Abstract
Anti-mitochondrial antibody (AMA) is considered the serological hallmark of primary biliary cirrhosis (PBC), but may be missing in a proportion of these patients. We assessed sensitivity and specificity of the currently available techniques for AMA detection in a large series of PBC patients and controls, and analysed their clinical and immunological features according to the AMA status. By indirect immunofluorescence on rat tissue sections and HEp-2 cells, Western immunoblot with bovine submitochondrial particles, and two ELISAs with AMA-specific recombinant proteins, we evaluated the presence of AMA in 127 PBC patients, 166 patients with type 1 autoimmune hepatitis and 100 with non alcoholic fatty liver disease. In PBC patients Western immunoblot detects AMA significantly more often than indirect immunofluorescence on HEp-2 cells (85% versus 72%, P = 0·02) or rodent tissue sections (71%, P = 0·01); both ELISAs are only slightly less sensitive than Western immunoblot (81% and 78%). Ten patients with non alcoholic fatty liver disease were AMA-positive by indirect immunofluorescence, but none recognized AMA-specific epitopes in Western immunoblot or in ELISAs. Twelve patients with type 1 autoimmune hepatitis were AMA-positive by indirect immunofluorescence, but only 6 (3·6%) reacted by Western immunoblot and ELISAs. Western immunoblot or ELISA should be regarded as first-line assay for the detection of AMA. Up to 15% of PBC patients are consistently AMA-negative, yet they share the same clinical, biochemical and histological features of AMA-positive PBC. Detection of AMA in type 1 autoimmune hepatitis might identify a subset of patients at risk of developing a hepatitic/cholestatic syndrome.
Keywords: liver, cholestasis, autoantibodies, Western immunoblotting, overlap syndrome
INTRODUCTION
Primary biliary cirrhosis (PBC) is a chronic liver disease characterized by cholestasis, inflammatory destruction of intrahepatic bile ducts, and presence of anti-mitochondrial antibody (AMA) [1]. The close association between AMA and PBC was first recognized in the sixties [2], but only some decades later were the major mitochondrial autoantigens identified as components of the 2 oxo acid dehydrogenase complex (2-OADC) such as the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2) [3], the E2 subunit of branched chain 2-oxo-acid dehydrogenase complex (BCOADC-E2) [4], the E2 subunit of oxoglutarate dehydrogenase complex (OGDC-E2) [5], the E1α subunit and the E3 binding protein of PDC (PDC-E1α and E3BP, respectively) [6–8].
Even if AMA is detectable in the vast majority of PBC patients, the existence of AMA negative cases is generally accepted, and in recent years many studies have focused on the clinical, histological and immunological features of these patients in comparison with classical AMA-positive PBC [9–13].
AMA is commonly detected with assays such as indirect immunofluorescence (IFL) on frozen sections of rat liver kidney and stomach sections and Hep2 cell lines, and only seldom by Western Immunoblot (W-IB) using submitochondrial particles of bovine or porcine heart as antigen source, and ELISA with the recombinant mitochondrial target proteins.
Given the high specificity and sensitivity of AMA as immunoserological hallmark of PBC, the determination of the best methodological approach for AMA detection is crucial. Aim of this study is the evaluation of the sensitivity and specificity of different substrates and techniques for the detection of reactivities against mitochondrial antigens expressed in different conditions (i.e. conformational native epitopes on rat tissue/cell line by IFL, linearized epitopes on mitochondrial preparatons by W-IB, conformational/linear epitopes on recombinant proteins by ELISA). In particular, we analysed a large number of PBC patients with an AMA-negative IFL test, to see if detectable levels of AMA may be revealed using additional assays.
In addition, we compared the clinical, biochemical and histological features of AMA-positive and AMA-negative PBC patients.
