Abstract
Neurogenic inflammation is regulated by sensory nerves and characterized by extravasation of plasma proteins and infiltration of neutrophils from post-capillary venules and arteriolar vasodilatation. Although it is well established that substance P (SP) interacts with the neurokinin 1 receptor (NK1R) to initiate neurogenic inflammation, the mechanisms that terminate inflammation are unknown. We examined whether neutral endopeptidase (NEP), a cell-surface enzyme that degrades SP in the extracellular fluid, terminates neurogenic inflammation in the colon. In NEP knockout mice, the SP concentration in the colon was ≈2.5-fold higher than in wild-type mice, suggesting increased bioavailability of SP. The extravasation of Evans blue-labeled plasma proteins in the colon of knockout mice under basal conditions was ≈4-fold higher than in wild-type mice. This elevated plasma leak was attenuated by recombinant NEP or the NK1R antagonist SR140333, and is thus caused by diminished degradation of SP. To determine whether deletion of NEP predisposes mice to uncontrolled inflammation, we compared dinitrobenzene sulfonic acid-induced colitis in wild-type and knockout mice. The severity of colitis, determined by macroscopic and histologic scoring and by myeloperoxidase activity, was markedly worse in knockout than wild-type mice after 3 and 7 days. The exacerbated inflammation in knockout mice was prevented by recombinant NEP and SR140333. Thus, NEP maintains low levels of SP in the extracellular fluid under basal conditions and terminates its proinflammatory effects. Because we have previously shown that intestinal inflammation results in down-regulation of NEP and diminished degradation of SP, our present results suggest that defects in NEP expression contribute to uncontrolled inflammation.
Numerous proinflammatory agents have been implicated in the acute and chronic phases of intestinal inflammation in humans and experimental animals. Prominent among these mediators is substance P (SP), a peptide expressed by extrinsic sensory and by enteric neurons (1). Sensory nerves release SP within the spinal cord to convey afferent information from peripheral tissues. During inflammation and injury they also release SP locally within the tissues they innervate and thereby participate in the efferent regulation of peripheral tissues. This “neurogenic inflammation” is characterized by arteriolar vasodilation and by extravasation of plasma proteins and neutrophils from postcapillary venules (2). SP mediates many aspects of neurogenic inflammation by interacting with the neurokinin-1 receptor (NK1R). Thus, SP interacts with the NK1R on endothelial cells of postcapillary venules in the airway and intestine to cause formation of gaps, which allows extravasation of plasma proteins (3, 4).
Neurogenic mechanisms involving SP and the NK1R contribute to intestinal inflammation. Direct evidence for a role of SP and the NK1R in acute intestinal inflammation derives from the observations that antagonism and deletion of the NK1R strongly suppress intestinal inflammation in rats and mice induced by toxin A from Clostridium difficile (5, 6). Moreover, this toxin induces release of SP and consequent internalization of the NK1R on enteric neurons, suggesting that neurogenic mechanisms participate in the inflammatory response (7). Furthermore, NK1Rs are markedly up-regulated on venules, arterioles, and lymph nodules in the inflamed human intestine (8). SP levels are increased in the inflamed intestine of patients with ulcerative colitis and in rats infected with Trichinella spiralis (9, 10). However, it is not known whether alterations in the SP content of inflamed tissue are due to altered synthesis or degradation.
Although it is well established that SP and the NK1R may initiate inflammation, less is known about how inflammation is terminated. The cell-surface enzyme neutral endopeptidase (NEP) degrades SP in the extracellular fluid and may thereby terminate its proinflammatory effects (11, 12). Administration of NEP inhibitors potentiates neurogenic inflammation in the airway by preventing the degradation of SP (13, 14). Genetic deletion of NEP leads to markedly elevated plasma extravasation in the small intestine that is prevented by administration of an NK1R antagonist, suggesting that this is mediated by diminished degradation of SP (15). In the inflamed intestine of rats infected with T. spiralis, NEP activity is markedly down-regulated, which results in diminished degradation of SP (16). These observations raise the possibility that down-regulation of NEP and diminished degradation of SP contribute to uncontrolled inflammation because of prolonged activation of the NK1R.
In the present investigation, we examined the hypothesis that absence of NEP exacerbates colitis because of diminished degradation of SP and continuous activation of the NK1R. We assessed colitis induced by dinitrobenzene sulfonic acid (DNBS) in wild-type (NEPwt) and NEP knockout (NEP−/−) mice. We administered recombinant NEP and a selective antagonist of the NK1R to determine whether the massive injury observed in NEP−/− mice was because of diminished degradation of SP and consequent overactivation of the NK1R.
