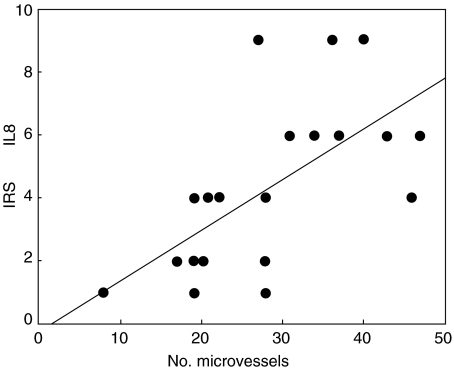
In addition to its chemoattractive function, CXCL8 is also known to increase angiogenesis in many carcinomas [17–20], which may promote tumour growth. Therefore, microvessel count (MVC) was compared with CXCL8 expression in gastric carcinoma. As shown in Fig. 3, a significant correlation between MVC and the IRS of CXCL8 was found (P < 0·01), suggesting that CXCL8 may indeed increase tumour angiogenesis in gastric carcinoma in vivo.
Fig. 3.
Relationship between immunoreactive score (IRS) of CXCL8 protein expression and microvessel counts (MVC) in intestinal- and diffuse-type gastric carcinoma (P < 0·01).
CXCL10 and CXCL9 expression by mononuclear cells in the tumour stroma correlates with tumour-infiltrating T cells in gastric carcinoma
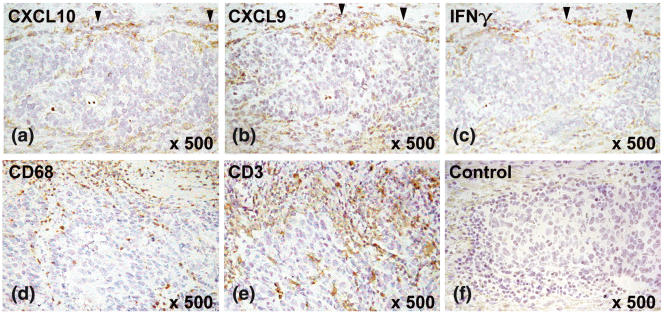
All gastric carcinomas investigated displayed a desmoplastic stroma reaction with tumour-infiltrating macrophages and T cells, both of which may contribute to tumour defence. CXCL10 and CXCL9 were expressed in seven of 10 patients with diffuse-type and in all patients (12/12) with intestinal-type gastric carcinoma (Figs 4a,b). CXCL10 and CXCL9 were expressed by tumour-infiltrating mononuclear cells, mainly macrophages, but not by the tumour cells themselves (Figs 4a,b). The number of CXCL10- and CXCL9-expressing cells increased close to the expanding tumour border. As CXCL10 and CXCL9 are known to be selective T cell attractants [11–13], their expression was compared with the distribution of T cells in the tumour stroma. CXCL10 and CXCL9 were produced mainly by macrophages in areas with high numbers of tumour-infiltrating T cells (Figs 4d,e). However, three patients with diffuse-type gastric carcinoma but without detectable CXCL10 and CXCL9 production also showed T cells and macrophages infiltrating the tumour stroma. This suggests, particularly for diffuse-type gastric carcinoma, that chemokines other than CXCL10 and CXCL9 may also be involved in the recruitment of tumour-infiltrating T cells.
Fig. 4.
CXCL10, CXCL9 and IFN-γ expression in gastric carcinoma by immunohistochemistry. The figure shows examplarily an intestinal-type gastric carcinoma. CXCL10 (a) and CXCL9 (b) were expressed by mononuclear cells, which infiltrate gastric carcinoma in areas with IFN-γ production (c). Expression was most pronounced in close proximity to the expanding tumour border (marked by arrowheads). Tumour cells themselves did not express CXCL10 and CXCL9. Serial sections stained with CD3 and CD68 identified cells at the tumour border as macrophages (d) and T cells (e). The negative control showed no staining of the mononuclear cells at the tumour border. Magnification (a–f) ×500.
As interferon-γ (IFN-γ) is known to induce CXCL10 and CXCL9 in vitro[11–13], expression of these two chemokines was compared with IFN-γ production in the peritumorous stroma. CXCL10- and CXCL9-producing macrophages were found only in close proximity to tumour-infiltrating T cells with high IFN-γ production (Fig. 4a–c). For the three diffuse gastric carcinoma patients without detectable CXCL10 and CXCL9 protein, IFN-γ was also not detected.
Expression of the CXCL8/CXCL1 receptors (CXCR1 and CXCR2) and the CXCL10/CXCL9 receptor (CXCR3) in gastric carcinoma
Immune cells are recruited by chemokines into tumour tissue only if they express the appropriate chemokine receptor that allows them to respond to locally presented chemokines.
