Abstract
It is believed that autoimmune phenomena and apoptosis contribute to CD4 depletion. We investigated 11 long-term (>20 years) HIV-infected haemophilia patients and 10 healthy controls. Using four-colour-fluorescence flow cytometry, we studied the proportions of CD3+CD4+ and CD3+CD4– blood lymphocytes that were CD95+, CD95L+, immune complex+ (IC+, consisting of IgM, IgG, C3d and/or gp120), and were viable or non-viable (propidium iodide+ = PI+). In addition, we studied viability of CD4+IgG+ patient lymphocytes using the apoptosis marker annexin and the permeability indicator 7-amino actinomycin D (7-AAD). HIV+ patients had a higher proportion of CD3+CD4+IgG+PI+ lymphocytes than healthy controls (median: 3·7%versus 0·3%; P = 0·00001). These non-viable IgG-coated lymphocytes might have been killed in vivo by ADCC or complement lysis; 9·1% of the circulating CD3+CD4+ blood lymphocytes were IgG+PI– (controls: 2·5%; P = 0·001). These viable IgG-coated lymphocytes might be targets for phagocytosis or anti-CD95 autoantibody-mediated apoptosis. Because HIV+ patients and healthy controls had similar proportions of PI+ or PI– CD3+CD4+ lymphocytes that carried CD95L on the surface, and because CD3+CD4+CD95L+ cells that were IgG+, C3d+ and/or gp120– were increased in HIV+ patients, the role of CD95L-induced apoptosis in long-term HIV-infected haemophilia patients remains unclear. The findings that HIV+ patients had higher proportions of CD3+CD4+CD95+ (PI+: 6·5%versus 1·4%; P = 0·00002; PI–: 55·8%versus 44·4%; P = 0·04) blood lymphocytes and that the proportion of CD4+IgG+Annexin+7-AAD– blood lymphocytes was associated inversely with peripheral CD4 counts (r = −0·636; P < 0·05) suggest that attachment of IgG to CD4+ blood lymphocytes (anti-CD95?) induces in some lymphocytes apoptosis with subsequent depletion of these IgG-coated apoptotic CD4+ lymphocytes from the circulation. We found supporting evidence for the contention that autoantibody-induced apoptotic and non-apoptotic mechanisms contribute to CD4 depletion in long-term HIV-infected haemophilia patients.
Keywords: apoptotic and non-apoptotic lymphocyte depletion mechanisms, autoantibody-induced CD4 depletion, CD8+ blood lymphocytes, four-colour-fluorescence flow cytometry, HIV-1 viral load, long-term HIV-infected haemophilia patients
INTRODUCTION
More than 20 years after the first report of AIDS in the literature, the pathogenesis of HIV-induced CD4 depletion is still unclear [1]. The interpretation of experimental findings is problematic because HIV induces many phenomena in vitro that are not observed in vivo. Moreover, immune phenomena observed for instance in lymph nodes, such as CD95 and CD95L expression on CD4+ lymphocytes, are different from those observed in peripheral blood [2]. It is now known that viral load and CD4 counts as measured in the peripheral blood are very useful markers for disease progression. In the present study, we tried to elucidate the mechanisms regulating the presence of CD3+CD4+ and CD3+CD4– lymphocytes circulating in the blood of haemophilia patients living with HIV for more than 20 years.
There is evidence that HIV regulates and mediates CD4 depletion by the induction of autoantibodies and autoreactive immune complexes (ICs) against CD4+ lymphocytes. Based on individual patient profiles, we observed in previous studies that IgM autoantibodies were formed early after HIV infection and appeared to deplete CD4+ lymphocytes very slowly, whereas complement-fixing IgG autoantibodies were generated at a later stage and depleted CD4+ lymphocytes more efficiently [3]. The presence of both gp120 (soluble gp120, complete virions, budding virus) and complement-fixing IgM and IgG antibodies on CD4+ lymphocytes was associated with very low CD4+ cell counts and coincided with progression to terminal disease. The occurrence of immune complexes on circulating CD4+ blood lymphocytes was associated with CD4 depletion as well as with impaired in-vitro function of IC-coated T lymphocytes and increased monocyte/macrophage activity in vivo[3–5]. Importantly, IC-coated CD4+ lymphocytes did not accumulate in the blood [6]. Rather, they were rapidly cleared from the circulation, suggesting an immunopathogenic role of CD4+ lymphocyte-reactive ICs in AIDS patients [7]. In advanced stages of HIV disease, ICs on CD4+ lymphocytes were associated with CD8 reduction [7]. Whereas we did not find an association between IgM and IgG autoantibodies on CD8+ with reduced CD8+ lymphocyte counts [7], others reported that lymphocyte-reactive autoantibodies seemed to facilitate the depletion of CD8+ T cells by macrophages [8]. Opsonized cells are rapidly cleared from the circulation [6], most probably by phagocytosis, antibody-dependent cellular cytotoxicity (ADCC) and complement lysis [8–10]. Some authors favour apoptosis as the main pathogeneic mechanism for CD4 depletion. Circulating ICs of HIV+ patients were shown to induce apoptosis in lymphocytes of healthy controls, and in a recent report highly active antiretroviral therapy (HAART) was associated with decreased CD4+ T cell apoptosis [11,12]. Moreover, anti-CD95 autoantibodies in sera of HIV+ children were associated with CD4+ cell depletion and it was speculated that the interaction of these antibodies with CD95-positive T cells might be involved in progressive T cell loss [13]. Others reported that anti-CD95 autoantibodies and soluble Fas levels concur in HIV type 1 infection-induced T cell depletion [14].
In the current study, we found further evidence for an important role of autoimmune mechanisms in the pathogenesis of AIDS. Using four-colour-fluorescence flow cytometry we analysed the proportions of CD3+CD4+ and CD3+CD4– blood lymphocytes that were supposed to be killed in vivo by (a) antibody-dependent cellular cytotoxicity (ADCC) or complement lysis (IgG+PI+), (b) phagocytosis (IgG+PI–), (c) anti-CD95 autoantibody-mediated apoptosis (IgG+PI– and IgG+Annexin+7-AAD–) or (d) CD95L-induced apoptosis (CD95L+PI–).
PATIENTS AND METHODS
Eleven consecutively studied HIV+ haemophilia patients and 10 consecutively measured healthy controls (eight male, two female) were investigated. They can be considered as random samples from their respective populations because they were recruited from consecutive referrals to the clinic. Laboratory staff and healthy blood donors, mainly medical students, donating blood in our department every 3 months, served as controls. Mean age of the patients was 37·9 years (range: 15–50 years), mean age of healthy controls 33·7 years (range: 20–60 years). The patients had been infected with HIV-1 before 1983 and were part of an ongoing longitudinal study. CD4+ and CD8+ blood lymphocyte counts as well as HIV-1 RNA load and antiretroviral treatment protocols of the patients are listed in Table 1. Patients were treated with combinations of nucleoside and non-nucleoside analogue reverse transcriptase inhibitors and/or protease inhibitors. The patients gave informed consent to the study.
Table 1.
CD4+ and CD8+ blood lymphocyte counts, HIV-1 load in plasma and antiretroviral therapy of 11 HIV+ haemophilia patients
| Antiretroviral drugs | ||||||
|---|---|---|---|---|---|---|
| Patient | CD4/l | CD8/l | HIV-1 RNA copies/ml | NRTI*(n =) | NNRTI† (n =) | PI‡ (n =) |
| A | 234 | 667 | 120 | 2 | 0 | 1 |
| B | 119 | 970 | 400 | 1 | 0 | 0 |
| C | 251 | 2914 | <80 | 1 | 1 | 0 |
| D | 66 | 805 | 27 000 | 2 | 0 | 0 |
| E | 122 | 599 | <80 | 2 | 0 | 2 |
| F | 276 | 793 | 3 200 | 2 | 0 | 1 |
| G | 26 | 110 | 140 000 | 2 | 1 | 1 |
| H | 284 | 376 | 16 000 | 2 | 0 | 2 |
| I | 383 | 252 | <80 | 0 | 1 | 2 |
| J | 436 | 575 | <80 | 1 | 0 | 1 |
| K | 733 | 2689 | 260 | 1 | 0 | 0 |
NRTI = nucleoside analogue reverse transriptase inhibitor.