METHODS
Animals.
NEP−/− mice back crossed to the C57BL/6 background were generated as described (Harvard Medical School) (17). Wild-type littermates and C57BL/6 mice (Taconic Farms) of the same background as the NEP−/− mice were used interchangeably as controls. Mice were maintained in a specific-pathogen-free facility, and males and females aged 10–20 weeks were used.
Materials.
Recombinant human NEP was from Khepri Pharmaceuticals (South San Francisco, CA). SR140333, which is an effective antagonist of the NK1R in the mouse (4), was from Sanofi (Paris). A SP enzyme-linked immunoassay kit was from Cayman Chemicals (Ann Arbor, MI). Osmotic pumps were from Alza. DNBS was from ICN. The sources of other reagents have been described (4).
Plasma Extravasation.
We used Evans blue to measure extravasation of plasma proteins (4, 15). Mice were anesthetized with xylazine (25 mg/kg i.m.) and ketamine (100 mg/kg i.m.). Evans blue (30 mg/kg) in 0.9% saline was injected into a femoral vein. Seven minutes later, mice were transcardially perfused with 70 ml of 100 mM PBS (pH 7.4) containing 100 units/ml heparin and 200 ml 4% paraformaldehyde in PBS. Evans blue was extracted from the colon by incubation in 1 ml of formamide for 48 hr and was quantified by measuring absorption at 620 nm. Results are expressed as ng of Evans blue per mg of dry tissue. Thiorphan, phosphoramidon (NEP inhibitors, 2.5 mg/kg i.v.), and SR 140333 (1 μmol/kg i.v.) was injected 5 min before Evans Blue. Recombinant human NEP (3 mg/kg i.v.) was injected 10 min before the Evans blue.
Induction of Colitis.
Because NEP−/− mice are sensitive to endotoxic shock (17), we induced colitis with a very low dose of DNBS (4 mg per mouse) by using a modification (18) of the method first described in rats (19). In preliminary experiments, this dose of DNBS was found to induce reproducible colitis without mortality. This low dose also allowed detection of increased inflammation in NEP−/− animals, which may have been masked if maximal doses of DNBS were used. Mice were anesthetized by Enflurane. DNBS (4 mg in 100 μl of 50% ethanol) was injected into the rectum through a catheter inserted 4–5 cm proximally to the anus. Carrier alone (100 μl of 50% ethanol) was administered in control experiments. Drugs were infused subcutaneously from osmotic pumps implanted beneath the back skin under Enflurane anesthesia 24 hr before administration of DNBS. NEP (3 mg⋅kg−1⋅d−1), SR140333 (50 mg⋅kg−1⋅d−1), or BSA (BSA, control, 3 mg⋅kg−1⋅d−1) was dissolved in saline and infused 24 hr before and for 3 or 7 days after induction of colitis. At 0, 3, 7, or 21 days after DNBS, the proximal colon was removed, and macroscopic damage was immediately scored. Tissues for histological examination were fixed in 10% formalin, and sections were stained with hematoxylin and eosin and scored for inflammation. Myeloperoxidase (MPO) activity (units/mg tissue) in colonic tissues was measured as an index of granulocyte infiltration (20, 21).
SP Measurement.
Full-thickness colon was processed to extract SP, which was quantified by using ELISA (pg of SP per mg of wet tissue).
Statistical Analysis.
Results are expressed as mean ± SEM (n > 6 mice per group) and are compared by using Student’s t test or ANOVA and Bonferroni t test.
RESULTS
Plasma Extravasation in the Colon.
We have previously shown that deletion of NEP induces extravasation of plasma proteins in the mouse duodenum under basal conditions, which is prevented by administration of NEP or an antagonist of the NK1R (15). To determine whether deletion of NEP also causes plasma extravasation in the colon, we measured the leak of Evans blue in the proximal and distal colon of NEP−/− and wt mice.