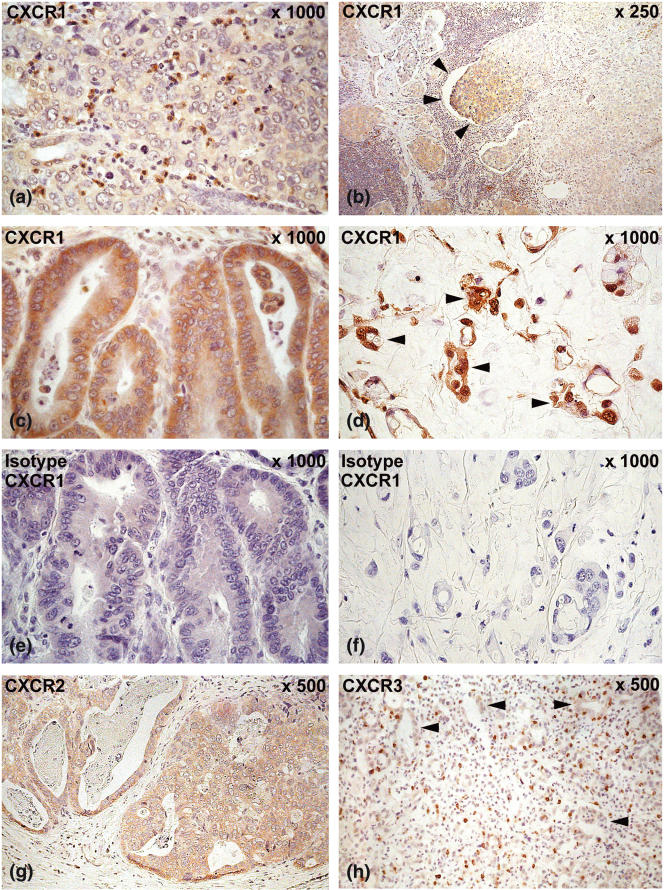
Most tumour-infiltrating neutrophils strongly expressed the CXCL8 receptor CXCR1 (Fig. 5a) whereas CXCR2, the receptor for both chemokines CXCL8 and CXCL1, was expressed only on a few neutrophils, suggesting that CXCL8 predominantly attracts neutrophils via CXCR1 into the tumour. Mononuclear cells representing macrophages and lymphocytes expressed both CXCR1 (Fig. 5a) and CXCR2 receptors. Furthermore, gastric carcinoma cells of all 22 patients expressed the CXCR1 receptor (Fig. 5b–f) and 17 of 22 patients expressed the CXCR2 receptor (Fig. 5g). Significant differences in the expression of the CXCR1 and CXCR2 receptor between diffuse and intestinal gastric carcinomas were not detected. CXCR1 and CXCR2 receptor expression was accentuated at the invasive front of the tumour (Fig. 5b). Localization of the two chemokine receptors on gastric carcinoma cells suggests that the corresponding chemokines CXCL8 and CXCL1 might exhibit an autocrine or paracrine function on the tumour cells themselves.
Fig. 5.
Expression of the chemokine receptors CXCR1, CXCR2 and CXCR3 in gastric carcinoma determined by immunohistochemistry. (a) CXCR1 was expressed strongly on neutrophils and some mononuclear cells, representing lymphocytes and macrophages infiltrating the tumour stroma. This was shown examplarily in an intestinal-type gastric carcinoma. (b–f): Carcinoma cells expressed the CXCR1 receptor (b,c): intestinal-type gastric carcinoma; (d): diffuse-type gastric carcinoma; carcinoma cells marked with arrowheads; (e,f): negative isotype controls for CXCR1). Expression was pronounced at the invasive edge (arrowheads) of the carcinoma (b). (g) Carcinoma cells expressed the CXCR2 receptor as examplarily shown in intestinal-type gastric carcinoma. Our data suggest that the corresponding chemokines CXCL8 and CXCL1 may act on carcinoma cells themselves by an autocrine or paracrine mechanism. (h) CXCR3, the receptor for CXCL10 and CXCL9, was expressed on tumour-infiltrating lymphocytes. Arrowheads mark gastric carcinoma glands. Magnification (a,c–f) ×1000; (b) ×250; (g,h) ×500.
CXCR3, the receptor for CXCL10 and CXCL9, was expressed strongly on tumour-infiltrating T lymphocytes (Fig. 5h), which gives CXCL10 and CXCL9 the capacity to recruit T lymphocytes into tumour tissue.
DISCUSSION
There is increasing evidence that CXC chemokines play an important role in tumour biology. While some CXC chemokines have antitumour activity by attracting immune cells into tumour tissue or by inhibition of tumour neovascularization, others may promote tumour growth, invasion and metastasis by direct growth stimulation, enhanced cell motility or stimulation of angiogenesis [1–5].