PATIENTS AND METHODS
Study population
From 1997 to 2002 at our Institution, which is a national referral centre for autoimmune liver diseases, we diagnosed 127 consecutive patients as having PBC on the basis of biochemical cholestasis, high IgM levels, and pathognomonic histological findings. AMA was detected by IFL on rat tissues in 91 out of 127 patients. All the remaining 38 AMA-negative patients, assessed by IFL, had liver biopsy findings indicative of PBC. The biliary tree of all these patients was also studied by Nuclear Magnetic Resonance cholangiography and/or by endoscopic retrograde colangiography, which in no case showed features of primary sclerosing cholangitis. Viral, obstructive and metabolic aetiologies were ruled out using appropriate tests. Drug aetiology was ruled out by a carefull drug history. Liver biopsy was available in 105 (83%) of 127 patients: 68 had evidence of initial disease (stages I/II), whereas in 37 a more advanced picture (stages III/IV) was observed. In 22 patients with a positive AMA test by routine IFL liver biopsy was not performed since the diagnosis of cirrhosis was clinical and/or instrumental (ascites, oesophageal varices).
All PBC patients have been tested at the time of diagnosis, before starting any treatment. Serum samples were stored at −20°C until use. Each patient gave his/her informed consent for this study.
Comparison population
A series of 166 patients with autoimmune hepatitis (AIH), diagnosed according to the criteria issued by the International Autoimmune Hepatitis Group (IAIHG) [14] and evaluated by the same investigator (A.J.C.) were studied. Of these, 141 reached the score of ‘definite’ and 25 of ‘probable’ AIH. All tested samples, stored at −20°C until the use, were collected at baseline, before starting immunosuppressive therapy.
As an additional control population, we studied a series of 100 patients, evaluated by the same investigator (P.L.), all with non alcoholic fatty liver disease (NAFLD), a diagnosis based on persistent cryptogenetic hypertransaminasemia and liver steatosis at ultrasonography.
Indirect immunofluorescence
Sera diluted 1 : 40 in phosphate buffered saline (PBS) were tested on cryostat sections of snap-frozen rat liver, kidney and stomach for 20 min in a humidified chamber. After three washing steps in PBS, the sections were incubated for 20 min with fluorescein-conjugated anti-human immunoglobulin (anti-human polyvalent immunoglobulin IgA, IgG, IgM FITC Conjugate, Sigma ImmunoChemicals, St. Louis, MO, USA), used as secondary antibody diluted 1 : 100. The immunofluorescence patterns were assessed under fluorescence microscope (Orthoplan, Leitz, Wetzlar, Germany). Each serum was also tested on commercially available HEp-2 cell lines (Kallestad, Chaska, MN, USA) at 1 : 100 dilution of the serum and of the secondary antibody. The typical ‘granular’ positivity of the cytoplasm is considered as AMA reactivity. Each sample was independently evaluated by two investigators (F.C. and P.M.).
Immunoblotting
All sera were tested by W-IB using as source of antigens a preparation of submitochondrial particles from bovine heart. Briefly, mitochondrial proteins (1 mg/ml) extracted from bovine heart were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in 10% mini gels (Mini-Protean II System, Bio-Rad Laboratories, Richmond, CA, USA) and transblotted onto nitrocellulose filters, which were then incubated in PBS containing 5% skimmed milk (blocking solution) for 1 h at room temperature. The filters were then cut into strips, and each strip was incubated with serum sample diluted 1 : 500 in blocking solution for 90 min on a rotor wheel at room temperature. After incubation, the strips were washed three times in PBS containing 0·1% Tween 20 and then incubated 1 h at room temperature in blocking solution containing the secondary antibody diluted 1 : 2000 (peroxidase-conjugated rabbit anti-human IgG, IgA and IgM, Dako, Copenhagen, Denmark). After further washing, the colourimetric reaction was developed with 4-chloro-1-naphtol for 10 min at room temperature.
ELISA
ELISA assay was carried out using as antigen the affinity purified recombinant fusion protein expressed by pML-MIT3 hybrid clones, as previously described in details [15], which express all the major mitochondrial epitopes such as PDC-E2, BCOADC-E2, OADC-E2.