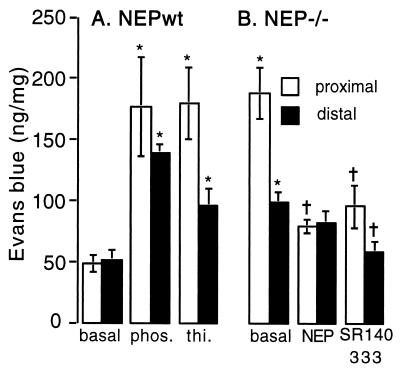
Under basal conditions, extravasation of Evans blue in the proximal colon of NEPwt mice was 49 ± 7 ng/mg (n = 7) and in NEP−/− mice was 188 ± 21 (n = 10, P < 0.05) (Fig. 1). The elevated basal leak in NEP−/− mice was markedly attenuated by intravenous administration of recombinant NEP (80 ± 6 ng/mg, n = 6) or SR14033 (96 ± 17 ng/mg, n = 6). Conversely, the low basal leak in NEPwt mice was elevated to that observed in NEP−/− mice by the NEP inhibitors phosphoramidon (177 ± 40 ng/mg, n = 6) and thiorphan (180 ± 29 ng/mg, n = 6). Similar results were obtained in the distal colon (Fig. 1). Thus, deletion or inhibition of NEP causes elevated plasma protein extravasation in the colon, which is probably cause by diminished degradation of SP. These results suggest that NEP terminates the proinflammatory effects of SP.
Figure 1.
Extravasation of Evans blue in the proximal and distal colon of NEPwt (A) and NEP−/− mice (B). NEPwt mice received the NEP inhibitors phosphoramidon (phos.) or thiorphan (thi.). NEP−/− mice received recombinant NEP and SR 140333. Extravasation is expressed as ng of Evans blue per mg of dry tissue. ∗, P < 0.05 compared with basal for NEPwt mice; †, P < 0.05 compared with basal for NEP−/− mice (ANOVA, Bonferroni t test). n = 6–11 mice per group.
Experimental Colitis.
The elevated basal extravasation of plasma proteins in the colon of NEP−/− mice suggests that these animals are more sensitive to inflammation. We investigated this possibility by inducing colitis.
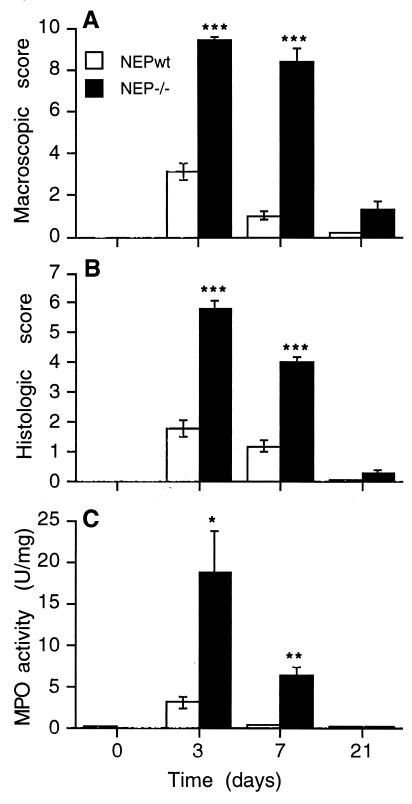
Before administration of DNBS, the colons of NEP−/− and NEPwt mice were macroscopically and histologically normal, and the MPO activity in colonic extracts was the same. Administration of carrier (100 μl of 50% ethanol) did not induce detectable inflammation in NEP−/− or wt mice (data not shown). However, 3 days after DNBS treatment, macroscopic evaluation of NEP−/− mice revealed massive ulceration of the colonic mucosa and severe intraabdominal adhesions. The macroscopic score in NEP−/− mice (9.4 ± 2.0, n = 8) was 3-fold higher than in NEPwt mice (3.1 ± 0.4, n = 8, P < 0.001) (Fig. 2A). At 7 days, most of the gross features of this acute injury and inflammation remained in NEP−/− animals (8.4 ± 0.6, n = 8), whereas inflammation in the NEPwt mice was almost fully resolved (1.0 ± 0.2, n = 8, P < 0.001). At 21 days, there was no significant difference in macroscopic evaluation of colitis in NEP−/− and NEPwt mice.
Figure 2.
DNBS-induced colitis in NEPwt and NEP−/− mice assessed by a macroscopic score (A), histological score (B), and measurement of MPO activity in the colon (C) at 0, 3, 7, or 21 days after DNBS. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.02 compared with NEPwt (Student’s t test). n = 6 (0, 21 days); n = 8 (3, 7 days).