NNRTI = non-nucleoside analogue reverse transriptase inhibitor.
PI = protease inhibitor.
Four-color-fluorescence analysis of CD3+CD4+ and CD3+CD4– blood lymphocytes coated with IgM, IgG, C3d or gp120 and positive or negative for PI or CD95L
Separation of PBLs
PBLs were separated from 10 ml heparinized whole blood using densitiy gradient centrifugation. Heparinized whole blood was diluted 1 : 2 with Hanks's balanced salt solution (HBSS) (Gibco BRL, Eggenstein-Leopoldshafen, Germany). Seven ml of diluted whole blood was added to 3 ml Ficoll (Eurobio, Les Ulis, France) and centrifuged at 2256 g for 8 min. Lymphocytes were removed, collected and washed with HBSS. Cells were centrifuged at 1316 g for 3 min. The supernatant was removed and the cell pellet was resuspended in 3 ml staining buffer (Gibco).
Staining protocol.
For four-colour-fluorescence flow cytometry, lymphocytes were stained with propidium iodide (PI) (Sigma) and mono- as well as polyclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridnine chlorophyll protein (PerCP) and allophycocyanin (APC). The following antibodies were used: mouse-IgG1/PE (Caltag, Hamburg, Germany), mouse-IgG1/FITC (Caltag), anti-CD95L/PE (Caltag), anti-CD3/APC (BD Biosciences, Heidelberg, Germany), anti-CD4/PerCP (BD), anti-CD4/FITC (BD), anti-CD4/PE (BD), anti-CD95/FITC (BD), goat-antihuman-IgG/FITC (Immuno-Research, Dianova, Hamburg, Germany), goat-antihuman-IgM/FITC (Caltag), rabbit-antihuman-C3d/FITC (Dako, Hamburg, Germany), sheep-antigp120 (Biochrom, Berlin, Germany) and rabbit-antisheep-Ig/FITC (Immuno-Research). Before use, anti-IgG/FITC was diluted 1 : 10 with phosphate buffered saline (PBS), anti-IgM/FITC and anti-C3d/FITC were diluted 1 : 40, and rabbit-antisheep-Ig/FITC 1 : 50. In order to adjust compensation of the four different fluorescence emissions, four tubes were prepared: tube A: 20 µl of anti-CD4/FITC; tube B: 20 µl of anti-CD4/PE, tube C: 20 µl of anti-CD4/PerCP and tube D: 5 µl of anti-CD3/APC. In addition, 11 tubes were prepared according to the scheme shown in Table 2. During the first step, antibodies 1–4 (Table 2) were pipetted into the tubes and incubated with 100 µl of lymphocyte suspension for 30 min at 22°C in the dark. Thereafter, the lymphocytes were washed with 2 ml of staining buffer, vortexed and centrifuged at 200 g for 5 min. The supernatant was removed. Five hundred µl of staining buffer was added to tubes 1–4, 6–9 and 11 (Table 2). Tubes were vortexed and incubated in the dark until further use. During step 2, 20 µl of rabbit-antisheep-Ig/FITC was added to tubes 5 and 10. Lymphocytes were vortexed, incubated for 30 min in the dark, washed with 2 ml of staining buffer and centrifuged at 200 g for 5 min. The supernatant was removed, 500 µl of staining buffer was added to each tube, and vortexed again. During the third step, 12·5 µl of PI was pipetted into tubes 6–11 (Table 2), vortexed and incubated for 5 min in the dark. Thereafter, lymphocytes were washed with 2 ml of staining buffer, vortexed and centrifuged at 200 g for 5 min. The supernatant was removed, 500 µl of staining buffer was added and the tubes were vortexed. The assays were now ready for four-colour-fluorescence flow cytometry.
Table 2.
Protocol for preparation of four-color-fluorescence flow cytometry assay
| 1st step | ||||||
|---|---|---|---|---|---|---|
| Tube no. | 1st antibody | 2nd antibody | 3rd antibody | 4th antibody | 2nd step Conjugate | 3rd step Viability |
| 1 | IgG1/PE | CD3/APC | CD4/PerCP | IgG1/FITC | – | – |
| (5 µl) | (5 µl) | (20 µl) | (5 µl) | |||
| 2 | CD95L/PE | CD3/APC | CD4/PerCP | IgG/FITC | – | – |
| (5 µl) | (5 µl) | (20 µl) | (10 µl) | |||
| 3 | CD95L/PE | CD3/APC | CD4/PerCP | IgM/FITC | – | – |
| (5 µl) | (5 µl) | (20 µl) | (50 µl) | |||
| 4 | CD95L/PE | CD3/APC | CD4/PerCP | C3d/FITC | – | – |
| (5 µl) | (5 µl) | (20 µl) | (50 µl) | |||
| 5 | CD95L/PE | CD3/APC | CD4/PerCP | Gp120 | Rabbit-antisheep-Ig/FITC | – |
| (5 µl) | (5 µl) | (20 µl) | (10 µl) | (20 µl) | ||
| 6 | CD95L/PE | CD3/APC | CD4/FITC | – | – | PI |
| (5 µl) | (5 µl) | (20 µl) | (12·5 µl) | |||
| 7 | – | CD3/APC | CD4/PE | IgG/FITC | – | PI |
| (5 µl) | (20 µl) | (10 µl) | (12,5 µl) | |||
| 8 | – | CD3/APC | CD4/PE | IgM/FITC | – | PI |
| (5 µl) | (20 µl) | (50 µl) | (12·5 µl) | |||
| 9 | – | CD3/APC | CD4/PE | C3d/FITC | PI | |
| (5 µl) | (20 µl) | (50 µl) | – | (12·5 µl) | ||
| 10 | – | CD3/APC | CD4/PE | Gp120 | Rabbit-antisheep-Ig/FITC | PI |
| (5 µl) | (20 µl) | (10 µl) | (20 µl) | (12·5 µl) | ||
| 11 | – | CD3/APC | CD4/PE | CD95/FITC | – | PI |
| (5 µl) | (20 µl) | (20 µl) | (12·5 µl) | |||
Four-Color-Fluorescence Flow Cytometry.
Four-colour-fluorescence was determined in a FACScalibur flow cytometer (BD) using CellQuest software (BD). Analysing tubes A–D, compensation of the four different fluorescence emissions was examined and adjusted to: FL1 versus FL2: 0·8%; FL2 versus FL1: 25·2%; FL2 versus FL3: 0%; FL3 versus FL2: 15·3%; FL3 versus FL4: 5·2%; FL4 versus FL3: 5·8%. After optimal adjustment, the patient samples were measured.
Gating strategies.
