Abstract
One approach to study the role of distinct cellular mechanisms in susceptibility/resistance to tuberculosis (TB) is to compare parameters of response to infection in the lungs of mouse strains exhibiting genetically determined differences in TB susceptibility/severity. Interstrain differences in antimycobacterial macrophage reactions, T cell responses & inflammation in the lungs of TB-susceptible I/St, TB-resistant A/Sn and (I/St × A/Sn)F1 mice were analysed following intratracheal inoculation of 103 CFUs of M. tuberculosis H37Rv. The antimycobacterial responses in the lungs of susceptible I/St mice were characterized by: (i) increased inflammatory infiltration by all major immune cell subsets; (ii) decreased type 1 cytokine production; (iii) impaired antimycobacterial activity of lung macrophages; (iv) unusually high proliferation of lung T lymphocytes. Differences in several parameters of anti-TB immunity between susceptible and resistant mice corresponded well to the polygenic pattern of TB control previously established in this mouse model. Importantly, lung macrophages isolated from noninfected mice were unable to respond to IFN-γ by increasing their mycobactericidal function, but between weeks 3 and 5 of the infection this capacity developed in all mice. However, by this time point susceptible but not resistant mice demonstrated a pronounced decrease in IFN-γ production by lung cells. This chain of events may explain the inability of I/St mice to control both early and chronic TB infection.
Keywords: mouse, tuberculosis, lung, macrophages, T lymphocytes
INTRODUCTION
There is substantial evidence for the role of genetic factors in human susceptibility to TB [1–3] confirmed by numerous recent studies in murine models [4–7]. Genetic factors may influence the host's ability either to restrict mycobacterial growth in the lungs or to avoid the excessive lung tissue damage that can result from the antimycobacterial immune response, or both. It is not known which is more important for protection of the host. The degree of mycobacterial multiplication in murine lungs does not always correlate with mortality [8]. Moreover, disease severity in terms of lung tissue destruction and liquefaction, the degree of cahexia, and time to death may depend more upon the inflammatory response of the host rather than the direct toxicity of mycobacteria and their products [9,10]. Thus, the host response to M. tuberculosis may be a major determinant of disease severity and outcome.
The natural route of infection with M. tuberculosis is via the respiratory tract. Mycobacteria are ingested by resident alveolar macrophages [11], cells that are the main candidate for conveyance engulfed bacilli from the alveolar space to the interstitium [12], or by dendritic cells [13]. In addition, mycobacteria are able to actively invade the lung epithelium and, after crossing its layer, to multiply within interstitial lung phagocytes [14–16]. If mycobacteria are not eradicated by the innate macrophage response within a short time (1–2 weeks postinfection), inflammatory cells are attracted to the site of infection. Following the establishment of the adaptive response, T cells specific for mycobacterial antigens are generated and migrate to the affected site(s) of the lung. A number of cytokines and chemokines are produced locally resulting in an increased local inflammatory reaction and granuloma formation [17–21]. During this phase of the disease (2–4 weeks), T lymphocytes contribute to antimycobacterial defence by stimulating infected macrophages to kill intracellular mycobacteria [11,21–24]. In addition, direct destruction of infected macrophages and continuing recruitment of blood-derived monocytes to the site of infection may also play a protective role [25,26].
If macrophages fail to completely eradicate mycobacteria, even after activation by mycobacteria-specific T cells, a successful host strategy to control the disease largely relies on the ability to form well-developed lung granulomata and thus to contain mycobacterial spreading [27,28]. In cases when this goal is not achieved, infection progresses and affects additional areas of the lung. At this phase, the disease is accompanied by destruction of the lung tissue due to the intense cellular immunity, whose protective function is diminishing while pathogenicity is growing. Rather, breakdown of TB foci leads to further spreading of infection via both lymphatics and blood stream [10,29]. Thus, three major elements of host reactivity determine the outcome of mycobacterial infection: (i) capacity of early infected cells to restrict replication of mycobacteria by innate response mechanisms; (ii) ability to establish a well-controlled type of adaptive T cell-mediated immunity; (iii) capacity to prevent breakdown of lung granulomata.
One of the approaches to establish the role of these factors in TB resistance is to study the course of infection in mice with genetically determined differences in susceptibility to and/or severity of infection. Earlier we reported that mice of the I/St strain are much more susceptible to intravenous (i. v.) TB infection than mice of the A/Sn strain or (I/St × A/Sn)F1 hybrids [30–32]. Susceptibility is inherited as a polygenic trait and is not controlled by the Nramp gene since both A/Sn and I/St mice carry the Nrampr allele [4]. Our observations concerning the functional differences between lung T cells and pulmonary macrophages from I/St, A/Sn and (A/Sn × I/St)F1 mice in response to mycobacteria were made in models that poorly mimic TB infection initiated via its natural aerogenic route [32,33]. Here, we characterize interstrain differences in disease severity, antimycobacterial macrophage reactions, T cell responses and granuloma formation between I/St, A/Sn and (I/St × A/Sn)F1 mice at intervals following intratracheal (i.t.) challenge with M. tuberculosis H37Rv. We also show for the first time that the capacity of lung macrophages to be activated for mycobacterial killing by IFN-γ is not intrinsic but rather is acquired relatively late in the infectious course – an important determinant of TB-susceptibility in I/St mice.
MATERIALS AND METHODS
Animals
Inbred mice of the I/StYCit (I/St) and A/JSnYCit (A/Sn) strains and their (A/Sn × I/St)F1 hybrids were bred under conventional conditions at the Animal Facilities of the Central Institute for Tuberculosis (Moscow, Russia), in accordance with guidelines from the Russian Ministry of Health ♯ 755, NIH Office of Laboratory Animal Welfare (OLAW) Assurance ♯A5502-01. Water and food were provided ad libitum. Male mice 2–4 months of age were used. All experimental procedures were approved by the institutional animal care committee.