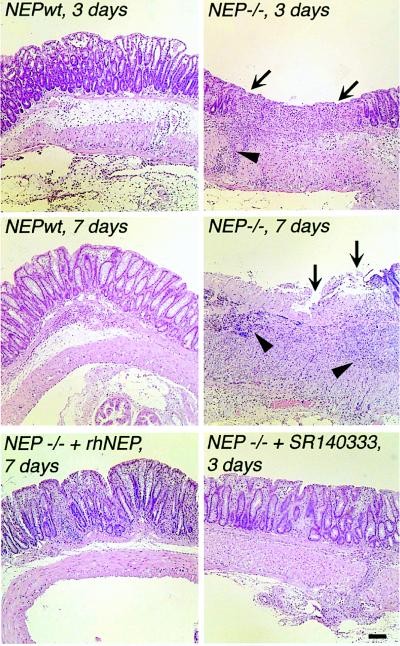
Histological examination of the colon 3 days after DNBS administration revealed massive infiltration of granulocytes throughout the muscle layers and a complete destruction of the mucosa of NEP−/− mice (score 5.8 ± 0.3, n = 8), whereas all of the characteristics of inflammation were diminished in NEPwt mice (1.8 ± 0.3, n = 8, P < 0.001) (Figs. 2B and 3). After 7 days, the histological score was still 4-fold higher in the NEP−/− mice (4 ± 0.2, n = 8), whereas the mucosa of NEPwt mice appeared normal (1 ± 0.2, n = 8, P < 0.001). At 21 days, there was no significant difference in the histological evaluation of the colons from NEP−/− and NEPwt mice, with the exception of a small residual ulcer found in one NEP−/− mouse.
Figure 3.
Histological examination of colon from NEPwt and NEP−/− mice at 3 and 7 days. Inflammation was markedly more severe in NEP−/− mice at 3 and 7 days. Note the severe mucosal ulceration and loss of mucosal architecture in NEP−/− mice at 3 and 7 days (arrows) and the large influx of granulocytes into the mucosa and muscle layers of NEP−/− mice at 7 days (arrowheads). Administration of recombinant human NEP (rhNEP) or SR140333 to NEP−/− mice diminished inflammation at 7 days and 3 days, respectively. (Bar = 100 μm.)
After 3 days, MPO activity in the colon of NEP−/− mice (18.7 ± 5 units/mg, n = 8) was 6-fold higher than in NEPwt animals (3.2 ± 0.7 units/mg, n = 8, P < 0.02) (Fig. 2C). After 7 days, MPO activity remained elevated in NEP−/− mice (6.3 ± 1.5 units/mg, n = 8), indicating persistent inflammation, whereas MPO activity in NEPwt mice (0.5 ± 0.1 units/mg, n = 8, P < 0.01) was similar to that observed on before administration of DNBS. After 21 days, MPO activity in NEP−/− and NEPwt mice was similar to that measured before administration of DNBS.
Thus, colitis in NEP−/− mice is significantly more severe at all time points than in NEPwt mice, which indicates that NEP serves to dampen DNBS-induced colitis.
Prevention of Colitis by Administration of Recombinant NEP.
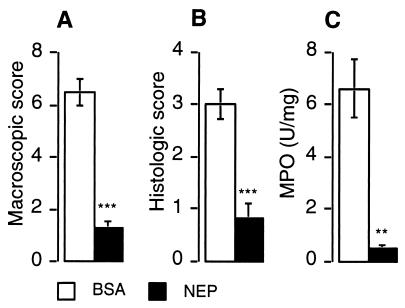
To determine whether administration of NEP attenuated the exacerbated colitis, we treated NEP−/− mice with recombinant NEP 24 hr before and for 7 days after inducing colitis. The macroscopic score in NEP−/− mice receiving BSA (control) was 6.5 ± 0.5 (n = 7) and in NEP-treated mice was 1.3 ± 0.2, n = 7, P < 0.001) (Fig. 4A). The histological score in control mice was 3.0 ± 0.3 (n = 7), and in NEP-treated mice was 0.8 ± 0.3, n = 7, P < 0.001) (Figs. 3 and Fig. 4B). MPO activity in control mice was 6.6 ± 1.1 (n = 7) and in NEP-treated mice was 0.5 ± 0.1, n = 7, P < 0.01) (Fig. 4C). Thus, NEP administration to NEP−/− mice reduced all indices of colitis to values observed in NEPwt mice.
Figure 4.
Effects of administration of recombinant NEP on DNBS-induced colitis in NEP−/− mice assessed by a macroscopic score (A), histological score (B), and measurement of MPO activity in the colon (C) at 7 days after DNBS. ∗∗∗, P < 0.001; ∗∗, P < 0.01 compared with control animals that received BSA (Student’s t test).