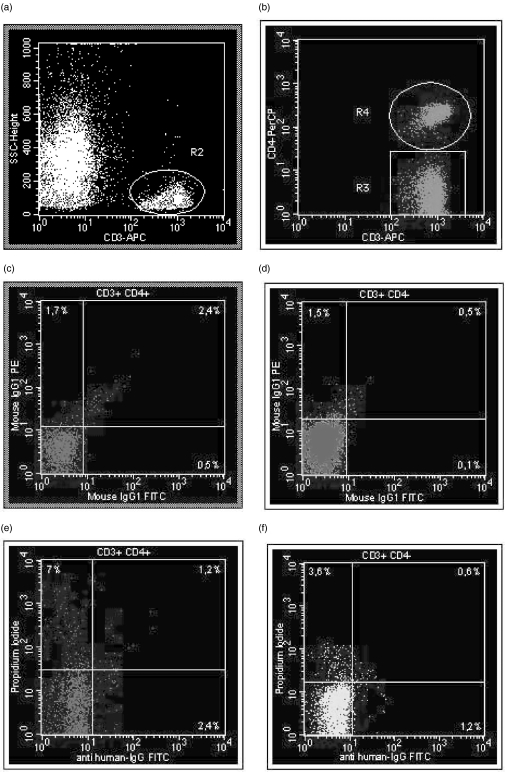
At first CD3+ lymphocytes (region R2) were defined in tube 1 using a CD3/APC versus side-scatter dot plot (SSC) (Fig. 1a). The CD3+ lymphocyte fraction of R2 was split into CD3+CD4+ (R4) and CD3+CD4– lymphocytes (R3) using a CD3/APC versus CD4/PE dot plot (Fig. 1b). Patient-specific background fluorescence of CD3+CD4+ (Fig. 1c) and CD3+CD4– (Fig. 1d) lymphocytes was determined using a previously defined standard gate setting for the analysis of the FITC- and PE-conjugated mouse-IgG1-isotype control in tube 1. Samples with high background staining were excluded from further analysis. Within the CD3+CD4+ lymphocyte subpopulation, IgG+ (tube 2), IgM+ (tube 3), C3d+ (tube 4) and gp120+ (tube 5) lymphocytes that were CD95L+ and IgG+ (tube 7) (Fig. 1e), IgM+ (tube 8), C3d+ (tube 9), and gp120+ (tube 10) lymphocytes that were PI+ (Fig. 1e) were examined using dot plot quadrant analysis. The same procedure was applied for determining these subpopulations within the CD3+CD4– lymphocyte population (Fig. 1f). Using this analysis protocol and summarizing IgG+, IgM+, C3d+ and/or gp120+ lymphocytes as IC+ cells, we obtained information on the percentages of CD3+CD4+ and CD3+CD4– blood lymphocytes that were IC+CD95L+, IC+CD95L–, IC–CD95L+, or IC–CD95L– (tubes 2–5), and IC+PI+, IC+PI–, IC–PI+, or IC–PI– (tubes 7–10). In addition, proportions of CD95L+PI+, CD95L–PI+, CD95L+PI–, CD95L–PI– (tube 6), and CD95+PI+, CD95+PI–, CD95–PI+ and CD95–PI– (tube 11) cells were determined.
Fig. 1.
(a–f) Gating strategies. At first CD3+ lymphocytes (region R2) were defined in tube 1 using a CD3/APC versus side-scatter dot plot (SSC) (a). Then CD3+ lymphocytes of R2 were split into CD3+CD4+ (R4) and CD3+CD4– lymphocytes (R3) using a CD3/APC versus CD4/PE dot plot (b). Patient-specific background fluorescence of CD3+CD4+ (c) and CD3+CD4– (d) lymphocytes was determined with a previously defined standard gate setting for the analysis of the FITC- and PE-conjugated mouse-IgG1-isotype control in tube 1. Samples with high background staining were excluded from further analysis. (e,f) Analysis of CD3+CD4+ and CD3+CD4– blood lymphocytes that were IgG+PI+. The patient had only 1·2% double-positive CD3+CD4+[CD3+CD4+IgG+PI+, (e)] and 0·6% double-positive CD3+CD4–[CD3+CD4–IgG+PI+, (f)] lymphocytes, suggesting that PI+ aggregates binding IgG unspecifically were excluded from the analysis by using the gating strategies shown in (a–d).
Interpretation of FACS results in Table 3 and 4.
Table 3.
Proportion of CD3+CD4+ blood lymphocytes coated with ICs (IgG, IgM, C3d, gp120) and stained with CD95L grouped according to suggested pathomechanism
| % Positive cells (median) in | |||
|---|---|---|---|
| Parameter | Patients (n = 11) | Controls (n = 10) | P |
| ADCC, complement lysis | |||
| CD3+CD4+IgG+PI+ | 3·7 (8·0) | 0·3 (0·2) | 0·00001 |
| CD3+CD4+IgM+PI+ | 5·3 (8·7) | 0·4 (0·6) | 0·000006 |
| CD3+CD4+C3d+PI+ | 3·5 (7·9) | 0·4 (0·5) | 0·000006 |
| CD3+CD4+gp120+PI+ | 1·5 (4·5) | 0·6 (0·6) | 0·00004 |
| CD3+CD4+IgG+CD95L– | 3·9 (6·1) | 1·9 (1·1) | 0·008 |
| CD3+CD4+IgM+CD95L– | 6·4 (15·4) | 1·5 (0·9) | 0·0003 |
| CD3+CD4+C3d+CD95L– | 13·9 (62·7) | 2·8 (1·8) | 0·000006 |
| CD3+CD4+gp120+CD95L– | 3·6 (1·9) | 2·1 (1·7) | 0·004 |
| CD3+CD4+PI+CD95L– | 1·9 (2·2) | 1·6 (0·7) | 0·5 |
| Anti-CD95-induced apoptosis, phagocytosis | |||
| CD3+CD4+IgG+PI– | 9·1 (10·5) | 2·5 (2·3) | 0·001 |
| CD3+CD4+IgM+PI– | 17·8 (18·9) | 2·3 (1·9) | 0·0002 |
| CD3+CD4+C3d+PI– | 24·2 (31·2) | 4·0 (1·5) | 0·0001 |
| CD3+CD4+gp120+PI– | 6·5 (13·8) | 2·6 (4·4) | 0·04 |
| CD3+CD4+CD95+PI– | 55·8 (16·3) | 44·4 (15·8) | 0·04 |
| CD95L-induced apoptosis | |||
| CD3+CD4+CD95+PI+ | 6·5 (5·9) | 1·4 (0·9) | 0·00002 |
| CD3+CD4+PI–CD95L+ | 5·6 (10·3) | 2·7 (10·3) | 0·1 |
| CD3+CD4+PI+CD95L+ | 6·0 (11·6) | 4·3 (5·7) | 0·2 |
| CD3+CD4+IgG+CD95L+ | 4·6 (15·5) | 0·8 (1·0) | 0·0001 |
| CD3+CD4+IgM+CD95L+ | 5·5 (11·1) | 1·4 (1·5) | 0·002 |
| CD3+CD4+C3d+CD95L+ | 8·7 (16·2) | 1·1 (1·9) | 0·001 |
| CD3+CD4+gp120+CD95L+ | 5·3 (9·6) | 1·5 (1·3) | 0·005 |
| CD3+CD4+IgG–CD95L+ | 2·7 (2·3) | 0·8 (0·3) | 0·0004 |
| CD3+CD4+IgM–CD95L+ | 2·4 (3·1) | 1·8 (2·1) | 0·9 |
| CD3+CD4+C3d–CD95L+ | 1·4 (2·4) | 0·6 (0·5) | 0·07 |
| CD3+CD4+gp120–CD95L+ | 1·9 (2·0) | 0·4 (0·5) | 0·0001 |
| IC-independent necrosis | |||
| CD3+CD4+IgG–PI+ | 8·5 (5·6) | 3·4 (3·0) | 0·00007 |
| CD3+CD4+IgM–PI+ | 6·9 (4·5) | 2·7 (1·6) | 0·001 |
| CD3+CD4+C3d–PI+ | 4·8 (1·9) | 3·0 (1·5) | 0·008 |
| CD3+CD4+gp120–PI+ | 9·1 (5·2) | 2·9 (2·8) | 0·0003 |
| CD3+CD4+CD95–PI+ | 3·7 (3·7) | 2·2 (2·8) | 0·04 |
| Viable lymphocytes | |||
| CD3+CD4+IgG–CD95L– | 84·9 (16·6) | 96·1 (2·3) | 0·000006 |
| CD3+CD4+IgM–CD95L– | 79·1 (26·7) | 94·2 (3·6) | 0·001 |
| CD3+CD4+C3d–CD95L– | 69·4 (68·3) | 94·7 (2·1) | 0·000006 |
| CD3+CD4+gp120–CD95L– | 88·2 (11·1) | 95·9 (1·9) | 0·0004 |
| CD3+CD4+PI–CD95L– | 85·4 (31·1) | 91·3 (15·6) | 0·05 |
| CD3+CD4+IgG–PI– | 75·6 (12·5) | 93·8 (3·4) | 0·000006 |
| CD3+CD4+IgM–PI– | 71·4 (24·5) | 94·7 (4·2) | 0·000006 |
| CD3+CD4+C3d–PI– | 67·5 (33·0) | 93·2 (2·0) | 0·000006 |
| CD3+CD4+gp120–PI– | 84·8 (17·5) | 93·8 (6·0) | 0·0004 |
| CD3+CD4+CD95–PI– | 26·9 (22·3) | 53·0 (11·6) | 0·00004 |
P-values were calculated using Mann–Whitney U-test. Adjustment for multiple testing was performed according to the method of Bonferroni (n = 40). P-values of ≤0·001 are considered significant and are shown in bold type. The interquartile range of each parameter is given in brackets.