Briefly, each well was coated overnight at 4°C with 100 µl (10 µg/ml) of the purified recombinant fusion protein. After three washing with PBS Tween 0·05%, 100 µl of blocking solution (PBS/bovine serum albumin 1%) was added to each well for 60 min at room temperature. One hundred microlitres of the test sera diluted 1 : 1000 in PBS/powder milk 3% were added in duplicate to each well and incubated for 60 min at room temperature. After three washing steps with PBS Tween 0·05%, 100 µl of rabbit peroxidase anti human immunoglobulin (Dako, Denmark) diluted 1 : 1000 in PBS/powder milk 3% was added to each well, incubated for 60 min at room temperature. After the washing steps the reaction was developed with o-phenylenediamine dihydrochloride (Sigma, St.Louis, USA) and H2O2 in citrate buffer for 15 min, and the colourimetric reaction was measured at 492 nm by spectrophotometry. The cut-off, in 80 healthy blood donors, was established as the median optical density plus 10× standard deviation (0·020 + {10 × 0·003} = 0·023).
All sera have been also assessed for reactivity against recombinant mitochondrial proteins PDC-E2, BCOADC-E2 and OADC-E2 by a commercially available semiquantitative ELISA assay (MBL Diagnostic, Nagoya, Japan) according to manufacturer's instructions.
Absorption test
To assess the specificities of the reactivities observed by W-IB, 5 representative AMA-positive sera (100 µl diluted 1 : 1000) were incubated overnight at 4°C with 100 µl of purified recombinant fusion protein (protein concentration: 300 µg/ml) expressing the three major mitochondrial epitopes (PDC-E2, BCOADC-E2 and OADC-E2).
Statistical analysis
The comparison of categorical variables was performed using Chi-square and Fisher's exact test when applicable. Unpaired t-test was used for the comparison of continuous data. Nominal variables were correlated by contingency table. A P-value < 0.05 was considered significant. Statistical analysis was performed using GraphPad InStat version 3·0a for Macintosh (GraphPad Software, San Diego, California, USA), and StatView 5·0.1 for Macintosh (SAS Institute Inc., Cary North, Carolina, USA).
RESULTS
Ninety-one (71·6%) PBC patients showed the typical AMA pattern on rat tissues (Fig. 1) and 72·4% had the characteristic ‘granular’ cytoplasmic positivity on HEp-2 cells. One hundred and three (81·1%) were AMA-positive by ELISA using the recombinant fusion protein as antigen, and 100 (78·7%) were positive using the commercially available ELISA kit. The detailed analysis of the optical density showed a high degree of correlation between the two ELISAs (commercial ELISA median optical density: 0·750, range 0·122–1·562; ‘in house’ ELISA median optical density: 0·768, range 0·059–1·596). In particular, only 6 sera had a difference in optical density values higher than 5% (of these, 3 were negative with the commercial ELISA kit, but positive with ‘in house’ ELISA). One hundred and eight (85%) were positive by W-IB and the mitochondrial proteins were recognized with the following hierarchy: PDC-E2 (70 kD) 93%, E3BP (52 kD) 93%, BCOADC-E2 (50 kD) 32%, OADC-E2 (46 kD) 41%. Anti-PDC-E2 and anti-E3BP reactivities were always detected together. Eight of 108 AMA positive samples (7%) only reacted with BCOADC-E2 (50 kD). An additional 40 kD reactivity, possibly anti-PDC-E1 alpha, was observed in 9 PBC patients; such a reactivity is never isolated, but always with other well-characterized AMA-specific bands in W-IB experiments. All the PBC sera positive for AMA by IFL on rat tissue were also positive by ELISA and W-IB, while a unique serum with the ‘granular’ cytoplasmic positivity on HEp2 cells was negative both by ELISA and by W-IB. Among 17 AMA negative by IFL, but positive by W-IB, 16 patients were positive for anti-PDC-E2 and PDC-E3 BP, and 2 for anti-BCOADC-E2 and anti-OADC-E2, variously associated. The detailed W-IB patterns and their corresponding optical density values are reported in Table 1.