Bacteria and infection
Mice were infected i.t. with 103 CFUs/mouse of mid-log-phase M. tuberculosis strain H37Rv Pasteur (initially, a kind gift of G. Marchal, Institute Pasteur, Paris, France) in 50 µl of PBS. The preparation of clump-free mid-log-phase mycobacteria suspensions has been described elsewhere [31,34]. The experimental surgical procedure to allow i.t. instillation of the challenge suspension has also been previously described [35,36]. Mice recovered with minimal visible trauma and only 2 deaths due to surgery were recorded. Control (sham-infected) mice were injected with 50 µl of PBS and housed separately from infected mice. To assess the mycobacterial load in spleens and lungs, 0·1 ml of serial 10-fold dilutions of whole-organ homogenates were plated onto Dubos agar, and colonies were counted after 18–20 days of incubation at 37°C.
Lung cell suspensions
At the indicated time points following challenge, mice were euthanized by i.p. injection of an overdose of thiopental (Biochemie GmbH, Vienna, Austria), and lung cell suspensions were prepared individually using the methods described previously [32,34]. Briefly, blood vessels were washed out, followed by repeated broncho-alveolar lavage with 0·02% EDTA-HBSS solution. Lungs were extracted from the chest and sliced with scissors into 1–3 mm3 pieces. The tissue was incubated at 37°C for 1·5 h in supplemented RPMI-1640 medium containing 10 mm HEPES, l-glutamine, nonessential amino acids, sodium pyruvate, 2% FCS (all components – HyClone, Carlington, the Netherlands), 200 U/ml collagenase and 50 U/ml DNAase-I (Sigma, St-Louis, MO, USA). Cell suspensions obtained by vigorous pipetting were washed twice and resuspended in supplemented RPMI-1640. Depending on the aim of the experiment, antibiotics (penicillin/streptomycin, HyClone) were or were not added to the medium. The FCS content was optimized for different experiments (see below).
Histopathology
At week 4 postinfection, lung tissue was examined for specific pathology. Mice were euthanized by thiopental overdose. After opening the chest, the lungs were filled in situ with 4% paraformaldehyde in PBS (PAF, pH = 7·2) via cannulated trachea, removed in toto and placed in 4% PAF for 24 h. After washing in running tap water and dehydrating by standard procedures, separated lung lobes were embedded in paraffin, and serial 6-µm thick sections were made across the widest area of the middle right lobe, always keeping an identical orientation. Sections were deparaffinated, stained with hematoxylin and eosin, and examined by an experienced pathologist without knowledge of the experimental groups.
Purification of interstitial lung macrophages
Purification was performed exactly as described previously [33]. Briefly, lung cells were incubated for 1·5 h at 37°C in petri dishes (Costar-Corning, Badhoevedorp, the Netherlands) in supplemented RPMI-1640. Non-adherent cells were removed by vigorous washing and, when appropriate, collected and used for further T-cell isolation. Adherent cells were detached by incubating dishes with PBS supplemented with 0·02% EDTA and 1 mm HEPES at room temperature for 30 min Cells were harvested, washed twice in antibiotic-free medium, resuspended in supplemented antibiotic-free RPMI-1640 containing 2% FCS and counted. The viability of cells, as determined by trypan blue exclusion, exceeded 93%, and the content of nonspecific esterase-positive cells was higher than 85%.
Purification of CD4+ and CD8+ lung T cells
CD4+ and CD8+ T-cell subsets were purified from plastic-nonadherent lung cells using the MACS method (Militenyi Biotec, Gladbach, Germany) as described earlier [37]. Briefly, cells were incubated with beads conjugated with rat-antimouse CD4 or rat-antimouse CD8 antibodies and positively selected on MS columns according to the manufacturer's instructions. The purity of recovered cells assessed by flow cytometry was >95% for CD4+ and >90% for CD8+ cells.
Staining of cell surface molecules
The bulk lung cell suspensions (3–5 × 105/sample) were washed with PBS supplemented with 0·5% BSA and 0·01% NaN3 and incubated for 5 min at 4°C with anti-CD16/CD32 mAbs (clone CT-17·1,17·2, Caltag, Burlingame, CA, USA) to block Fc receptors. Cells were then double-stained with directly conjugated Abs according to the manufacturer's instructions. The following Abs were used in different combinations: FITC- or PE-labelled anti-CD4 (clone CT-CD4, Caltag), FITC- or PE-labelled anti-CD8 (clone CT-CD8a, Caltag), PE-anti-CD62L (clone MEL-14, Caltag), PE-anti-F4/80 (clone CI:A3-1, Caltag), FITC-anti-CD44 (clone IM7, PharMingen, San Diego, CA), FITC-anti-I-Ap (clone 7–16·17, PharMingen), FITC-anti-CD11c (clone HL3, PharMingen). Stained cells were washed twice, fixed with 1% paraformaldehide and analysed by EPICS XL flow cytometer (Coulter Corporation, Miami, FL, USA) and MultiGraph software (Coulter). At least 104 cells of each sample were analysed.
Proliferative response
An aliquot of 105 purified CD4+ or CD8+ lung T cells was mixed with 3 × 105 irradiated (12 Gy) syngenic antigen-presenting spleen cells per well in a 96-well flat-bottom plate (Costar-Corning) and cultured at 37°C in 5% CO2 in the presence or absence (control) of 10 µg/ml H37Rv sonicate. Culture medium was supplemented RPMI-1640 containing 5% FCS and antibiotics. Cultures were pulsed with 0·5 µCi of [3H]-thymidine (Isotop, Sent-Petersburg, Russia) for the last 18 h of a 72-h incubation. The label uptake was measured in a liquid scintillation counter (Wallac, Turku, Finland) after harvesting each well's content onto fibreglass filters (Scatron, Oslo, Norway).