Table 4.
Proportion of CD3+CD4– blood lymphocytes coated with ICs (IgG, IgM, C3d, gp120) and stained with CD95L grouped according to suggested pathomechanism
| % Positive cells (median) in | |||
|---|---|---|---|
| Parameter | Patients(n = 11) | Controls (n = 10) | P |
| ADCC, complement lysis | |||
| CD3+CD4–IgG+PI+ | 0·3 (0·4) | 0·2 (0·2) | 0·2 |
| CD3+CD4–IgM+PI+ | 0·5 (0·8) | 0·1 (0·2) | 0·02 |
| CD3+CD4–C3d+PI+ | 0·6 (0·6) | 0·2 (0·4) | 0·005 |
| CD3+CD4–gp120+PI+ | 0·3 (0·2) | 0·1 (0·2) | 0·07 |
| CD3+CD4–IgG+CD95L– | 3·8 (2·8) | 2·7 (3·8) | 0·2 |
| CD3+CD4–IgM+CD95L– | 6·7 (15·0) | 2·2 (1·4) | 0·01 |
| CD3+CD4–C3d+CD95L– | 11·8 (21·0) | 3·1 (6·5) | 0·002 |
| CD3+CD4–gp120+CD95L– | 3·3 (1·4) | 2·2 (1·9) | 0·09 |
| CD3+CD4–PI+CD95L– | 0·9 (1·6) | 1·0 (0·8) | 0·9 |
| Anti-CD95-induced apoptosis, phagocytosis | |||
| CD3+CD4–IgG+PI– | 2·1 (1·6) | 1·5 (3·2) | 0·30 |
| CD3+CD4–IgM+PI– | 5·4 (12·7) | 1·7 (1·0) | 0·005 |
| CD3+CD4–C3d+PI– | 12·0 (17·6) | 3·1 (4·9) | 0·0003 |
| CD3+CD4–gp120+PI– | 2·4 (2·9) | 1·2 (1·4) | 0·1 |
| CD3+CD4–CD95+PI– | 83·6 (13·0) | 45·4 (28·0) | 0·0002 |
| CD95L-induced apoptosis | |||
| CD3+CD4–CD95+PI+ | 2·1 (1·4) | 1·9 (2·3) | 0·6 |
| CD3+CD4–PI–CD95L+ | 0·9 (2·2) | 1·2 (1·1) | 0·8 |
| CD3+CD4–PI+CD95L+ | 1·3 (1·3) | 1·1 (1·2) | 0·9 |
| CD3+CD4–IgG+CD95L+ | 1·2 (1·0) | 0·3 (0·2) | 0·003 |
| CD3+CD4–IgM+CD95L+ | 1·3 (0·9) | 1·1 (0·5) | 0·1 |
| CD3+CD4–C3d+CD95L+ | 2·0 (1·3) | 0·9 (0·6) | 0·02 |
| CD3+CD4–gp120+CD95L+ | 1·1 (0·6) | 1·2 (0·8) | 0·9 |
| CD3+CD4–IgG–CD95L+ | 1·2 (1·4) | 0·6 (0·5) | 0·6 |
| CD3+CD4–IgM–CD95L+ | 0·9 (1·6) | 0·9 (1·3) | 1·0 |
| CD3+CD4–C3d–CD95L+ | 0·9 (1·4) | 0·7 (1·2) | 0·9 |
| CD3+CD4–gp120–CD95L+ | 0·4 (0·9) | 0·5 (0·2) | 0·4 |
| Necrosis | |||
| CD3+CD4–IgG–PI+ | 1·3 (1·3) | 2·3 (0·7) | 0·04 |
| CD3+CD4–IgM–PI+ | 1·5 (1·0) | 3·2 (2·1) | 0·02 |
| CD3+CD4–C3d–PI+ | 1·8 (1·3) | 2·8 (1·1) | 0·001 |
| CD3+CD4–gp120–PI+ | 2·9 (0·8) | 2·9 (0·8) | 0·8 |
| CD3+CD4–CD95–PI+ | 0·3 (0·8) | 1·1 (1·1) | 0·01 |
| Viable lymphocytes | |||
| CD3+CD4–IgG–CD95L– | 93·3 (6·2) | 95·5 (4·9) | 0·1 |
| CD3+CD4–IgM–CD95L– | 89·5 (19·8) | 96·0 (1·7) | 0·4 |
| CD3+CD4–C3d–CD95L– | 85·4 (20·0) | 95·0 (5·3) | 0·0001 |
| CD3+CD4–gp120–CD95L– | 95·5 (3·4) | 95·7 (1·2) | 0·4 |
| CD3+CD4–PI–CD95L– | 95·9 (3·5) | 96·4 (2·6) | 0·8 |
| CD3+CD4–IgG–PI– | 95·7 (4·2) | 95·6 (3·1) | 1·0 |
| CD3+CD4–IgM–PI– | 92·1 (13·6) | 94·6 (2·5) | 0·9 |
| CD3+CD4–C3d–PI– | 85·1 (18·3) | 93·4 (4·7) | 0·002 |
| CD3+CD4–gp120–PI– | 94·7 (2·7) | 95·2 (2·4) | 0·3 |
| CD3+CD4–CD95–PI– | 14·8 (9·2) | 49·5 (29·2) | 0·0002 |
P-values were calculated using Mann–Whitney U-test. Adjustment for multiple testing was performed according to the method of Bonferroni (n = 40). P-values of ≤0·001 are considered significant and are shown in bold type. The interquartile range of each parameter is given in brackets.
Circulating CD3+CD4+ blood lymphocytes were analysed as to whether they (a) expressed CD95 and were PI+ (b) had attached/expressed CD95L and were PI+ (c) had attached ICs and were PI+, or (d) had attached ICs and were CD95L+ (Table 3). The same parameters were studied within the CD3+CD4– lymphocyte subpopulation (Table 4). Each lymphocyte subpopulation in Tables 3 and 4 represents one of the four quadrants of a FACS dot plot analysis. CD3+CD4+ (Table 3) and CD3+CD4– (Table 4) lymphocytes were characterized for two additional fluorescence parameters as shown in Fig. 1e,f. For a certain parameter combination, the sum of stained lymphocytes in the four quadrants of the FACS dot plot analysis should be approximately 100%. The different lymphocyte subpopulations in Tables 3 and 4 are grouped according to the supposed killing mechanism of CD3+CD4+ and CD3+CD4– blood lymphocytes.