In our study, chemotactic signals were derived not only from carcinoma cells themselves, but also from tumour-infiltrating immune cells. Tumour cells expressed CXCL8 and CXCL1, whereas tumour-infiltrating immune cells expressed CXCL10 and CXCL9. CXCL8 and CXCL1 expression differed significantly between the two types (diffuse and intestinal) of gastric carcinoma. Tumour cells of diffuse-type gastric carcinoma expressed CXCL8 significantly stronger than tumour cells of the intestinal type. CXCL1 was expressed almost exclusively by diffuse-type gastric carcinoma but not by intestinal-type gastric carcinoma.
In the gastric mucosa infected by H. pylori, CXCL8 and CXCL1 expression correlates with neutrophil infiltration [21] and therefore it seems likely that these two chemokines recruit neutrophils into the gastric mucosa. In contrast, in gastric carcinoma CXCL8 and CXCL1 expression did not correlate with neutrophil infiltration in our study, suggesting that the function of these chemokines in gastric carcinoma may exceed their originally described roles as neutrophil attractants.
In many malignant tumours, CXCL8 and CXCL1 are known to be strong angiogenic factors [17–20]. Recently, transfection of CXCL8 resulted in an increased angiogenesis and tumourigenesis of human gastric carcinoma cells in mice [22]. In our study CXCL8 protein expressed by human gastric carcinoma cells correlated with the number of tumour vessels. This confirms former data on the m-RNA level [23] and extends this to the protein level, suggesting that CXCL8 is indeed an angiogenetic factor in human gastric carcinoma in vivo.
CXCL10 and CXCL9, which are produced by macrophages in the stroma of gastric carcinoma, are both strong angiostatic factors [1–4,11–13]. These two chemokines, which derive from tumour-infiltrating mononuclear cells, may be able to antagonize the angiogenetic capacity of CXCL8 and CXCL1 produced by gastric carcinoma cells themselves. A complex interplay between the angiostatic CXC chemokines CXCL10 and CXCL9 produced in the tumour stroma and the angiogenetic CXC chemokines CXCL8 and CXCL1 may modulate tumour vascularization in gastric carcinoma.
The expression pattern of CXCL10 and CXCL9 correlated in intestinal-type gastric carcinoma with tumour-infiltrating T lymphocytes expressing the corresponding chemokine receptor CXCR3. These data suggest that CXCL10 and CXCL9 may regulate the positioning of T cells between carcinoma cells. However, in diffuse-type gastric carcinoma, 30% of the patients in this study produced no detectable CXCL10 and CXCL9 despite a dense infiltration of the tumour stroma by T lymphocytes. Therefore, in diffuse-type gastric carcinoma, other chemokines with T cell attractant capacity as CXCL11 (I-TAC), may be additionally involved in the recruitment of T cells. CXCL8 and CXCL1 are often considered to be specific for neutrophils, although some reports confirm the ability of these chemokines to induce chemotactic activity in T cells [24,25]. We were able to detect strong expression of CXCL8 and CXCL1 by tumour cells of diffuse-type gastric carcinoma and a clear expression of the corresponding receptors CXCR1 and CXCR2 on lymphocytes. This suggests that CXCL8 and CXCL1 may contribute in addition to CXCL10 and CXCL9 to the attraction of tumour-infiltrating T lymphocytes in gastric carcinoma.
CXCL10- and CXCL9-producing mononuclear cells were found only in close proximity to tumour-infiltrating T cells with IFN-γ production. This confirms previous in vitro data, that IFN-γ induces CXCL10 and CXCL9 expression, both of which may enhance the T cell-mediated immunity directed against gastric carcinoma cells.
However, the clear difference in expression of CXCL8 and CXCL1 between diffuse- and intestinal-type gastric carcinoma cannot be explained sufficiently by the traditional functions of these chemokines. Recent in vitro data suggest that chemokines, such as CXCL8 and CXCL1, modulate growth and invasive behaviour of malignant tumours [1–5,20,26].
In addition to CXCL8 and CXCL1 the corresponding chemokine receptors (CXCR1 and CXCR2) were expressed on gastric carcinoma cells, which may give these chemokines the ability to act on carcinoma cells in vivo. Therefore, one may speculate that the significantly different expression of CXCL8 and CXCL1 between intestinal- and diffuse-type gastric carcinoma may also modulate infiltrative and diffuse growth-pattern of gastric carcinoma in vivo via the corresponding chemokine receptors CXCR1 and CXCR2. Therefore, one may speculate that also in vivo the infiltrative and diffuse growth pattern of gastric carcinoma may be modulated by the significantly different expression of CXCL8 and CXCL1 between intestinal- and diffuse-type gastric carcinoma. These chemokine signals may be mediated via the corresponding chemokine receptors CXCR1 and CXCR2.