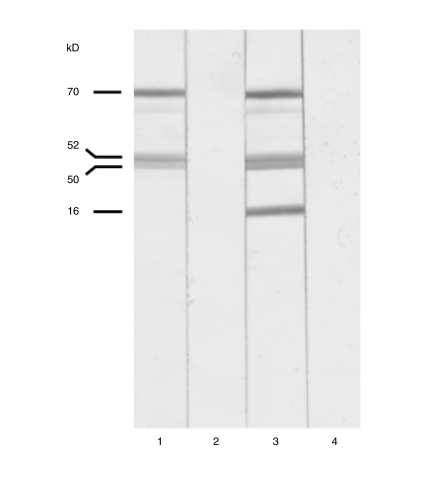
Fig. 1.
Western immunoblotting with bovine heart mitochondria. Lanes 1 and 3: representative anti-mitochondrial positive sera; lanes 2 and 4: same sera as lanes 1 and 3, after absorption (see text for details). Anti-PDC-E2 (70 kD), Anti-E3BP (52 kD), Anti-BCOAGDC-E2 (50 kD), Anti-OGDC-E2 (46 kD).
Table 1.
W-IB reactivities and ELISA OD in PBC Patients with AMA-negative IFL test
| Pts. | W-IB reactivities | ‘In house’ ELISA (OD) | Commercially ELISA (OD) |
|---|---|---|---|
| 1 | Anti-PDC-E2, Anti-E3BP | neg | neg |
| 2 | Anti-PDC-E2, Anti-E3BP | neg | neg |
| 3 | Anti-PDC-E2, Anti-E3BP, Anti-BCOADC-E2 | neg | neg |
| 4 | Anti-PDC-E2, Anti-E3BP | neg | neg |
| 5 | Anti-PDC-E2, Anti-E3BP | 0·811 | 0·789 |
| 6 | Anti-PDC-E2, Anti-E3BP | 0·098 | neg |
| 7 | Anti-BCOADC-E2 | 0·561 | 0·578 |
| 8 | Anti-PDC-E2, Anti-E3BP, Anti-OGDC-E2 | 0·128 | 0·132 |
| 9 | Anti-PDC-E2, Anti-E3BP | 0·521 | 0·542 |
| 10 | Anti-PDC-E2, Anti-E3BP | 0·768 | 0·735 |
| 11 | Anti-PDC-E2, Anti-E3BP | 0·257 | neg |
| 12 | Anti-PDC-E2, Anti-E3BP | neg | neg |
| 13 | Anti-PDC-E2, Anti-E3BP | 0·059 | neg |
| 14 | Anti-PDC-E2, Anti-E3BP | 0·344 | 0·336 |
| 15 | Anti-PDC-E2, Anti-E3BP | 0·671 | 0·658 |
| 16 | Anti-PDC-E2, Anti-E3BP, Anti-OGDC-E2 | 0·901 | 0·884 |
| 17 | Anti-PDC-E2, Anti-E3BP | 0·714 | 0·686 |
W-IB, Western Immunoblot; OD, Optical Density; PDC-E2, E2 subunit of the pyruvate dehydrogenase complex; BCOADC-E2, E2 subunit of branched chain 2-oxo-acid dehydrogenase complex; OGDC-E2, E2 subunit of oxoglutarate dehydrogenase complex E3BP: E3 Binding protein.
The detection rate of AMA in PBC patients was significantly higher using W-IB in comparison with IFL on rat tissues or HEp-2 cells (P = 0·01 and P = 0·02, respectively, Table 2).
Table 2.
Sensitivity and specificity of different techniques for AMA detection in PBC patients
| Sensitivity (127 cases) | Specificity (166 + 100 cases) | |
|---|---|---|
| W-IB | 85%†* | 97·8% |
| ‘In-House’ ELISA | 81·1% | 97·8% |
| Commercial ELISA | 78·8% | 97·8% |
| IFL on rat tissue | 71·6%* | 97·4% |
| IFL on HEp-2 cells | 72·4%† | 93·3% |
W-IB, Western immunoblotting; IFL, indirect immunofluorescence. The sensitivity has been evaluated in 127 PBC cases, the specificity in 166 type 1 autoimmune hepatitis patients and in 100 patients with non alcoholic fatty liver disease.