Determination of CD4+IgG+Annexin+7-AAD– Lymphocytes in the Blood
In a second series of experiments using four-colour fluorescence flow cytometry, we studied whether IgG on CD4+ blood lymphocytes induces apoptosis that could be determined by staining with annexin and 7-AAD. Freshly obtained blood lymphocytes of 10 long-term HIV-infected haemophilia patients were investigated. All individuals were consecutively studied outpatients and 2 of them had been also investigated during the first series of experiments. All patients were male. Mean age was 34·1 ± 6·6 years (mean ± s.d., range: 24–46 years).
Ten tubes were prepared [tube 1: no antibodies (autofluorescence); tube 2: 20 µl mouse-IgG/FITC (BD) + 20 µl mouse-IgG/PE (BD) + 5 µl mouse-IgG/PerCP (BD) + 10 µl mouse-IgG/APC (BD); tube 3: 5 µl anti-CD4/APC (BD) + 50 µl goat-antihuman-IgG/FITC (Caltag; diluted 1 : 40 with PBS); tube 4: 5 µl anti-CD4/APC + 50 µl goat-antihuman-IgM/FITC (Caltag; diluted 1 : 40 with PBS); tube 5: 5 µl anti-CD4/APC + 50 µl rabbit-antihuman-C3d/FITC (Dako, diluted 1 : 40 with PBS); tube 6: 5 µl anti-CD8/APC (BD) + 50 µl goat-antihuman-IgG/FITC (diluted 1 : 40 with PBS); tube 7: 5 µl anti-CD8/APC + 50 µl goat-antihuman-IgM/FITC (diluted 1 : 40 with PBS); tube 8: 5 µl anti-CD8/APC + 50 µl rabbit-antihuman-C3d/FITC (diluted 1 : 40 with PBS); tube 9: 5 µl anti-CD4/APC + 20 µl anti-CD14/PE (BD); tube 10: 5 µl anti-CD8/APC + 20 µl anti-CD16/PE (BD)]. One hundred µl heparinized whole blood was added to each tube. Tubes were vortexed and incubated for 30 min at room temperature in the dark. Two ml PharM lysing solution (BD, diluted 1 : 10 with aqua dest.) was pipetted into each tube and the tubes were vortexed again. After 10 min incubation time at room temperature in the dark, the tubes were centrifuged at 200 g for 5 min. The supernatant was removed and the tubes were filled with 2 ml annexin V binding buffer (BD, diluted 1 : 10 with aqua dest.), vortexed and centrifuged at 200 g for 5 min. The supernatant was removed and 100 µl annexin V binding buffer (diluted 1 : 10 with aqua dest.) was pipetted into each tube. Five µl annexin V/PE and 5 µl 7-AAD were filled into tubes 3–8. The tubes were vortexed and incubated for 15 min at 4°C. Then 400 µl annexin V binding buffer (diluted 1 : 10 with aqua dest.) was added to each tube and samples were measured within 1 h after sample preparation using a FACScalibur flow cytometer (BD).
Determination of HIV-1 RNA copies in plasma
HIV-1 RNA was measured using the NucliSens QT kit (Organon Teknika, Heidelberg, Germany). According to the manufacturer's instructions, HIV-1 RNA was quantified using a 1 ml plasma sample. Quantification is based on co-amplification of HIV-1 wild-type RNA with three internal calibrators (QA,QB,QC) differing only by sequence randomization of a 20 nucleotide fragment of the wild-type RNA, thereby ensuring efficiency of isolation and amplification. RNA is amplified directly and based selectively on the activity of reverse transcriptase, RNaseH and T7RNA polymerase. The quantity of the amplified product was measured by electron-chemiluminescence using the automated NASBA QR System. Sensitivity of the assay is >80 copies using samples of 1 ml plasma.
Statistical analysis
The Mann–Whitney U-test was applied for comparison of lymphocyte subpopulations in patients and controls and Spearman's rank correlation test was applied for calculating associations of CD4 and CD8 counts with the different lymphocyte subpopulations. The Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) was used. Adjustment for multiple testing was performed according to the method of Bonferroni (n = 40). P-values of ≤0·001 are considered significant and are printed in bold type in Tables 3 and 4.
RESULTS
CD3+CD4+IC+ blood lymphocytes − evidence for ADCC, complement lysis, anti-CD95 induced apoptosis and phagocytosis
The most prominent finding was that more than one-fourth of the circulating CD3+CD4+ lymphocytes in HIV+ patients were coated with IgM, IgG, C3d and/or gp120, in contrast to only <5% in healthy controls (Table 3). Especially IgG+PI+ (median: 3·7%versus 0·3%; P = 0·00001), IgM+PI+ (5·3%versus 0·4%; P = 0·000006), C3d+PI+ (3·5%versus 0·4%; P = 0·000006) and gp120+PI+ (1·5%versus 0·6%; P = 0·00004), but also IgG+PI– (9·1%versus 2·5%; P = 0·001), IgM+PI– (17·8%versus 2·3%; P = 0·0002) and C3d+PI– (24·2%versus 4·0%; P = 0·0001) CD3+CD4+ lymphocytes were increased strongly in HIV+ patients. We therefore speculate that approximately one-third of the IC-coated CD3+CD4+ lymphocytes were killed in the circulation by ADCC or complement lysis (IC+PI+), whereas two-thirds of the IC-coated CD3+CD4+ lymphocytes were viable and might be targets for elimination from the circulation by phagocytes in lymphnodes and the spleen or were killed by anti-CD95 autoantibody-induced apoptosis (IC+PI–). CD3+CD4+ lymphocytes (4·6%) were coated with IgG and expressed CD95L, which was significantly different from healthy controls (0·8%; P < 0·0001), indicating that CD3+CD4+ lymphocytes that were probably killed by CD95L-induced apoptosis bound autoreactive IgG in addition.
CD95L+ lymphocytes and PI+ lymphocytes − evidence for CD95L-induced apoptosis and necrosis
CD3+CD4+CD95+PI+ lymphocytes represent dead cells expressing the apoptosis-associated CD95 receptor, whereas CD3+ CD4+CD95L+PI+ and CD3+CD4+CD95L+PI– lymphocytes represent cells that have attached or expressed CD95L. CD3+CD4+CD95L+PI– cells represent lymphocytes that were probably killed by CD95L-induced apoptosis in vivo. Although HIV+ patients had significantly higher proportions of CD3+CD4+CD95+PI+ (6·5%versus 1·4%; P = 0·00002; Table 3) cells than healthy controls, there was only an insignificant trend towards higher proportions of CD3+CD4+CD95L+PI+ (6·0%versus 4·3%; P = 0·2) and CD3+CD4+CD95L+PI– (5·6%versus 2·7%; P = 0·1) blood lymphocytes in HIV+ patients (Table 3). The data suggest that activated CD95-expressing CD3+CD4+ lymphocytes are killed in HIV-infected patients, whereas the increase of CD95L-induced apoptosis of CD3+CD4+ lymphocytes measured by CD95L+PI– staining did not reach statistical significance in this series of long-term infected patients.
In contrast, HIV+ patients had higher proportions of CD3+CD4+CD95L+ lymphocytes that were IgG– (2·7%versus 0·8%; P = 0·0004) and gp120– (1·9%versus 0·4%; P = 0·0001), and higher proportions of IgG–PI+ (8·5%versus 3·4%; P = 0·00007), IgM–PI+ (6·9%versus 2·7%; P = 0·001) and gp120–PI+ (9·1%versus 2·9%; P = 0·0003; Table 3) CD3+CD4+ lymphocytes than healthy controls. These findings suggest that part of the circulating CD3+CD4+ blood lymphocytes in HIV+ patients were killed in vivo by mechanisms unrelated to IC-coating, for example by CD95L-induced apoptosis or necrosis-inducing mechanisms.
IC–, CD95L– and PI– CD3+CD4+ lymphocytes
Because HIV+ patients had increased proportions of IC+, CD95L+ and/or PI+ blood lymphocytes, they had significantly decreased proportions of viable CD3+CD4+ lymphocytes that were IC–, CD95L– and/or PI– (P< 0·001; Table 3).