Prevention of Colitis by Administration of an NK1R Antagonist.
Deletion of NEP markedly exacerbates colitis whereas replenishment of NEP reduces inflammation, suggesting that colitis is mediated by an NEP substrate, such as SP. To evaluate this possibility, we administered a specific antagonist of the NK1R to NEP−/− and NEPwt mice 24 hr before and for 3 days after inducing colitis, when the inflammation was maximal in both groups.
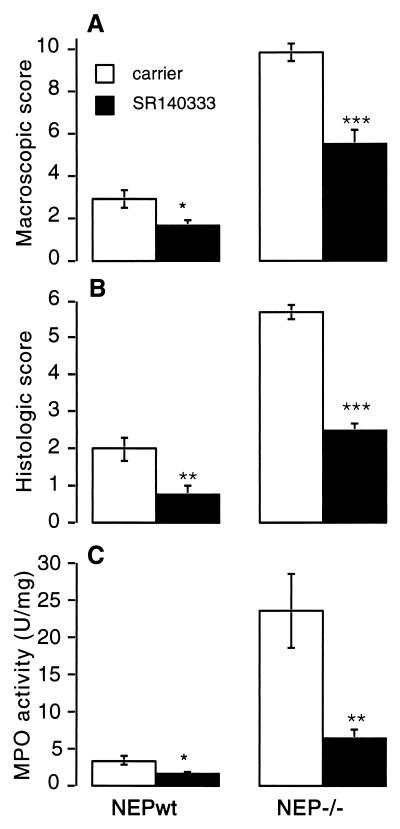
Antagonism of the NK1R significantly diminished inflammation in NEPwt and NEP−/− mice. In NEPwt and NEP−/− mice, the macroscopic score was diminished approximately 2-fold by SR140333 (NEPwt: carrier, 2.9 ± 0.4; SR140333, 1.6 ± 0.3; n = 8, P < 0.05; NEP−/−: carrier, 9.8 ± 0.4; SR140333, 5.5 ± 0.6; n = 8, P < 0.001) (Fig. 5A). Histological assessment showed that SR140333 diminished mucosal damage in both groups only (Fig. 3). The histological score in SR140333-treated NEPwt and NEP−/− mice (respectively 0.8 ± 0.2, n = 8, P < 0.01; 2.5 ± 0.2, n = 8, P < 0.001) was 2-fold lower than in carrier-treated controls (respectively 2.0 ± 0.3; 5.7 ± 0.2) (Fig. 5B). Similarly, colonic MPO activity in SR140333-treated NEPwt and NEP−/− mice (respectively, 1.5 ± 0.3 units/mg, n = 8, P < 0.02; 6.3 ± 1.3 units/mg, n = 8, P < 0.01) was significantly lower than in carrier-treated NEPwt and NEP−/− mice (respectively, 3.3 ± 0.6 units/mg; 23.5 ± 5 units/mg) (Fig. 5C). Thus, antagonism of the NK1R in NEPwt and NEP−/− mice markedly reduces colitis, suggesting that SP is a major mediator of DNBS-induced colitis. The extent of the inhibition was slightly larger in NEPwt mice, which suggests that other NEP substrates, which do not interact with the NK1R, also contribute to colitis in the NEP−/− mice.
Figure 5.
Effects of administration of SR140333 on DNBS-induced colitis in NEPwt and NEP−/− mice assessed by a macroscopic score (A), histological score (B), and measurement of MPO activity in the colon (C) at 3 days after DNBS. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05 compared with control animals that received carrier (Student’s t test).
SP Concentration in Inflamed Colon.
To assess whether deletion of NEP resulted in increased bioavailability of SP within the colon, we measured SP levels. Before inducing colitis, the SP concentration in the colon of NEP−/− mice (70.3 ± 7.6 pg/mg, n = 6) was significantly greater than in NEPwt mice (28.3 ± 10.7 pg/mg, n = 6, P < 0.05). Three days after inducing colitis, colonic SP levels were increased in the NEP−/− (91 ± 23 pg/mg, n = 6), but this was not significantly different from levels in the uninflamed colon. In contrast, SP levels in the inflamed colon of NEPwt mice significantly decreased (9.1 ± 1.5 pg/mg, n = 6). Thus, in mice lacking NEP, SP levels in the colon are initially higher and remain elevated in inflamed tissues, whereas in NEPwt mice they decline. There is a higher bioavailability of SP in NEP−/− mice.