P = 0·01;
P = 0·02.
The main biochemical and immunological parameters of the PBC patients, according to their AMA status in W-IB and ELISA assays, are very similar, and are reported in Table 3.
Table 3.
Main biochemical liver parameters in AMA-positive and AMA-negative PBC
| AMA +ve PBC (108 patients) | AMA –ve PBC (19 patients) | |
|---|---|---|
| Gender (M/F) | 14/94 | 0/19 |
| Age (years) | 58·1 ± 14 | 57·2 ± 12·5 |
| ALT (x unl) | 1·6 ± 1·5 | 2·3 ± 1·7 |
| ALP (x unl) | 2·3 ± 2·2 | 3·2 ± 2·6 |
| Bilirubin (mg/dl) | 1·3 ± 2·6 | 1·3 ± 1·5 |
| Albumin (g/l) | 37 ± 5 | 39 ± 4 |
| γ-globulins (g/l) | 18 ± 4 | 18 ± 4 |
| IgM (mg/dl) | 544 ± 402 | 461 ± 387 |
| Histological stage I-II | 56/86 (65%) | 12/19 (63%) |
| Histological stage III-IV | 30/86 (35%) | 7/19 (37%) |
ALT, alanino aminotransferase; ALP, alkaline phosphatase; unl, upper normal level. Data are reported as mean ± Standard Deviation.
In the NAFLD series 2 (2%) patients were AMA-positive by IFL on rat tissues and 10 (10%) on HEp-2 cells, but none of them was AMA-positive by W-IB or ELISA. The liver biopsy performed in all 10 AMA-positive cases showed a picture of steatosis, but not signs of duct/ductular injury.
In the AIH series 5 out of 166 (3%) patients were AMA-positive by IFL on rat sections and 8 showed a ‘granular’ cytoplasmic positivity on HEp-2 cells; 6 of 166 (3·6%) were AMA-positive both by ELISA and W-IB. All 5 AIH sera with AMA by IFL on rat sections were also reactive by ELISA and W-IB assays, whereas only 1 of 8 sera giving the ‘granular’ cytoplasmic positivity on HEp-2 cells was also reactive by ELISA and W-IB. Five AMA-positive AIH patients recognized only PDC-E2 and E3 BP, whereas one recognized also BCOADC-E2.
The absorption of the AMA reactivity with the recombinant mitochondrial proteins led to complete disappearance of the specific bands (Fig. 1), confirming the specificity of the W-IB technique.
All of the 6 AMA-positive AIH patients had a cumulative IAIHG score of ‘definite’ AIH; the histological features were those of AIH, in particular no bile duct lesions were observed. The HLA profile, available for 5 of them, was typical of AIH; in particular all these patients had at least one or more HLA alleles characteristic of AIH (i.e. A1, B8, DR3 or DR4)
DISCUSSION
AMA reactivity is considered the serological ‘gold standard’ of PBC diagnosis, and the lack of its detection often delays the recognition of this disease and the prompt initiation of the appropriate therapy. It is generally accepted that AMA positivity, even before the appearing of biochemical cholestasis, strongly points to the diagnosis of PBC in an early stage, many years before the biochemical and the clinical expression of the disease [16]. At the light of its prominent diagnostic value, it is essential to identify the most sensitive and specific technique for AMA detection.
Historically, AMA is detected in most laboratories by IFL on rat tissue, an approach considered to balance sensitivity and specificity, even if false-positive reactivities sometimes may occur, due to other specificities such as anti-cardiolipin antibody [17].