CD3+CD4– blood lymphocytes
Our hypothesis that opsonization with autoreactive IgG or gp120/IgG complexes is (a) specific for CD4+ blood lymphocytes of HIV+patients and (b) a pathomechanism of CD4 depletion issupported by the observation that increased IgG-coating was not found on CD3+CD4– lymphocytes of HIV+ patients (IgG+PI+: 0·3%versus 0·2%; P = 0·2; IgG+PI–: 2·1%versus 1·5%; p = 0·3; Table 4). HIV+ patients showed a trend towards increased proportions of IgM+ and C3d+ CD3+CD4– lymphocytes; however, these cells were predominantly PI– (C3d+PI–: 12·0%versus 3·1%; P = 0·0003; Table 4). It is noteworthy that PI– CD3+CD4–CD95+ lymphocytes were increased strongly in HIV+ patients (83·6%versus 45·4%; P = 0·0002), whereas proportions of PI+ CD3+CD4–CD95+ lymphocytes (2·1%versus 1·9%; P = 0·6) were similar in HIV+ and HIV– individuals. These data imply that HIV+ patients had an increased pool of activated CD95-expressing CD3+CD4– lymphocytes. These cells are viable, in contrast to the high proportion of PI+ CD3+CD4+CD95+ lymphocytes in the same patients. Because CD95L+PI+ and CD95L+PI– CD3+CD4– lymphocytes were similar in HIV+ and HIV– individuals (PI+: 1·3%versus 1·1%; P = 0·9; PI–: 0·9%versus 1·2%; P = 0·8), we conclude that cell death of CD95L+ lymphocytes, especially CD95L-induced apoptosis of CD3+CD4– lymphocytes, is not increased in long-term HIV-infected patients.
To assess the clinical relevance of the results, we analysed which of the lymphocyte subpopulations were associated with CD4 or CD8 depletion in the blood or with high plasma viral load.
Association with CD8+ blood lymphocyte counts
There was no significant association of CD4+ blood lymphocyte count or viral load with any of the studied lymphocyte subpopulations. In contrast, CD8+ blood lymphocyte count was associated positively with CD3+CD4–CD95+PI– blood lymphocytes (r = 0·891; P = 0·004; data not shown) and negatively with CD3+CD4–CD95–PI– blood lymphocytes (r = − 0·873; P = 0·02; data not shown), suggesting that increases of CD8 counts were associated with increased activation of these lymphocytes.
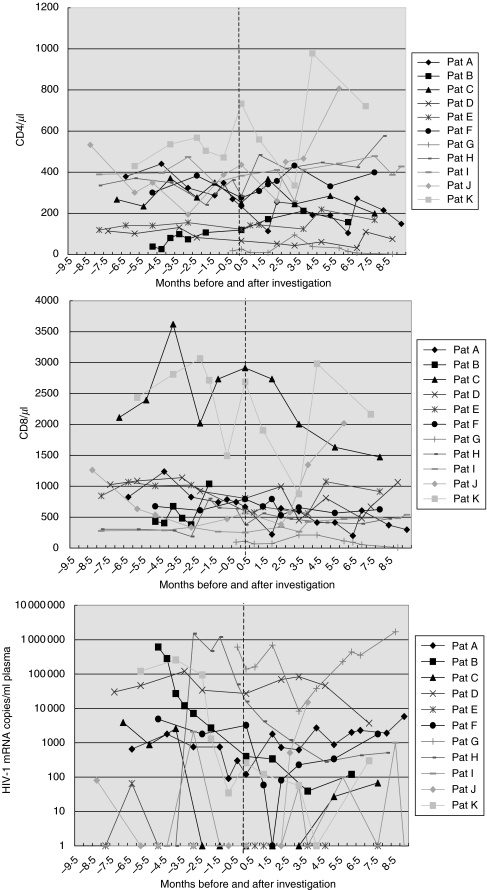
Association with a subsequent CD4 and CD8 decrease or viral load increase
The analysis shown above does not consider whether there was a trend towards increasing or decreasing CD4 counts at the time of investigation. Figure 2 shows that CD4 counts decreased in five of 11 HIV+patients within 2 months after the initial investigation of lymphocyte subpopulations with four-colour-fluorescence flow cytometry. These five patients had slightly higher proportions of CD3+CD4+IgM–CD95L+ lymphocytes than the six patients with a subsequent CD4 increase (median: 3·7 versus 0·8%; P = 0·03, data not shown). When CD8 counts were analysed, patients with a subsequent CD8 decrease (n = 7) had slightly higher CD3+CD4–IgG+CD95L– (4·9%versus 2·5%; P = 0·07) and slightly lower CD3+CD4–IgG–CD95L– (92·4%versus 95·7%; P = 0·07) as well as slightly lower CD3+CD4–IgG–PI– (95·1%versus 96·6%; P = 0·07) lymphocytes than patients with a subsequent CD8 increase (n = 4) (data not shown). These data show that CD4 decreases are associated weakly with CD95L expression/attachment on/to CD3+CD4+ cells, whereas CD8 count decreases are weakly associated with higher proportions of IgG-coated and lower proportions of IgG-free CD3+CD4– lymphocytes. Patients with an increasing viral load after the investigation (n = 3) had a higher proportion of CD3+CD4+IgG+PI– (19·5%versus 6·6%; P = 0·05) and a lower proportion of CD3+CD4+IgG–PI– lymphocytes (68·8%versus 78·8%; P = 0·05) than patients with a decreasing or a stable viral load below the detection limit of the test (>80 HIV-1 mRNA copies/ml plasma) (data not shown). The results suggest that an increasing viral load was associated with an increase of IgG-coated viable CD3+CD4+ blood lymphocytes and a decrease of IgG-free viable CD3+CD4+ blood lymphocytes. However, these results represent only a trend because the associations were no longer statistically significant after Bonferroni correction.
Fig. 2.
(a–c) CD4 counts (a), CD8 counts (b) and viral load (c) of 11 HIV+ patients before and after the investigation with four-colour-fluorescence flow cytometry. All patients were studied in August and September 2001. Before the investigation CD4 counts decreased in six patients and CD8 counts in six patients, viral load increased in two patients. After the determination CD4 counts decreased in five patients and CD8 counts in seven patients, viral load increased in three patients. A viral load below the detection limit of the test (>80 HIV-1 mRNA copies/ml plasma) was depicted as 1 copy/ml in (c). Time of investigation (time point 0) is indicated by a vertical line.
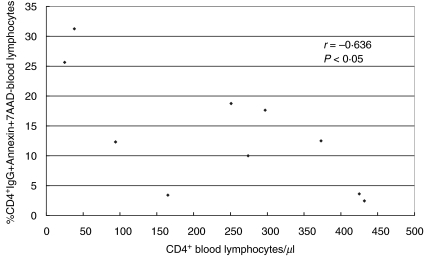
CD4+ blood lymphocyte counts and CD4+IgG+annexin+7-AAD– blood lymphocytes
When the induction of apoptosis and necrosis was studied in 10 HIV+ patients using annexin binding and 7-AAD staining, CD4+ blood lymphocyte counts were associated negatively with the proportion of CD4+IgG+annexin+7-AAD– lymphocytes in the blood (r = −0·636; P < 0·05; Fig. 3). This result suggests that, in part of these lymphocytes, attachment of IgG to CD4+ blood lymphocytes induces apoptosis and subsequent depletion of these IgG-coated apoptotic CD4+ lymphocytes from the peripheral blood.
Fig 3.
Blood CD4+ lymphocyte counts and proportions of CD4+IgG+annexin+7-AAD– lymphocytes in the blood of 10 long-term HIV-infected haemophilia patients. CD4 counts were negatively associated with the proportion of CD4+IgG+annexin+7-AAD– blood lymphocytes (Spearman's rank correlation test: r = −0·636; P < 0·05), suggesting that attachment of IgG to CD4+ blood lymphocytes induces apoptosis in some lymphocytes with subsequent depletion of these IgG-coated apoptotic CD4+ lymphocytes from the circulation.