DISCUSSION
Genetic deletion and pharmacological inhibition of the SP-degrading enzyme NEP led to increased bioavailability of SP in colonic extracts, elevated extravasation of plasma proteins in the colon, and markedly exacerbated colitis. Administration of recombinant NEP or an NK1R antagonist attenuated plasma extravasation and colitis. Thus, deletion or inhibition of NEP results in diminished degradation of SP in the extracellular fluid, increased availability of SP, and prolonged activation of the NK1R. These results indicate that NEP serves to maintain a low concentration of SP in the extracellular fluid under basal circumstance and to terminate its proinflammatory effects. NEP is widely distributed and may serve this role in multiple tissues. Because we have previously shown that intestinal inflammation results in marked down-regulation of NEP and diminished degradation of SP (16), our present results support the possibility that defects in mechanisms that terminate signaling by SP contribute to uncontrolled inflammation.
NEP Terminates the Proinflammatory Effects of SP.
Deletion of NEP or administration of two different NEP inhibitors resulted in an ≈4-fold elevation in extravasation of plasma proteins in the colon that was attenuated by administration of NEP and an NK1R antagonist. We have previously reported similar findings in the duodenum and urinary bladder of the mouse (15), which supports our results in the colon. Other proteases also degrade SP in the extracellular fluid. Angiotensin-converting enzyme (ACE, EC 3.4.15.1) degrades SP (22), and administration of ACE inhibitors induces widespread plasma extravasation that is prevented by an NK1R antagonist (23). The elevated plasma extravasation observed in mice lacking NEP or treated with inhibitors of NEP and ACE may be mediated by diminished degradation of other peptides in addition to SP. NEP and ACE degrade bradykinin, which induces plasma extravasation directly, by activating the bradykinin-2 receptor on endothelial cells, and indirectly by stimulating SP release from sensory nerves (4). The elevated plasma extravasation observed in mice lacking NEP or treated with NEP or ACE inhibitors is reduced by antagonism or deletion of the bradykinin-2 receptor, indicating that diminished degradation of bradykinin also contributes to the extravasation of plasma proteins (23). It is therefore likely that SP and bradykinin are continuously released under basal circumstances and that NEP and ACE maintain low levels of these peptides in extracellular fluid. Our observation that SP levels were >2-fold higher in the colon of NEP−/− mice that in NEPwt animals supports this suggestion. However, despite our findings that deletion of NEP resulted in increased bioavailability of SP and elevated extravasation of plasma proteins under basal conditions, there were no signs of spontaneous full-blown inflammation. Thus, diminished degradation and increased bioavailability of SP or other NEP substrates per se will not cause fulminant inflammation. Agents other than NEP substrates must initiate inflammation. Our results clearly indicate SP contributes to the development of colitis and that without NEP the proinflammatory effects of SP are markedly enhanced.
In mice lacking NEP, DNBS-induced colitis was markedly more severe than in wild-type animals. The carrier for DNBS, 50% ethanol, did not induce colitis in NEP−/− mice, indicating that the severe DNBS-induced colitis observed in these animals represents inflammation and not nonspecific injury. This exacerbated colitis was apparent at 3 days, when the inflammatory response in wild-type animals was maximal, and also at 7 days, when inflammation in wild-type animals was mostly resolved. Thus, deletion of NEP affects both the magnitude and duration of the inflammatory response, suggesting that NEP dampens inflammation. Administration of recombinant NEP to NEP−/− mice markedly diminished all indices of inflammation to levels similar to those observed in wild-type animals, suggesting that the exaggerated inflammation was mediated by a NEP substrate. This substrate is probably, but not exclusively, SP, because treatment with an NK1R antagonist also markedly reduced inflammation in the NEP−/− mice. Furthermore, SP levels in the colon were over 2-fold higher in NEP−/− mice both under basal conditions and during colitis, suggesting an increased bioavailability of SP in the absence of NEP. During colitis, NEP must function to degrade newly released SP in the extracellular fluid and terminate its proinflammatory effects. Although diminished degradation of SP is a major cause of the exacerbated colitis, other peptides such as bradykinin may also contribute to colitis because treatment of NEP−/− mice with the NK1R antagonist did not suppress inflammation to levels observed in NEPwt mice.