In our experience, the sensitivity of IFL was only 71–72%, therefore nearly 30% of our PBC patients resulted to be AMA-negative using standard IFL assays; in addition, given the high number of the false-positive IFL results, particularly frequent using HEp2 cells as substrate, a more sensitive and more specific confirmation test is required.
On the other hand, W-IB and ELISA assays were similarly sensitive (more than 80%) and specific (98%) for AMA detection, W-IB being positive in up to 85% of our PBC series. In addition, W-IB allowed the detection of AMA in nearly half (17 of 36) of patients with a previously negative IFL test on rat tissue. However, the significant advantage of the ELISA assay is the standardization of the procedure.
Despite the use of all the currently available techniques for AMA detection, a subset of patients still exist with all the PBC features, but without detectable serum levels of AMA. In recent years a number of studies addressed the clinical significance of AMA-negative PBC: Michieletti et al. [9] observed lower IgM serum level in AMA-negative PBC, Gordon et al. [18] proposed antibodies to carbonic anhydrase as a novel immunological marker of such a condition, but we and others did not confirm this observation [19–21].
We recently demonstrated a high prevalence of anti-nuclear reactivities in AMA negative PBC [22], some of which, such as anti-sp100 and anti-gp210, appear to be specific for PBC, and may therefore be considered as surrogate markers of the disease in AMA-negative patients.
From the clinical standpoint, however, no biochemical, clinical or histological differences were observed between AMA-positive and AMA-negative PBC [10,13]. In keeping with these previous studies, in the present series the clinical, biochemical and histological features at the time of the diagnosis were similar between AMA-positive and AMA-negative patients, an observation supporting the view that AMA-positive and AMA-negative PBC is one and the same disease.
Interestingly, genuine AMA reactivities have been detected in 3·6% patients with pure type 1 AIH. Recently, Lohse and collaborators suggested the diagnosis of a PBC/AIH ‘overlap syndrome’ for those patients who had a biochemical and genetic profile typical of AIH, but with AMA reactivity [23]. In our study, all AMA-positive AIH patients had a diagnosis of ‘definite’ AIH according to the IAIHG score, including the HLA profile, therefore we suggest that these patients may truly have a ‘serological overlap syndrome’. At the light of the notion that AMA may appear even decades before the biochemical and histological expression of the liver disease [16], a strict follow-up is thus recommended in such patients. The need for biliary acids therapy in this setting remains to be evaluated.
REFERENCES
- 1.Kaplan MM. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–80. doi: 10.1056/NEJM199611213352107. [DOI] [PubMed] [Google Scholar]
- 2.Doniach D, Roitt IM, Walker JG, et al. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol. 1966;1:237–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Gershwin ME, Mackay IR, Sturgess A, et al. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–31. [PubMed] [Google Scholar]
- 4.Surh CD, Danner DJ, Ahmed A, et al. Reactivity of primary biliary cirrhosis sera with a human fetal liver cDNA clone of branched-chain alpha-keto acid dehydrogenase dihydrolipoamide acyltransferase, the 52 kD mitochondrial autoantigen. Hepatology. 1989;9:63–8. doi: 10.1002/hep.1840090110. [DOI] [PubMed] [Google Scholar]
- 5.Fregeau DR, Prindiville T, Coppel RL, et al. Inhibition of alpha-ketoglutarate dehydrogenase activity by a distinct population of autoantibodies recognizing dihydrolipoamide succinyltransferase in primary biliary cirrhosis. Hepatology. 1990;11:975–81. doi: 10.1002/hep.1840110611. [DOI] [PubMed] [Google Scholar]