DISCUSSION
While the exact mechanism of CD4 depletion is still unclear, it is now accepted that autoimmune phenomena and apoptosis contribute to CD4 depletion. Three alternatives are discussed: (a) elimination of cells by CD95L-induced apoptosis, (b) elimination of cells coated with autoreactive ICs by non-apoptotic mechanisms such as phagocytosis, ADCC or complement lysis or (c) apoptotic mechanisms such as anti-CD95 autoantibody-mediated apoptosis. Phagocytosis, ADCC, complement lysis and apoptosis were studied mainly in vitro with (a) HIV-infected cell lines, (b) lymphocytes of HIV-infected individuals that were stimulated with mitogens in vitro, (c) lymphocytes of healthy controls that were coated with recombinant HIV glycoproteins or (d) transfected cell lines expressing virus peptides. The various authors postulated that their in vitro findings were relevant for the pathogenesis of the CD4 depletion in HIV-infected individuals. In contrast, we studied freshly obtained lymphocytes of patients living with HIV for more than 20 years. Within the CD4+ and CD8+ lymphocyte subpopulations we studied the proportions of viable (PI–) and dead (PI+) cells expressing the apoptosis-associated activation marker CD95 (CD95+). One would expect that part of the dead CD95+ cells would have died by activation-induced cell death (CD95+PI+). Moreover, we investigated the proportion of cells that were killed by CD95L-induced apoptosis (CD95L+PI–), anti-CD95 autoantibody-mediated apoptosis (IgG+PI– and IgG+annexin+7-AAD–) or, alternatively, ADCC or complement lysis (IC+PI+). Viable IC-coated CD3+CD4+IC+PI– lymphocytes were considered as target cells for phagocytosis.
Our data show that the proportion of CD3+CD4+CD95L+PI+ as well as that of CD3+CD4+CD95L+PI– lymphocytes was similar in HIV+ patients and healthy controls, whereas the proportion of CD3+CD4+CD95L+ lymphocytes that were IgG+, C3d+ and/or gp120– were increased in HIV+ patients. Cell death of CD95L+ lymphocytes and the role of CD95L-induced apoptosis for CD4 depletion in HIV+ long-term surviving haemophilia patients remains unclear. The association of CD4+ and CD8+ lymphocyte counts with CD95L+ T lymphocytes in HIV-infected patients probably reflects the physiological lymphocyte turnover mediated by CD95L-induced apoptosis [15–17]. Ten of the 11 patients had abnormal CD4 counts of <500/µl. In five patients CD4+ lymphocytes decreased further within the following 2 months. However, the changes in CD4 counts were usually small. Six of 11 patients showed increases or decreases of <60 cells/µl (Fig. 2) during these 2 months. Therefore, highly significant associations of changes in CD4 counts with IC load could not be expected. The proportion of CD3+CD4+IC+PI+ blood lymphocytes was significantly higher in HIV+ patients than healthy controls (P = 0·000006). It is reasonable to assume that this lymphocyte subpopulation was killed by ADCC or complement lysis. The proportion of this subset in the peripheral blood was 5% of circulating CD3+CD4+ lymphocytes. An additional 10–25% of circulating CD3+CD4+ blood lymphocytes were coated with ICs but PI–. We hypothesize that a part of these IC-coated cells is viable and represents targets for elimination from the circulation by phagocytosis, whereas another part is killed by anti-CD95 autoantibody-mediated apoptosis.
Another argument for a predominant role of ICs in CD4 depletion during HIV infection is the finding that ICs on CD8+ lymphocytes were not associated strongly with cell death, in contrast to ICs on CD4+ lymphocytes. HIV+ patients had less than 2% CD3+CD4–IC+ lymphocytes that were PI+ and/or CD95L+, but 2–12% CD3+CD4–IC+ cells that were PI– and/or CD95L–. We speculate that part of the ICs bound to CD3+CD4– lymphocytes unspecifically via Fc receptors (FcR). FcR binding of immunoglobulins is predominantly a phenomenon of CD8+ cells because FcR for IgG, for example Fc gamma RII (CDw32), are more frequent on CD8+ lymphocytes (frequency: 27%– 69%) than on CD4+ cells (frequency: 10%– 14%) [18]. Such IC-coated lymphocytes would notbe target cells for phagocytosis, ADCC, complement lysis or anti-CD95 autoantibody-mediated apoptosis. However, CD3+CD4– lymphocytes that specifically bind Ics can be killed by phagocytosis, as shown previously [8].
Recent studies indicate that soluble Fas may modulate T cell apoptosis as it inhibits Fas-ligand-mediated cytotoxicity in vitro[19]. This finding agrees with our previous observation that sFas plasma levels increased in long-term surviving HIV+ haemophilia patients even though patients were treated with HAART and showed increasing CD4+ lymphocyte counts [20]. Other authors described that serum titres of sFas and anti-Fas were correlated linearly in 17 severely lymphopenic subjects [14], and reported a negative correlation between anti-CD95 autoantibody levels and CD4+ T cell counts in patients observed longitudinally [13]. The authors speculated that a high release of soluble Fas by T cells during chronic HIV-induced immune activation primes a humoral response against this Fas epitope due to its high antigenicity [21]. The same authors published that functional Fas-ligand expression on T cells from HIV-1 infected patients is unrelated to CD4+ lymphopenia [22,23], which is in accordance with our data showing that HIV+ patients and healthy controls have similar proportions of CD3+CD4+CD95L+PI+ as well as CD3+CD4+CD95L+PI– blood lymphocytes and, as reported previously, that sFasL plasma levels are not associated with CD4 counts in long-term HIV-infected patients [20]. The finding of others that HIV-induced apoptosis of activated CD4+ T cells in vitro is confined to productively infected cells and is not mediated by a Fas–FasL interaction points in the same direction [24]. Apoptotic T cells of HIV+ patients were bound by antiphosphatidylserine antibodies in vitro, suggesting that the ICs on CD4+ lymphocytes are heterogeneous and cover a wide spectrum of different target epitopes on the cell membrane [25]. The binding of antiphosphatidylserine antibodies to apoptotic T lymphocytes might explain the attachment of IgG to CD95L+ CD3+CD4+ as well as CD3+CD4– lymphocytes in our experiments.
In a previous study, 19 of 71 HIV+ haemophilia patients had IgM–IgG–gp120–, 29 had IgM+IgG–gp120–, 19 had IgM+IgG+gp120– and four had IgM+IgG+gp120+ CD3+CD4+ blood lymphocytes, suggesting that CD3+CD4+IgG+ lymphocytes are coated in addition with IgM, and that CD3+CD4+gp120+ cells are coated in addition with IgM and IgG [4]. The observation that IgG autoantibodies were present predominantly on CD3+CD4+ but rarely on CD3+CD4– blood lymphocytes confirms our previous findings [3–5] and the reports of others [9,10], and further supports the notion that autoreactive ICs are involved in CD4 depletion. One-third of circulating CD3+CD4+ lymphocytes in long-term HIV-infected patients are coated with ICs. According to the results presented in this report, approximately 5% of the CD3+CD4+ lymphocytes seem to be eliminated from the circulation by ADCC or complement lysis, and 10–25% by phagocytosis or anti-CD95 autoantibody-mediated apoptosis. The role of CD95L-induced apoptosis as a pathomechanism for CD4 depletion in HIV+ long-term surviving patients remains unclear. The finding that ICs on CD3+CD4– lymphocytes are not associated with cell death supports our hypothesis that IC coating specifically induces the depletion of CD4+ blood lymphocytes in HIV infection [3,6]. Although the measurements reported in this study reflect only a momentary picture of the interactions of the immune system, they suggest strongly that autoimmune pathomechanisms contribute to the depletion of CD4+ lymphocytes in HIV infection. CD4 depletion in HIV+ long-term surviving haemophilia patients appears to be mediated in part by autoantibody-induced apoptotic and non-apoptotic mechanisms.