Deletion of NEP results in elevated plasma extravasation and exacerbated colitis that are mediated by SP and the NK1R, indicating that degradation of SP in the extracellular fluid is a major and important mechanism for attenuating cellular responses to SP. Responses to SP are also terminated by mechanisms operating at the level of the NK1R (24). NK1R phosphorylation and interaction with β-arrestins uncouples the receptor from G proteins and terminates signal transduction (25, 26), and NK1R endocytosis depletes the plasma membrane of receptors available to bind SP in the extracellular fluid (3, 27). In view of the existence of these additional mechanisms for terminating signaling by the SP, it is perhaps surprising that the increased bioavailability of SP in NEP−/− mice results in exaggerated responses to SP. Presumably, SP degradation is the major mechanism for terminating the proinflammatory effects of SP. It is also possible that the proinflammatory responses to SP rapidly resensitize even in the continued presence of SP. In cell lines expressing the NK1R, responses to SP rapidly resensitize by mechanisms that involve endocytosis, dephosphorylation, and recycling of the NK1R (28). The effects of SP on plasma extravasation also resensitize efficiently (3). Although long-term exposure to agonists often results in down-regulation of G protein-coupled receptors as a result of diminished synthesis and increased degradation (24), little is known about the down-regulation of the NK1R in the continued presence of SP.
Neurogenic Mechanisms of Intestinal Inflammation.
Our observation that deletion of NEP results in exacerbated inflammation suggests that neurogenic mechanisms that are mediated by SP and the NK1R play an important role in DNBS-induced colitis. This suggestion is supported by the findings that stimulation of sensory nerves with capsaicin or administration of exogenous SP induces plasma extravasation in the intestine that is mediated by the NK1R (4). Antagonism or deletion of the NK1R strongly inhibits acute intestinal inflammation induced by toxin A from C. difficile (5, 6), providing further support for the role of neurogenic mechanisms in intestinal inflammation. Neurogenic mechanisms may also contribute to inflammatory bowel disease because there are alterations of innervation and expression of SP and the NK1R in the inflamed human colon (8, 9).
SP has many proinflammatory effects. It stimulates gap formation between endothelial cells of postcapillary venules to permit extravasation of plasma proteins (2, 3) and induces expression of adhesion molecule by endothelial cells to enable attachment and infiltration of immune cells into inflamed tissues (29). SP also causes degranulation of intestinal mast cells and thereby facilitates the release of other inflammatory mediators (30). These effects of SP are mediated by the NK1R, because they are effectively blocked by receptor antagonists. Our observation that deletion of NEP causes increased extravasation of plasma proteins and exacerbated colitis, assessed by macroscopic and microscopic evaluation and measurement of neutrophil infiltration, indicate that NEP terminates many of these proinflammatory effects of SP.
Down-regulation of NEP in the intestine may contribute to uncontrolled intestinal inflammation. SP levels declined in the inflamed colon of wild-type mice treated with DNBS, suggesting decreased degradation and perhaps up-regulation of NEP in this model. However, we have previously reported that NEP activity is diminished by 84-fold in the mucosa and circular muscle and by 12-fold in the longitudinal muscle layer in the inflamed intestine of rats infected with Trichinella spiralis, which results in a 2- to 6-fold reduction in the rate of SP degradation (16). Coupled with our observation of exaggerated colitis in NEP−/− mice, these findings suggests that down-regulation of NEP contributes to intestinal inflammation in experimental animals. It remains to be determined whether down-regulation of NEP contributes to inflammation in the human intestine.
Acknowledgments
This work was supported by DK39957 (N.W.B.), DK52388 (E.F.G., N.W.B., and S.M.C.), and the Crohns and Colitis Foundation of America (N.W.B.).