- 6.Fussey SP, Bassendine MF, Fittes D, et al. The E1 alpha and beta subunits of the pyruvate dehydrogenase complex are M2′d’and M2′e′ autoantigens in primary biliary cirrhosis. Clin Sci (Lond) 1989;77:365–8. doi: 10.1042/cs0770365. [DOI] [PubMed] [Google Scholar]
- 7.Surh CD, Roche TE, Danner DJ, et al. Antimitochondrial autoantibodies in primary biliary cirrhosis recognize cross-reactive epitope(s) on protein X and dihydrolipoamide acetyltransferase of pyruvate dehydrogenase complex. Hepatology. 1989;10:127–33. doi: 10.1002/hep.1840100202. [DOI] [PubMed] [Google Scholar]
- 8.Dubel L, Tanaka A, Leung PS, et al. Autoepitope mapping and reactivity of autoantibodies to the dihydrolipoamide dehydrogenase-binding protein (E3 BP) and the glycine cleavage proteins in primary biliary cirrhosis. Hepatology. 1999;29:1013–8. doi: 10.1002/hep.510290403. [DOI] [PubMed] [Google Scholar]
- 9.Michieletti P, Wanless IR, Katz A, et al. Antimitochondrial antibody negative primary biliary cirrhosis. a distinct syndrome of autoimmune cholangitis. Gut. 1994;35:260–5. doi: 10.1136/gut.35.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman ZD, McNally PR, Davis DR, et al. Autoimmune cholangitis. a variant of primary biliary cirrhosis. Clinicopathologic and serologic correlations in 200 cases. Dig Dis Sci. 1995;40:1232–42. doi: 10.1007/BF02065530. [DOI] [PubMed] [Google Scholar]
- 11.Lacerda MA, Ludwig J, Dickson ER, et al. Antimitochondrial antibody-negative primary biliary cirrhosis. Am J Gastroenterol. 1995;90:247–9. [PubMed] [Google Scholar]
- 12.Kinoshita H, Omagari K, Whittingham S, et al. Autoimmune cholangitis and primary biliary cirrhosis – an autoimmune enigma. Liver. 1999;19:122–8. doi: 10.1111/j.1478-3231.1999.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P, Crosignani A, Battezzati PM, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and – negative primary biliary cirrhosis. Hepatology. 1997;25:1090–5. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report. review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 15.Moteki S, Leung PS, Coppel RL, et al. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97–103. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 16.Neuberger J, Thomson R. PBC and AMA – what is the connection? Hepatology. 1999;29:271–6. doi: 10.1002/hep.510290126. [DOI] [PubMed] [Google Scholar]
- 17.Meroni PL, Harris EN, Brucato A, et al. Anti-mitochondrial type M5 and anti-cardiolipin antibodies in autoimmune disorders: studies on their association and cross-reactivity. Clin Exp Immunol. 1987;67:484–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon SC, Quattrociocchi-Longe TM, Khan BA, et al. Antibodies to carbonic anhydrase in patients with immune cholangiopathies. Gastroenterology. 1995;108:1802–9. doi: 10.1016/0016-5085(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 19.Muratori P, Muratori L, Lenzi M, et al. Antibodies to carbonic anhydrase in autoimmune cholangiopathy. Gastroenterology. 1997;112:1053–4. doi: 10.1053/gast.1997.v112.agast971053. [DOI] [PubMed] [Google Scholar]
- 20.Invernizzi P, Battezzati PM, Crosignani A, et al. Antibody to carbonic anhydrase II is present in primary biliary cirrhosis (PBC) irrespective of antimitochondrial antibody status. Clin Exp Immunol. 1998;114:448–54. doi: 10.1046/j.1365-2249.1998.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comay D, Cauch-Dudek K, Hemphill D, et al. Are antibodies to carbonic anhydrase II specific for anti-mitochondrial antibody-negative primary biliary cirrhosis? Dig Dis Sci. 2000;45:2018–21. doi: 10.1023/a:1005548126211. [DOI] [PubMed] [Google Scholar]
- 22.Muratori P, Muratori L, Ferrari R, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431–7. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 23.Lohse AW, zum Buschenfelde KH, Franz B, et al. Characterization of the overlap syndrome of primary biliary cirrhosis (PBC) and autoimmune hepatitis: evidence for it being a hepatitic form of PBC in genetically susceptible individuals. Hepatology. 1999;29:1078–84. doi: 10.1002/hep.510290409. [DOI] [PubMed] [Google Scholar]