Acknowledgments
We would like to acknowledge the specialized expertise and enthusiasm of Edda Schaller-Süfling in establishing the protocol for four-color-fluorescence flow cytometry. We also acknowledge the skillful technical assistance of Roland Seidel and Regina Seemuth, who determined T lymphocyte subpopulations and HIV-1 viral load.
REFERENCES
- 1.Quagliarello V. The Acquired Immunodeficiency Syndrome: current status. Yale J Biol Med. 1982;55:443–52. [review]. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Adegboyega PA. Down-regulation of Fas expression in the lymph nodes of patients infected with human immunodeficiency virus. Arch Pathol Lab Med. 2002;126:28–32. doi: 10.5858/2002-126-0028-DROFEI. [DOI] [PubMed] [Google Scholar]
- 3.Daniel V, Süsal C, Weimer R, et al. Sequential occurrence of IgM, IgM/IgG, and gp120–IgM/IgG–complement complexes on CD4+ lymphocytes in relation to CD4+ blood lymphocyte depletion in HIV+ hemophilia patients: results of a 10-year study. Immunol Lett. 1995;47:97–102. doi: 10.1016/0165-2478(95)00081-f. [DOI] [PubMed] [Google Scholar]
- 4.Daniel V, Susal C, Weimer R, et al. Association of T cell dysfunction with the presence of IgG autoantibodies on CD4+ lymphocytes in haemophilia patients; results of a 10-year study. Clin Exp Immunol. 1996;104:4–10. doi: 10.1046/j.1365-2249.1996.d01-640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel V, Schimpf K, Opelz G. Lymphocyte autoantibodies and alloantibodies in HIV-positive haemophilia patients. Clin Exp Immunol. 1989;75:178–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel V, Melk A, Susal C, et al. CD4 depletion in HIV-infected haemophilia patients is associated with rapid clearance of immune complex-coated CD4+ lymphocytes. Clin Exp Immunol. 1999;115:477–84. doi: 10.1046/j.1365-2249.1999.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel V, Susal C, Weimer R, et al. CD8+ lymphocyte decrease in HIV disease: association with anti-CD4+ but not with anti-CD8+ lymphocyte autoantibodies. Vox Sang. 1996;70:86–91. doi: 10.1111/j.1423-0410.1996.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZQ, Horowitz HW, Orlikowsky T, Dudhane A, Weinstein A, Hoffmann MK. Lymphocyte-reactive autoantibodies in human immunodeficiency virus type 1-infected persons facilitate the deletion of CD8 T cells by macrophages. J Infect Dis. 1998;178:404–12. doi: 10.1086/515623. [DOI] [PubMed] [Google Scholar]
- 9.Gerencer M, Burek V, Crowe BA, Barrett NP, Dorner F. The role of complement and gp120-specific antibodies in virus lysis and CD4+ T cell depletion in HIV-1-infected patients. Microb Pathog. 1998;25:253–66. doi: 10.1006/mpat.1998.0233. [DOI] [PubMed] [Google Scholar]
- 10.Parker SJ, Sadlon TA, Gordon DL. Enhancement of NK cell-mediated antibody-dependent lysis of recombinant gp120-coated CD4 cells by complement. J Infect Dis. 1995;171:186–9. doi: 10.1093/infdis/171.1.186. [DOI] [PubMed] [Google Scholar]
- 11.Aceituno E, Castanon S, Jimenez C, et al. Circulating immune complexes from HIV-1+ patients induce apoptosis on normal lymphocytes. Immunology. 1997;92:317–20. doi: 10.1046/j.1365-2567.1997.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roger PM, Breitmayer JP, Arlotto C, et al. Highly active anti-retroviral therapy (HAART) is associated with a lower level of CD4+ T cell apoptosis in HIV-infected patients. Clin Exp Immunol. 1999;118:412–6. doi: 10.1046/j.1365-2249.1999.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stricker K, Knipping E, Bohler T, Benner A, Krammer PH, Debatin KM. Anti-CD95 (APO-1/Fas) autoantibodies and T cell depletion in human immunodeficiency virus type 1 (HIV-1)-infected children. Cell Death Differ. 1998;5:222–30. doi: 10.1038/sj.cdd.4400332. [DOI] [PubMed] [Google Scholar]
- 14.Silvestris F, Grinello D, Del Prete A, Cafforio P, Quarto M, Dammacco F. Anti-Fas (CD95/Apo-I) autoantibodies and soluble Fas levels concur in T cell depletion in HIV type 1 infection. AIDS Res Hum Retroviruses. 2001;17:603–14. doi: 10.1089/088922201300119707. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S. Molecular and biochemical pathways of apoptosis in lymphocytes from aged humans. Vaccine. 2000;18:1596–601. doi: 10.1016/s0264-410x(99)00492-2. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 17.Phelouzat MA, Laforge T, Arbogast A, Quadri RA, Boutet S, Proust JJ. Susceptibility to apoptosis of T lymphocytes from elderly humans is associated with increased in vivo expression of functional Fas receptors. Mech Ageing Dev. 1997;96:35–46. doi: 10.1016/s0047-6374(97)01883-6. [DOI] [PubMed] [Google Scholar]
- 18.Mantzioris BX, Berger MF, Sewell W, Zola H. Expression of the Fc receptor for IgG (Fc gamma RII/ (CDw32) by human circulating T and B lymphocytes. J Immunol. 1993;150:5175–84. [PubMed] [Google Scholar]
- 19.Mouawad R, Khayat D, Soubrane C. Plasma Fas ligand, an inducer of apoptosis, and plasma soluble Fas, an inhibitor of apoptosis, in advanced melanoma. Melanoma Res. 2000;10:461–7. doi: 10.1097/00008390-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Daniel V, Susal C, Weimer R, Zimmermann R, Huth-Kuhne A, Opelz G. Increased soluble Fas in HIV-infected hemophilia patients with CD4+ and CD8+ cell count increases and viral load and immune complex decreases. AIDS Res Hum Retroviruses. 2001;17:329–35. doi: 10.1089/08892220150503690. [DOI] [PubMed] [Google Scholar]
- 21.Silvestris F, Cocco T, Cafforio P, Calvani N, Dammacco F. Immunogenicity of an eight amino acid domain shared by Fas (CD95/Apo-I) and HIV-1 gp120. I. Structural and antigenic analysis. Mol Med. 2000. pp. 494–508. [PMC free article] [PubMed]
- 22.Silvestris F, Cafforio P, Camarda G, Tucci M, Frassanito MA, Dammacco F. Functional Fas-ligand expression on T cells from HIV-1-infected patients is unrelated to CD4+ lymphopenia. Int J Clin Laboratory Res. 1998;28:215–25. doi: 10.1007/s005990050048. [DOI] [PubMed] [Google Scholar]
- 23.Silvestris F, Camarda G, Cafforio P, Dammacco F. Upregulation of Fas ligand secretion in non-lymphopenic stages of HIV-1 infection. AIDS. 1998;12:1103–4. [PubMed] [Google Scholar]
- 24.Noraz N, Gozlan J, Corbeil J, Brunner T, Spector SA. HIV-induced apoptosis of activated primary CD4+ T lymphocytes is not mediated by Fas–Fas ligand. AIDS. 1997;14:1671–80. doi: 10.1097/00002030-199714000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cafforio P, Camarda G, Del Prete A, Silvestris F. Antiphosphatidylserine antibodies in HIV-1+ patients bind apoptotic T cells in vitro. Eur J Histochem. 1997;41(Suppl. 2):65–6. [PubMed] [Google Scholar]