Cytokine assays
Lung cells were cultured at 2 × 106/ml in supplemented RPMI-1640 medium containing 5% FCS and antibiotics. Cells were either stimulated with 10 µg/ml H37Rv sonicate or left unstimulated. IL-4, IL-10, IL-12, TNF-α, and IFN-γ were measured in 48 h culture supernatants using sandwich ELISA assays. The following ELISA kits were purchased from PharMingen and used according to the manufacturer's instructions: OptEIA mouse TNF-α Set (sensitivity, 31 pg/ml), OptEIA mouse IL-4 Set (125 pg/ml), OptEIA mouse IL-12 Set (31 pg/ml), OptEIA mouse IL-10 Set (63 pg/ml), OptEIA mouse IFN-γ Set (63 pg/ml).
Antimycobacterial activity of macrophages
To evaluate the antimycobacterial activity of interstitial lung macrophages, the growth of mycobacteria in vitro in the presence or absence of these cells was estimated using the[3H]-uracil uptake assay [33,38], a surrogate method that provides >90% correlation with mycobacterial CFU counts [33]. Lung macrophages were recovered from uninfected or infected mice and plated at 4 × 104 cells/well in 250 µl of supplemented RPMI-1640 medium containing 2% of FCS without antibiotics in the wells of flat-bottom 96-well plates. Cells were allowed to adhere for 1 h, and M. tuberculosis H37Rv were added at multiplicity of infection (MOIs) between 10 and 1 in triplicates. Murine recombinant IFN-γ (Peprotech, London, UK) was added to some cultures at a final concentration of 100 U/ml. Mycobacteria cultured without macrophages and macrophages cultured in the absence of mycobacteria served as controls. 50 µl samples of the culture supernatants were removed 36 h after starting the culture and used for NO evaluation. Cultures were pulsed with 1 µCi/well [3H]-uracil (Isotop) for the last 18 h of the 72-h incubation. Cultures were terminated by freezing the plates at −30°C overnight, with subsequent thawing and harvesting the wells’ contents onto fibreglass filters. The [3H]-uracil uptake by mycobacteria was measured in a liquid scintillation counter. To kill the mycobacteria potentially present in macrophages recovered from infected mice, digestion of lung tissue and isolation of macrophages from these mice were performed using medium containing antibiotics. After isolation, macrophages were washed in antibiotic-free HBSS with 2% FCS and further cultured in antibiotic-free medium, as described above for macrophages from naive mice. Neither[3H]-uracil uptake nor colony formation were detected in these cultures, unless mycobacteria were added exogenously.
Nitric oxide production by, and lactate dehydrogenase (LDH) release from, macrophages
To assess production of nitric oxide by M. tuberculosis-infected macrophages, macrophages were loaded with mycobacteria as described above, and 50 µl supernatant samples were collected 36 h later. The concentration of nitrite, a stable metabolite of NO, was determined using the Griess reaction [39], by measuring absorbance at 550 nm in a micro-ELISA reader (Sigma), using a 620-nm reference filter.
To measure macrophage lysis following in vitro infection with M. tuberculosis, the content of LDH in culture supernatants was determined by its enzymatic activity using the CytoTox 96 kit (Promega, Madison, WI, USA) as recommended by the manufacturer. The percentage of specific lysis was calculated according to the formula: [(absorbance at 490 nm (A490) in experimental wells – A490 after spontaneous release)/(A490 total lysis – A490 after spontaneous release)] × 100.
Statistical analysis
The significance of the differences between mouse strains was estimated by the Student's t-test (CFU counts, mean survival time, T-cell proliferation) and the Mann–Whitney test (cell yield, LDH assay). Figures represent means ± SEM unless indicated otherwise. P < 0·05 was considered statistically significant.
RESULTS
Interstrain differences in disease severity
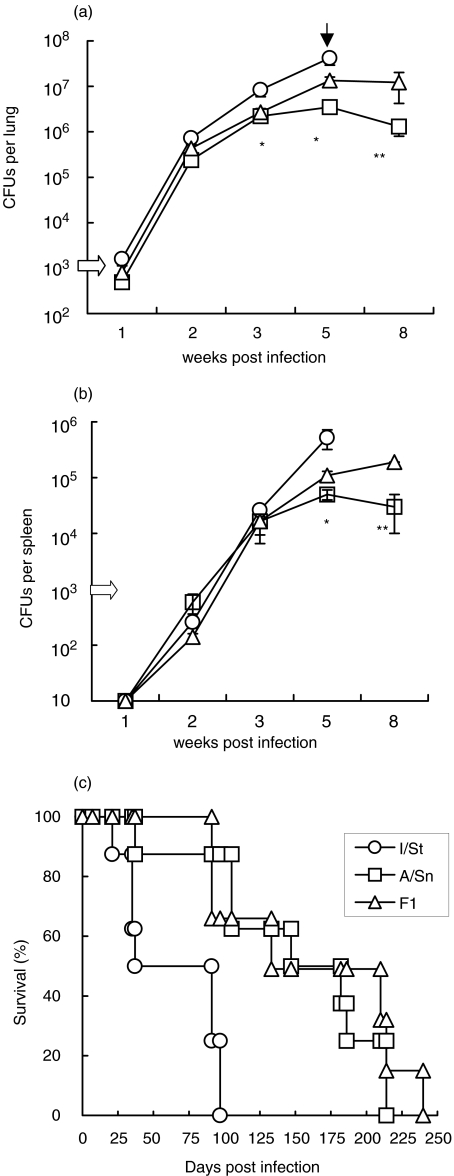
Previously, I/St, A/Sn and F1 mice have been categorized as, respectively, susceptible, resistant and highly resistant to TB infection using the i.v. challenge model [30,31]. Here we followed the course of TB infection induced by i.t. injection of 103M. tuberculosis CFUs. As shown in Fig. 1, during the first 2 weeks of infection mycobacterial growth was similar in the lungs of all three strains of mice (Fig. 1a). Mycobacteria appeared in spleens in week 2 of infection and had increased in numbers by about 15-fold by week 3 (Fig. 1b). In week 3 of infection, resistant A/Sn mice acquired the ability to control mycobacterial growth, as indicated by stable CFU counts between weeks 3 and 8, whereas in susceptible I/St mice there was a progressive increase in the mycobacterial population and these mice died between weeks 5 and 8 of infection. F1 mice exhibited an intermediate phenotype between that of the parental mice strains at week 5 postchallenge (P < 0·05, Fig. 1a,b). Mortality data (Fig. 1c) confirmed a high level of TB susceptibility in I/St mice, with these mice exhibiting a significantly (P < 0·01) shorter mean survival time (MST) compared to A/Sn and F1 mice. Contrary to that which has been demonstrated in a high-dose i.v. challenge model [31], F1 mice did not survive longer than A/Sn mice.