ABBREVIATIONS
- SP
substance P
- NK1R
neurokinin-1 receptor
- NEP
neutral endopeptidase
- NEPwt
wild-type mice
- NEP−/−
knockout mice
- DNBS
dinitrobenzene sulfonic acid
- MPO
myeloperoxidase
References
- 1.Otsuka M, Yoshioka K. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 2.McDonald D M, Bowden J J, Baluk P, Bunnett N W. Adv Exp Med Biol. 1996;410:453–462. [PubMed] [Google Scholar]
- 3.Bowden J J, Garland A M, Baluk P, Lefevre P, Grady E F, Vigna S R, Bunnett N W, McDonald D M. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figini M, Emanueli C, Grady E F, Kirkwood K, Payan D G, Ansel J, Gerard C, Geppetti P, Bunnett N W. Am J Physiol. 1997;272:G785–G793. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
- 5.Pothoulakis C, Castagliuolo I, LaMont J T, Jaffer A, O’Keane J C, Snider R M, Leeman S E. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard N P, Pothoulakis C. J Clin Invest. 1998;101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantyh C R, Pappas T N, Lapp J A, Washington M K, Neville L M, Ghilardi J R, Rogers S D, Mantyh P W, Vigna S R. Gastroenterology. 1996;111:1272–1280. doi: 10.1053/gast.1996.v111.pm8898641. [DOI] [PubMed] [Google Scholar]
- 8.Mantyh C R, Gates T S, Zimmerman R P, Welton W L, Passaro E P, Vigna S R, Maggio J E, Kruger L, Mantyh P W. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Dig Dis Sci. 1989;34:754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- 10.Swain M G, Agro A, Blennerhassett P, Stanisz A, Collins S M. Gastroenterology. 1992;102:1913–1919. doi: 10.1016/0016-5085(92)90313-n. [DOI] [PubMed] [Google Scholar]
- 11.Matsas R, Fulcher I S, Kenny A J, Turner A J. Proc Natl Acad Sci USA. 1983;80:3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto A, Lovett M, Payan D G, Bunnett N W. Biochem J. 1994;299:683–693. doi: 10.1042/bj2990683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umeno E, Nadel J A, Huang H-T, McDonald D M. J Appl Physiol. 1988;66:2647–2652. doi: 10.1152/jappl.1989.66.6.2647. [DOI] [PubMed] [Google Scholar]
- 14.Umeno E, Nadel J A, McDonald D M. J Appl Physiol. 1990;69:2131–2136. doi: 10.1152/jappl.1990.69.6.2131. [DOI] [PubMed] [Google Scholar]
- 15.Lu B, Figini M, Emanueli C, Geppetti P, Grady E F, Gerard N P, Ansel J C, Payan D G, Gerard C, Bunnett N W. Nat Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- 16.Hwang L, Leichter R, Okamoto A, Payan D, Collins S M, Bunnett N W. Am J Physiol. 1993;264:G735–743. doi: 10.1152/ajpgi.1993.264.4.G735. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Gerard N P, Kolakowski L F, Bozza M, Zurakowski D, Finco O, Carroll M C, Gerard C. J Exp Med. 1995;181:2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu B S, Vallence B A, Blennerhassett P A, Collins S M. Gastroenterology. 1998;114:A1065. [Google Scholar]
- 19.Wallace J L, Le T, Carter L, Appleyard C B, Beck P L. J Pharmacol Toxicol Methods. 1995;33:237–239. doi: 10.1016/1056-8719(95)00001-x. [DOI] [PubMed] [Google Scholar]
- 20.Bradley P P, Priebat D A, Christensen R D, Rothstein G. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 21.Boughton-Smith N K, Wallace J L, Whittle B J. Agents Actions. 1988;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 22.Skidgel R, Defendini R, Erdos E. In: Angiotensin Converting Enzyme and Its Role in Neuropeptide Metabolism. Turner A, editor. Chichester, England: Ellis Horwood; 1987. pp. 165–182. [Google Scholar]
- 23.Emanueli C, Grady E F, Madeddu P, Figini M, Bunnett N W, Parisi D, Regoli D, Geppetti P. Hypertension. 1998;31:1299–1304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- 24.Böhm S, Grady E F, Bunnett N W. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwatra M M, Schwinn D A, Schreurs J, Blank J L, Kim C M, Benovic J L, Krause J E, Caron M G, Lefkowitz R J. J Biol Chem. 1993;268:9161–9164. [PubMed] [Google Scholar]
- 26.McConalogue K, Corvera C units, Gamp P D, Grady E F, Bunnett N W. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grady E F, Garland A G, Gamp P D, Lovett M, Payan D G, Bunnett N W. Mol Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garland A M, Grady E F, Lovett M, Vigna S R, Frucht M M, Krause J E, Bunnett N W. Mol Pharm. 1996;49:438–446. [PubMed] [Google Scholar]
- 29.Quinlan K L, Song I S, Bunnett N W, Letran E, Steinhoff M, Harten B, Olerud J E, Armstrong C A, Caughman S W, Ansel J C. Am J Physiol. 1998;275:C1580–1590. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Stanisz A M, Wershil B K, Galli S J, Perdue M H. Am J Physiol. 1995;269:G85–92. doi: 10.1152/ajpgi.1995.269.1.G85. [DOI] [PubMed] [Google Scholar]