In human gastric carcinoma cell lines CXCL8 is shown to decrease expression of the epithelial cell adhesion molecule E-cadherin by autocrine or paracrine mechanisms [27]. In gastric carcinoma, low or absent E-cadherin expression is associated with disintegration of tissue architecture. Consequently, E-cadherin is absent in the diffuse type of gastric carcinoma [28].
It is therefore tempting to speculate, that CXCL8 may down-regulate E-cadherin in vivo via CXCR1/2 expressed on the tumour cells and so contribute − in addition to E-cadherin mutations or altered methylation of the E-cadherin promotor − to the infiltrative and diffuse growth patterns of gastric carcinoma.
In summary, our study suggests that for gastric carcinoma, CXC chemokines show pleiotropic effects on tumour biology that go beyond their originally described functions as leucocyte chemoattractants. The complex interplay between the CXC chemokine signals derived not only from gastric carcinoma cells (CXCL8, CXCL1), but also from tumour-infiltrating immune cells in the stroma (CXCL10, CXCL9), may influence tumour growth, invasive behaviour and progression. Therefore, future chemokine research in gastric carcinoma may not only be of scientific interest, but might also be of future benefit for the treatment of tumour patients.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft grant EC 203/01. We thank E. Bachmann and E. Schmitt for their excellent technical assistance. We also thank H.P. Vollmers for reading the manuscript and B. Puppe for the statistical analysis.
REFERENCES
- 1.Wang JM, Deng X, Gong W, et al. Chemokines and their role in tumor growth and metastasis. J Immunol Meth. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 2.Moore BB, Arenberg DA, Addison F, et al. Tumor angiogenesis is regulated by CXC chemokines. J Lab Clin Med. 1998;132:97–103. doi: 10.1016/s0022-2143(98)90004-x. [DOI] [PubMed] [Google Scholar]
- 3.Belperio JA, Keane MP, Douglas AA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 4.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Strieter RM. Chemokines: not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001;2:285–6. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]
- 6.Kupers H, Adami HO, Trichopoulos S. Infection as a maior preventable cause of human cancer. J Int Med. 2000;248:171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 7.Lauren P. The two histopathological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Patholog Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Luster AD. Chemokines − chemotactic cytokines that mediate inflammation. New Engl J Med. 1998;338:436–47. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 9.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 10.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–73. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Liao BF, Rabin RL, Yannelli JR, et al. Human MIG chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–14. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loetscher M, Gerber B, Loetscher P, et al. Chemokine receptor specific for IP-10 and MIG. structure, function and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber JM. MIG and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 14.Remmele W, Stegner HE. Vorschlag zur einheitlichen Definierung eines immunreaktiven Score (IRS) für den immunhistochemischen Östrogenrezeptornachweis (ER-ICA) im Mammakarzinomgewebe. Pathologe. 1987;8:138–40. [PubMed] [Google Scholar]
- 15.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis − correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 16.Tanigawa N, Amaya H, Matsumura M, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res. 1996;56:2671–6. [PubMed] [Google Scholar]
- 17.Smith DR, Polverini PJ, Kunkel SL, et al. Inhibition of IL-8 attentuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–15. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda J, Kuniyasu H, Crispens MA, et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto J, Sakaguchi H, Aoki I, et al. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–5. [PubMed] [Google Scholar]
- 20.Inoue K, Slaton JW, Eve BY, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–19. [PubMed] [Google Scholar]
- 21.Eck M, Schmausser B, Scheller K, et al. CXC chemokines Groα/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. 2000;122:192–9. doi: 10.1046/j.1365-2249.2000.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitadai Y, Takahashi Y, Haruma K, et al. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647–53. doi: 10.1038/sj.bjc.6690742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitadai Y, Haruma K, Sumii K, et al. Expression of interleukin-8 correlates with vascularity in human gastric cancer. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen CG, Anderson AO, Appella E, et al. The neutrophil- activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–6. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 25.Tani K, Su SB, Utsonomiya I, et al. Interferon gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–7. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Luca M, Huang S, Gershenwald JE, et al. Expression of interleukin-8 by human melanoma cells upregulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–13. [PMC free article] [PubMed] [Google Scholar]
- 27.Kitadai Y, Haruma K, Mukaida N, et al. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin Canc Res. 2000;6:2735–40. [PubMed] [Google Scholar]
- 28.Mayer B, Johnson JP, Leitl F, et al. E-Cadherin expression in primary and metastastic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–5. [PubMed] [Google Scholar]