Fig. 1.
Mycobacterial growth in organs and mortality of mice following i.t. challenge. Groups of I/St, A/Sn and (A/Sn × I/St)F1 male mice were infected i.t. with 103 CFUs of M. tuberculosis and mycobacterial burden in (a) lungs and (b) spleens was assessed as described in Materials and Methods. Combined means ± SEM from 4 independent experiments (4–6 mice per time point in each experiment, total n = 20 per time point) are presented. Significant (P < 0·05, Student's t-test) differences between I/St – A/Sn and A/Sn – F1 mice are marked by * and **, respectively. Black arrow indicates that mortality reached approximately 50% in I/St mice by week 5 postinfection, thus CFU counts in I/St mice remaining alive by week 8 were not estimated. (c) Mortality curves were obtained in groups of 10 mice of each strain. Results of one out of two similar experiments are displayed.
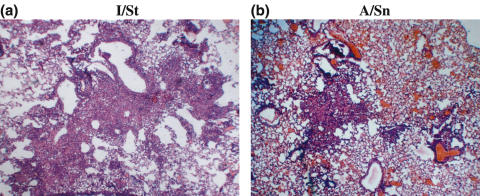
The degree of lung tissue pathology is considered an important characteristic in TB susceptibility/resistance analysis [5,40]. Almost no signs of lung tissue inflammation were evident at week 2 following challenge (data not shown). At week 4 postinfection, the lungs of I/St mice (Fig. 2a) contained abundant infiltrates, and large granulomata formed massive pneumonic foci. In contrast, the lungs of A/Sn mice (Fig. 2b) were mildly infiltrated, and pneumonic foci in the parenchyma were small and few.
Fig. 2.
Lung pathology in (a) I/St and (b) A/Sn mice at week 4 postchallenge. Mice were infected i.t. with 103 CFUs M. tuberculosis. Lung sections were stained with hematoxylin-eosin (magnification × 150).
Accumulation of inflammatory cells in the lungs
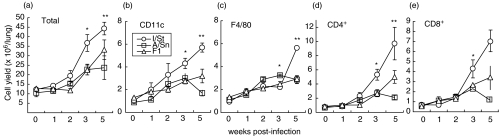
To quantitatively characterize inflammatory reactions in the lungs of infected mice, suspensions of lung cells were obtained and the number of macrophages (F4/80), dendritic cells (CD11c+) and T cells (CD4+ and CD8+) was assessed during the course of infection. Neither total cellularity of the lungs, nor the number of each cell type differed between I/St, A/Sn and F1 mice before challenge (Fig. 3, week 0). Starting at week 3 following challenge, the total number of cells, as well as the number of dendritic cells, CD4+ and CD8+ T cells significantly (P < 0·001–0·01) increased in the lungs of I/St mice compared to A/Sn and F1 mice. These results correlate well with the histopathological picture (Fig. 2).
Fig. 3.
Accumulation of inflammatory cells in the lungs of I/St (○), A/Sn (□) and F1 mice (▵) challenged i.t. with 103 CFUs M. tuberculosis. At indicated time points, lung cell suspensions were prepared from individual mice by enzymatic disruption (see Materials and methods) and analysed for the content of inflammatory cells using staining with subset-specific antibodies followed by flow cytometry. Results are combined means ± SEM from three independent experiments (3 mice per point in each, total n = 9 per point). Cells were counted (total), per cent of cells belonging to each subset was estimated by flow cytometry, and the cell yield in absolute numbers was calculated. Significant differences between I/St and A/Sn mice are labeled with asterisks: *P <0·05, **P <0·001, Mann–Whitney U-test.
T cell activation in the lungs of infected mice occurs during the second week of infection
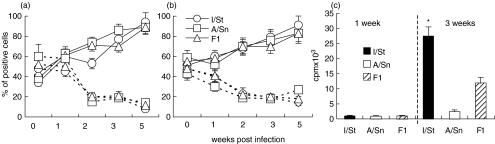
The dynamics of lung T-cell activation and the capacity of lung T cells to proliferate in response to mycobacterial antigens were also assessed during the course of infection. T-cell activation was estimated as an increase in CD44 and a decrease in CD62L expression within the CD4+ and CD8+ subsets. As shown in Fig. 4, starting in week 2 of infection, both CD4+ (Fig. 4a) and CD8+ (Fig. 4b) lymphocytes gradually up-regulated CD44 and down-regulated CD62L expression, resulting in 80–90% of cells being activated by week 5 post-infection. No interstrain differences with respect to lung T cell activation were detected.
Fig. 4.
Dynamics of activation (a, b) and proliferative response to mycobacterial sonicate (c) of lung T cells from I/St, A/Sn and F1 mice. At indicated time points, lung cells from individual mice (n = 3 per time point) were double-stained with (a) PE-anti-CD4 or (b) PE-anti-CD8, combined with FITC-anti-CD44 (—) or FITC-anti-CD62L (---) mAbs and analysed by flow cytometry. Results of one out of two similar experiments are depicted. Proliferation of lung cells was assessed after positive selection of CD4+ cells using MACS (Miltenyi). Cells were stimulated in vitro with H37Rv sonicate in the presence of irradiated APCs. No proliferation of purified CD8+ cells was observed. Bars represent mean ± SD of triplicate determinations in one out of two similar experiments. *P < 0·01, Mann–Whitney U-test; differences in proliferative response between I/St, A/Sn and F1 cells.
Assessment of proliferation indicated that mycobacteria-specific T cells first appeared in the lungs about 2 weeks postchallenge. In I/St and F1 mice, mycobacteria-specific proliferation of lung T cells was weak at week 2, but increased by week 3 postchallenge (Fig. 4c). Magnetic separation of lung CD4+ and CD8+ cells revealed that proliferation was restricted to the CD4+ subset. In full agreement with our previous results [32], only marginal proliferation of lung T cells was detected in A/Sn mice (Fig. 4c). Apparently, this was not due to the absence of mycobacteria-specific T cells in their lungs since these cells expressed an activated phenotype (Fig. 4a,b) and produced proinflammatory cytokines in an antigen-specific manner (see below). Thus, the proliferative activity of mycobacteria-specific T cells could be dissociated from their activation and effector functions.
Cytokine production by lung cells
Numerous studies in knock-out mouse models have clearly demonstrated that the type 1 cytokines IFN-γ, TNF-α and IL-12 are central elements of antimycobacterial immune defence (for review, see [29]), whereas the type 2 cytokines IL-4 and IL-10 are nonprotective [41]. To determine if lung cells from I/St, A/Sn and F1 mice displayed distinct patterns of cytokine production, the cells were cultured in vitro in the presence or absence H37Rv sonicate, and the cytokine concentrations in the culture supernatants were determined using ELISA.
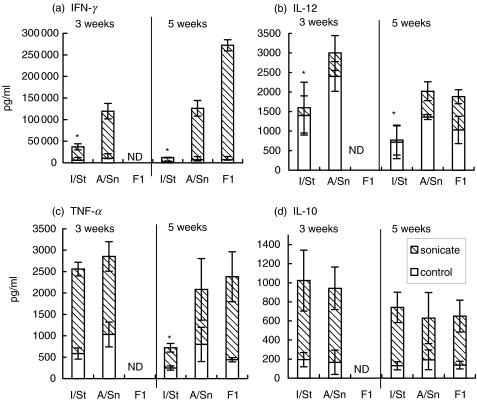
At week 1 postinfection, the production of all cytokines in all mice was low and did not depend upon secondary antigen stimulation (data not shown). As shown in Fig. 5, I/St lung cells produced significantly less IFN-γ than their A/Sn counterparts; moreover, IFN-γ production by I/St but not A/Sn cells decreased as the disease progressed (weeks 3 and 5, Fig. 5a). At week 5 postchallenge, I/St lung cells secreted 12 and 27 times less IFN-γ compared to A/Sn and F1 cells, respectively (P < 0·001). Similar, although less pronounced, interstrain differences were observed in IL-12 production which, however, was largely antigen-independent (Fig. 5b). Levels of TNF-α, although identical at early stages of infection in susceptible and resistant animals, dropped significantly (P < 0·01) by week 5 postchallenge in I/St but not A/Sn or F1 mice (Fig. 5c). No interstrain differences were observed in IL-10 production, and IL-4 was not detected in all groups. Thus, the overall production of type 1 cytokines by I/St lung cells was significantly decreased compared to cells from resistant animals, and this difference became increasingly evident as the disease progressed. It is noteworthy that the dynamics of cytokine production outside the lung differed profoundly from that described above. For example, IFN-γ production by splenocytes from infected animals neither differed between mouse strains, nor decreased in I/St animals as the infection progressed. The concentrations of IFN-γ in sonicate-stimulated splenocyte culture supernatants from I/St, A/Sn and F1 mice were, 9·7, 9·9 and 11·2 ng/ml (week 3) and 22·1, 21·4 and 22·9 ng/ml (week 5), respectively. Thus, a defect in the type 1 response in I/St mice is restricted to the infected lung tissue.
Fig. 5.
Production of type 1 cytokines by lung cells is markedly reduced in susceptible I/St compared to resistant A/Sn and F1 mice. 2 × 106/ml lung cells from individual mice were cultured, in the presence ( ) or absence (□) of 10 µg/ml H37Rv sonicate, for 60 h. Cytokine content in supernatants was determined using ELISA. Results are combined means ± SEM from two independent experiments (3 mice per time point in each, total n = 6 per point). *P < 0·01, Mann–Whitney U-test; differences between I/St and two other mouse strains.
) or absence (□) of 10 µg/ml H37Rv sonicate, for 60 h. Cytokine content in supernatants was determined using ELISA. Results are combined means ± SEM from two independent experiments (3 mice per time point in each, total n = 6 per point). *P < 0·01, Mann–Whitney U-test; differences between I/St and two other mouse strains.
Different antimycobacterial activity of lung macrophages from susceptible and resistant mice
The results described above indicate that mycobacteria-specific T cells appear in the lung no earlier than about the second week of infection. These T lymphocytes interact with mycobacteria-containing macrophages, changing significantly the effector functions of the latter. Thus, it was important to evaluate how the functional activity of lung macrophages changes during infection, especially since we have demonstrated recently that lung macrophages from naive I/St mice infected in vitro fail to restrict mycobacterial growth and die more readily compared to infected A/Sn macrophages [33].
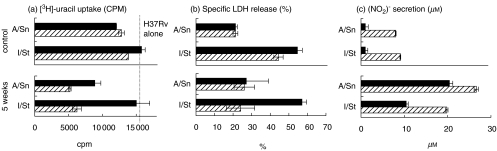
The gradual activation of lung macrophages was indicated by the increasing proportion of Class II-positive cells within the F4/80 lung cell population between week 2 (60–70%) and week 5 (90–95%) postinfection in all mice. To characterize the effector functions of lung macrophages, we estimated their capacity to combat mycobacteria and to resist their cytopathic effect using in vitro assays. Plastic-adherent cells were recovered from the lungs of naive mice (control) and mice infected with mycobacteria for 1 or 5 weeks. Cells were incubated in medium containing antibiotics to kill ‘endogenous’ mycobacteria, washed and infected with mycobacteria in vitro at various MOIs in antibiotic-free medium. In full agreement with our earlier results, control I/St macrophages were less efficient in restricting mycobacterial growth than their A/Sn counterparts and could not be activated to inhibit mycobacterial growth by addition of exogenous IFN-γ(Fig. 6a, upper panel). Similarly, IFN-γ failed to activate lung macrophages recovered from I/St mice at day 7 postchallenge (data not shown). However, at week 5 of infection, I/St lung macrophages became responsive to the activating action of IFN-γ. In the presence of IFN-γ, both A/Sn and I/St macrophages inhibited[3H]-uracil incorporation by mycobacteria significantly better than in its absence (P < 0·05, Fig. 6a, bottom panel). Importantly, as infection progressed, A/Sn macrophages increased their capacity to restrict mycobacterial growth in the absence of exogenous stimuli while I/St cells, although acquiring responsiveness to IFN-γ, remained poorly mycobacteriostatic per se.
Fig. 6.
Effect of exogenous IFN-γ on (a) mycobacterial growth, (b) cytopathic effect and (c) NO production in I/St and A/Sn lung macrophages following in vitro infection. Lung macrophages were recovered from control and infected mice and plated at 4 × 104 macrophages/well. Live M. tuberculosis H37Rv were added at MOIs between 10 and 1 in triplicates, and infected macrophages were incubated in the absence (▪) or presence (hatched bars) of 100 u/ml rIFN-γ. Results for MOI = 10 are displayed. Mycobacterial growth was assessed (a) by [3H]-uracil uptake and (b) by its cytopathic effect on macrophages by specific LDH release into culture supernatants after 72 h of culture. (c) After 36 h of culture, 50 µl samples of each supernatant were removed to evaluate nitrite content. Results of one out of three (mycobacterial growth) or two (LDH release and NO production) similar independent experiments are depicted as mean ± SD for triplicate determinations. As assessed by Mann–Whitney U-test, addition of IFN-γ significantly increased NO production by all macrophages (P < 0·001) and inhibited mycobacterial growth in macrophages recovered from infected mice (P < 0·01).
Interstrain differences between I/St and A/Sn lung macrophages were readily confirmed when the capacity of these cells to survive in vitro challenge was assessed using a common LDH release assay (Fig. 6b). The level of specific LDH release from infected I/St macrophages was significantly (P < 0·01) higher, indicating the stronger cytopathic effect of mycobacteria on these cells. Apparently, the capacity of A/Sn lung macrophages to better survive infection is an intrinsic trait, since neither disease progression in vivo, nor addition of exogenous IFN-γ in vitro quantitatively changed their ‘resistant’ phenotype (Fig, 6B). By contrast, the viability of TB-experienced I/St macrophages extracted at week 5 of infection significantly (P < 0·01) increased when exogenous IFN-α was added to cultures.
Given that the production of reactive nitrogen intermediates (RNIs) is generally considered a pivotal mechanism used by murine macrophages for mycobacterial killing [42–44], we estimated nitric oxide (NO) production in mycobacteria-infected I/St and A/Sn lung macrophage cultures. The results, depicted in Fig. 6, indicate that lung macrophages from naive mice of both strains produced negligible amounts of NO (Fig. 6c, upper panel). Although addition of IFN-γ resulted in a slight increase in NO production, the level of RNIs apparently remained below the threshold required for mycobacterial killing, as indicated by [3H]-uracil incorporation (Fig. 6a). However, by week 5 of infection, NO production by macrophages significantly increased and could be additionally stimulated by exogenous IFN-γ (Fig. 6c, bottom panel). Importantly, the level of NO production by TB-experienced A/Sn macrophages reached approximately 20 µm/ml in the absence of exogenous IFN-γ. This appeared to be sufficient to confer a level of antimycobacterial activity that could be only slightly increased by further stimulation with IFN-γ (Fig. 6a,b). On the other hand, I/St cells reached the ‘effective’ level of NO production only if stimulated with IFN-γ in vitro, as is clearly reflected by the results of the [3H]-uracil and LDH assays.
Taken together, these results suggest that: (i) ‘unprimed’ lung macrophages from both I/St and A/Sn mice are resistant to the activating antimycobaterial effect of IFN-γ, although these two cell populations display significantly different abilities to inhibit mycobacterial growth and different sensitivities to the cytopathic action of mycobacteria; and (ii) in the course of M. tuberculosis infection both I/St and A/Sn lung macrophages acquire responsiveness to IFN-γ; however, the interstrain differences in their capacity to inhibit mycobacterial growth are retained except when IFN-γ is added to the experimental system exogenously.
DISCUSSION
In the present work we studied the dynamics of M. tuberculosis-triggered disease following i.t. infection of I/St and A/Sn inbred mice and their F1 hybrids. It has been shown by others that mycobacterial delivery directly to the lung induces a more severe disease compared to that induced by intravenous challenge, and that only 1% of mycobacteria delivered i.v. are deposited in the lung [8,45]. This corresponds well with our data. I.t. injection of 103 CFUs and i.v. injection of 105 CFUs resulted in a similar mycobacterial content in the lungs of infected mice between weeks 2 and 5 of infection (data not shown); however, i.t. challenge caused a more rapid death of mice; about 50% of I/St mice died by week 5 following i.t. infection (Fig. 1), whereas no mortality among the mice challenged by the i.v. route was observed at this time point [32].
Development of M. tuberculosis infection is accompanied by a complex chain of local and systemic reactions, including the generation of antigen-specific T lymphocytes and their migration to the lung, inflammatory lung tissue infiltration and activation of interstitial lung macrophages. At an early stage of infection, mycobacteria showed progressive growth in the lungs (Fig. 1a), but the inflammatory response in the lung tissue was practically undetectable. Starting at week 3 postchallenge, the speed of mycobacterial multiplication in the lungs was reduced (Fig. 1), presumably, at the cost of progressive inflammatory infiltration of the lung tissue, which was particularly evident in susceptible I/St mice (Fig. 2). Activation of lung T lymphocytes occurred no earlier than week 2 following challenge (Fig. 4a,b) which corresponds well with previous observations [32,37,46]. Shortly after this, lung macrophages increased their MHC Class II expression and acquired the ability to increase antimycobacterial function in response to exogenous IFN-γ (Fig. 6). Development of inflammatory reactions and activation of macrophages coincided with the gradual accumulation of immune T cells in the lungs and are possibly the consequence of T cell functional activity.
T cell activation of macrophages via IFN-γ is generally considered to be a pivotal protective response against mycobacteria [25,47,48]. However, in our experimental system, exogenous IFN-γ did not stimulate the antimycobacterial activity of lung macrophages if cells were recovered from a noninfected host [33, Fig. 6]. The inability of IFN-γ to increase the antimycobacterial activity of human alveolar macrophages has also been reported [49]. Thus, questions arise about the mechanisms of lung macrophage activation and the role of T cells (and IFN-γ) in this process. Our results might help to clarify these issues.
We have found that lung macrophages recovered from TB-infected animals, unlike cells obtained from noninfected mice, are readily activated by exogenous IFN-γ (Fig. 6). BCG vaccination induces similar changes in lung macrophage responsiveness to IFN-γ (unpublished observation). It is reasonable to assume that in the course of infection lung macrophages pass through the process that could be termed ‘macrophage maturation’ or ‘macrophage priming’. Irrespective of the nature and number of factors potentially involved in the maturation process [50,51], it is likely that IFN-γ finalises rather than initiates the maturation process. The fact that one of the key sources of IFN-γ, i.e. immune-stimulated T cells, do not accumulate in the lungs before weeks 2/3 of infection may be considered as indirect evidence in favour of this hypothesis.
At the late, immune phase of infection, I/St lung cells produced significantly less type 1 cytokines than their A/Sn and F1 counterparts, with IFN-γ deficiency being the most pronounced (Fig. 5). Given the ability of IFN-γ to stimulate the antimycobacterial activity of macrophages from mice with advanced TB (Fig. 6), these quantitative differences may account for the ability of A/Sn, but not I/St mice, to control mycobacterial multiplication late in the infection course (Fig. 1). The most convincing evidence for the important role of IFN-γ in TB resistance comes from studies in mice with a genetically disrupted IFN-γ-encoding gene [52] and from human family studies of missense mutations in IFN-γ- or IFN-γ R-encoding genes [53]. Our results suggest that not only a complete lack of function but also quantitative differences in the level of IFN-γ production could significantly influence the progress of TB infection. Importantly, spleen cells recovered from M. tuberculosis-infected I/St and A/Sn mice produced similar levels of IFN-γ (see Results). This suggests that (i) I/St mice carry a nondefective allele of the Ifng gene and (ii) the decrease in IFN-γ production is lung tissue-specific and might be a consequence of the progress of infection, rather than its cause.
These results generate important questions concerning the causal links within a network of host responses that function to maintain an appropriate balance between effective host protection and fatal pathology. Such questions will require a substantial number of genetic studies to provide answers. It is well established that a number of QTLs participate in the control of resistance/susceptibility to TB in mice [4–7]. Future studies will determine which of these immunological and inflammatory responses are controlled by these loci.
Acknowledgments
We thank Dr David McMurray for critically reading the manuscript. This work was supported by HHMI grant 753–01564101 (to ASA as a Howard Hughes International Research Scholar), by R-01 grant HL 68532–02 from NIH, and by grant 01-04-49095 from Russian Foundation for Basic Research.
REFERENCES
- 1.Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–7. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 2.Comstock GW. Tuberculosis in twins. a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–6. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 3.Newport M, Levin M. Genetic susceptibility to tuberculosis. J Infect. 1999;39:117–21. doi: 10.1016/s0163-4453(99)90002-6. [DOI] [PubMed] [Google Scholar]
- 4.Lavebratt C, Apt AS, Nikonenko BV, Schalling M, Schurr E. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J Inf Dis. 1999;180:150–5. doi: 10.1086/314843. [DOI] [PubMed] [Google Scholar]
- 5.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsos E, Cardon LR, Fortin A, Ryan L, LaCourse R, Nort h RJ, Gros P. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 2000;1:467–73. doi: 10.1038/sj.gene.6363712. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez F, Radaeva TV, Nikonenko BV, et al. Multigenic control of desease severity after Mycobacterium tuberculosis infection in mice. Infect Immun. 2003;71:126–31. doi: 10.1128/IAI.71.1.126-131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.North RJ. Vaccinated mice remain more susceptible to Mycobacterium tuberculosis infection initiated via the respiratory route than via the intravenous route. Infect Immun. 1999;67:2010–2. doi: 10.1128/iai.67.4.2010-2012.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rook GAW, Hernandez-Pando R. The pathogenesis of tuberculosis. Ann Rev Microbiol. 1996;50:259–82. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 10.Dannenberg AJ. Pathogenesis of pulmanory tubercolosis. Am Rev Respir Dis. 1982;125:25–9. doi: 10.1164/arrd.1982.125.3P2.25. [DOI] [PubMed] [Google Scholar]
- 11.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 12.Henderson HJ, Jr, Dannenberg AM, Lurie MB. Phagocytosis of tubercle bacilli by rabbit pulmonary alveolar macrophages and its relation to native resistance to tuberculosis. J Immunol. 1963;90:553–5. [PubMed] [Google Scholar]
- 13.Mohagheghpour N, van Vollenhoven A, Goodman J, Bermudez LE. Interaction of Mycobacterium avium with human monocyte-derived dendritic cells. Infect Immun. 2000;68:5824–9. doi: 10.1128/iai.68.10.5824-5829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonough KA, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–11. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–6. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobos KM, Spotts EA, Quinn FD, King CH. Necrosis of lung epithelial cells during Infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect Immun. 2000;68:6300–10. doi: 10.1128/iai.68.11.6300-6310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orme IM, Roberts AD, Griffin JP, Adams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–25. [PubMed] [Google Scholar]
- 18.Cooper AM. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–6. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 19.Rhoaders ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–7. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–90. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 22.North RJ. T cell dependence of macrophage activtion and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974;10:66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme IM, Anderson P, Boom WH. Tcell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 24.Strenger S, Hanson DA, Teitelbaum R, et al. An amtimicroboal activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 25.Daniel TM, Boom WH. Immunology of Tuberculosis. In: Reichman LR, Hershfield ES, editors. TuberculosisA comprehensive international approach. New York: Marcel Dekker; 2000. pp. 67–82. [Google Scholar]
- 26.Serbina N, Liu C-CV, Scanga CA, Flynn JL. CD8 CTL from lungs of Mycobacterium tuberculosis- infected mice express perforine in vivo and lyse infected macrophages. J Immunol. 2000;165:353–63. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 27.Ellner JJ, Wallis RS. Immunologic aspects of mycobacterial infections. Rev Inf Dis. 1989;11(Suppl. 2):S455. doi: 10.1093/clinids/11.supplement_2.s455. [DOI] [PubMed] [Google Scholar]
- 28.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–6. [PubMed] [Google Scholar]
- 29.Dannenberg AJ. Immunopathogenesis of pulmanory tuberculosis. Hosp Pract. 1993;28:51–8. doi: 10.1080/21548331.1993.11442738. [DOI] [PubMed] [Google Scholar]
- 30.Nickonenko BV, Apt AS, Mezhlumova MB, Avdienko VG, Yeremeev VV, Moroz AM. Influence of the mouse Bcg, Tbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of BCG vaccination. Clin Exp Immunol. 1996;104:37–43. doi: 10.1046/j.1365-2249.1996.d01-643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikonenko BV, Averbakh MM, Lavebratt C, Schurr E, Apt AS. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tubercule Lung Dis. 2000;80:15–25. doi: 10.1054/tuld.1999.0225. [DOI] [PubMed] [Google Scholar]
- 32.Lyadova IV, Eruslanov EB, Yeremeev VV, et al. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant and hyperresistant to Mycobacterium tuberculosis-triggered disease. J Immunol. 2000;165:5921–31. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 33.Majorov KB, Lyadova IV, Kondratieva TK, Eruslanov EB, Rubakova EI, Orlova MO, Mischenko VV, Apt AS. Different innate ability of I/St and A/Sn mice to combat virulent M. tuberculosis: phenotypes expressed in lung and extra-pulmonary macrophages. Infect Immun. 2003;71:697–707. doi: 10.1128/IAI.71.2.697-707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyadova IV, Yeremeev VV, Majorov KB, Nikonenko BV, Khaidukov SV, Kondratieva TK, Kobets NV, Apt AS. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect Immun. 1998;66:4981–8. doi: 10.1128/iai.66.10.4981-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondratieva TK, Kobets NV, Khaidukov SV, Yeremeev VV, Lyadova IV, Apt AS, Tam MF, Stevenson MM. Characterization of T cell clones derived from lymph nodes and lungs of Pseudomonas aeruginosa-susceptible and resistance mice following immunization with heat-killed bacteria. Clin Exp Immunol. 2000;121:275–82. doi: 10.1046/j.1365-2249.2000.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson MM, Kondratieva TK, Apt AS, Tam MF, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–102. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyadova IV, Eruslanov EB, Vordermeier M, Khaidukov S, Apt AS, Hewinson G. Intranasal BCG vaccination protects BALB/c mice against virulent Mycobacterium bovis and accelerates production of IFN-γ in their lungs. Clin Exp Immunol. 2001;126:274–9. doi: 10.1046/j.1365-2249.2001.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stach JL, Gros P, Forget A, Skamene E. Phenotypic expression of genetically controlled natural resistance to Mycobacterium bovis (BCG) J Immunol. 1984;132:888–92. [PubMed] [Google Scholar]
- 39.Migliorini P, Corradin G, Corredin SB. Macrophages NO2- production as a sensitive and rapid assay for the quantitation of murine IFN. J Immunol Meth. 1991;139:107–15. doi: 10.1016/0022-1759(91)90357-l. [DOI] [PubMed] [Google Scholar]
- 40.Orrell JM, Brett SJ, Ivanyi J, Coghill G, Grant A, Beck JS. Morphometric analysis of Mycobacterium tuberculosis infection in mice suggests a genetic influence on the generation of the granulomatous inflammatory response. J Pathol. 1992;166:77–82. doi: 10.1002/path.1711660112. [DOI] [PubMed] [Google Scholar]
- 41.North RJ. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–8. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermudez LE. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–81. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated mutine macropahges. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.North RJ. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J Infect Dis. 1995;172:1550–3. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]
- 46.Feng CG, Bean AG, Hooi H, Briscoe H, Britton WJ. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67:3242–7. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa JP, Rastogi N. Comparative ability of human monocytes and macrophages to control the intracellular growth of Mycobacterium avium and Mycobacterium tuberculosis: effect of interferon-gamma and indomethacin. FEMS Microbiol Immunol. 1992;4:329–34. doi: 10.1111/j.1574-6968.1992.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 48.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–4. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aston C, Rom WN, Talbot AT, Reibman J. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am J Respir Crit Care Med. 1998;157:1943–50. doi: 10.1164/ajrccm.157.6.9705028. [DOI] [PubMed] [Google Scholar]
- 50.Franke-Ullmann G, Prortner C, Walter P, Steinmuller C, Lohmann-Matthes M, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol. 1996;157:3097–104. [PubMed] [Google Scholar]
- 51.Johanson A, Lundborg M, Lundahl J, Tornling G, Eklund A, Camner P. Functional, morphological, and phenotypical differences between rat alveolar and interstitial macrophages. Am J Respir Cell Mol Biol. 1997;16:582–8. doi: 10.1165/ajrcmb.16.5.9160840. [DOI] [PubMed] [Google Scholar]
- 52.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova J. Il-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]