Abstract
The inflammatory response plays a major role in the induction of several neonatal diseases. We hypothesize that an imbalance between the pro- and anti-inflammatory response is crucial for the previously shown enhanced production of proinflammatory cytokines in term and preterm infants during infection. To test this hypothesis, we compared the capacity to produce the main anti-inflammatory cytokines IL-10 and TGF-β in term infants, preterm infants and adults at different levels of synthesis by quantitative real time reverse-transcribed PCR, flow cytometry, as well as enzyme-linked immunoassay. Term and preterm infants showed a profoundly diminished IL-10 mRNA-expression and IL-10 production after stimulation. In addition, the amount of TGF-β-positive lymphocytes was significantly less in neonates than adults. Furthermore, there was a considerably lower inhibition of production of IL-1α, IL-6, IL-8 and TNF-α by the use of recombinant IL-10 in term and preterm infants compared with adults. These results demonstrate not only a diminished anti-inflammatory capacity but also a reduced response to anti-inflammatory stimuli in term and preterm infants. From these data we conclude that neonates display an immature compensatory anti-inflammatory response syndrome (CARS) which may predispose preterm infants to harmful effects of proinflammatory cytokines resulting in severe organ sequelae during infection.
Keywords: IL-10, TGF-β, SIRS, CARS, neonates
INTRODUCTION
There is growing evidence that the fetal inflammatory response plays a major role in the induction of several neonatal diseases. It has previously been demonstrated that elevated levels of proinflammatory cytokines in cord blood and lung lavage can predict neonatal brain damage and chronic lung disease in preterm infants [1–3]. However, the neonatal inflammatory response has been considered to be intrinsically hyporesponsive [4–9]. This is contradictory to the clinical observation that neonates more often develop a severe systemic inflammatory response during sepsis than children and adults [10–12]. Recently, we could demonstrate an enhanced capacity to produce proinflammatory cytokines in term and preterm infants [13]. Even fetuses produced reasonable amounts of proinflammatory cytokines after endotoxin challenge during early gestation [14]. This is of special interest because fetuses and preterm infants are especially vulnerable to diverse detrimental effects of the systemic inflammatory response syndrome (SIRS) [15–18]. Current hypotheses on the pathophysiology of sepsis suggest that proinflammatory molecules that initiate SIRS trigger the release of anti-inflammatory molecules to limit inflammation [15,19]. The anti-inflammatory response which is primarily mediated by interleukin (IL)-10 and transforming-growth-factor-β (TGF-β) is referred to as the compensatory anti-inflammatory response syndrome (CARS) [19]. We hypothesize that an imbalance between SIRS and CARS is responsible for the exaggerated inflammatory response [13,14,20] and consequently for the high morbidity and mortality of preterm infants during infection [21–25]. To test this hypothesis, we investigated the capability of neonates to produce the predominant anti-inflammatory cytokines IL-10 and TGF-β [15,18,19] at different levels of synthesis.
METHODS
Study population
Heparinized venous cord blood samples were obtained after informed consent and approval of the institutional review board from preterm infants and healthy term infants immediately after delivery. The one-minute Apgar score was > 7 in all preterm and term infants. The median gestational age of preterm infants was 28·3 weeks (range: 25·5–30·4 weeks), the median birth weight 1078 g (range: 700–1610 g). There was no evidence of congenital malformation, growth retardation or infection. Healthy adult volunteers served as a control. All blood samples were collected in Lithium-Heparin tubes (Sarstedt, Nürnbrecht, Germany) and were stored at room temperature for no longer than 24 h before processing [26].
Culture and stimulation of cells
Heparinized whole blood was suspended in RPMI 1640 supplemented with 1% penicillin/streptomycin, 2 mm glutamine, 1 mm pyruvate and nonessential amino acids (Seromed Biochrome, Berlin, Germany) at a concentration of 5 × 106 leucocytes/ml. Aliquots of blood were stimulated for 5 h in multiwell plates at 37°C, 5% CO2 with 30 ng/ml lipopolysaccharide (LPS) (Sigma, Deisenhofen, Germany) to induce IL-1α, IL-6, IL-8 and TNF-α production, for 5, 10, 18 and 24 h with 100 ng/ml LPS to induce IL-10, and for 24 h with 3 µg/ml PMA and 3 µm ionomycin (Sigma) to induce TGF-β. An unstimulated control was added in each experiment. Recombinant IL-10 or TGF-β was added one hour before stimulation at a concentration of 0·01, 0·1 and 1 ng/ml or 1, 10 and 100 ng/ml, respectively. Supernatants were aliquoted and frozen at −80°C until cytokine determination by ELISA. For total RNA preparation, whole blood leucocytes were separated by adding a haemolysis buffer as described earlier [27]. The resulting RNA was resuspended in 300 µl RNAse free water and stored at −80°C until use. For intracytoplasmic cytokine detection cells were exposed to 3 µm monensin (Sigma) during the whole stimulation period, followed by fixation with 4% paraformaldehyde (Riedel de Haen, Seelze, Germany), as described previously [28].
Assessment of IL-10 mRNA expression by real-time QRT-PCR
For the relative quantification of IL-10 mRNA, a real-time PCR technique was applied that was recently established in our laboratory [27]. In brief, the PCR reaction mixture contained 25 µl Mastermix (Taqman, Perkin Elmer, Foster City, CA, USA), 100 nm of forward and reverse primer, 100 nm fluorogenic probe, 20 U RNAse-Inhibitor (Gibco BRL, Eggenstein, Germany), 25 U Murine Leukaemia Virus (MuLV) – reverse transcriptase (Perkin Elmer, Foster City, CA, USA), 1·25 U Ampli-Taq Gold-DNA-Polymerase (Perkin Elmer, Foster City, CA, USA) and 20 µl of water control or unknown RNA-template in a total volume of 50 µl. Sequence-specific primer pairs and fluorogenic probes were commercially obtained from Perkin Elmer Cetus, Foster City, USA (Human IL10 PDAR Kit; β-actin kit for cDNA samples). All PCR reactions were performed in triplicate in optical reaction tubes (Applied Biosystems, Foster City, USA) and conditions were 2 min at 50°C and 30 min at 48°C for reverse transcription, 10 min at 95°C for DNA-polymerase activation, followed by 40 cycles of 15 s at 95°C and 1 min 30 s at 60°C with a final 25°C hold.
All PCR reactions for cytokine mRNA quantification were performed on an ABI PRISM 7700 Sequence Detector System (Applied Biosystems, Foster City, CA, USA). The fluorescence signals of each well were collected every seven seconds and threshold cycles (CT) were calculated by determining the point at which the fluorescence intensity was 10 times larger than the standard deviation of the baseline fluorescence. For each sample, the CT value for IL-10 was substracted from the CT value of β-actin to generate the ΔCT value. The relative values for gene amplification were calculated using the equation: 2–ΔΔCT (System APSD, 1997). ΔΔCT is the normalized ΔCT value of the target gene that is generated by subtracting ΔCT values for each sample from that of the designated normalizer sample.
Intracellular staining of cytokines
Cells were washed in HBSS and resuspended in a buffer consisting of HBSS, 0·1% saponin (Riedel de Haen) and 0·01 m HEPES buffer (Seromed Biochrome). 200 L aliquots of cells were added to tubes containing 0·5 µg/10 µl of monoclonal antibodies (MAbs) against CD3 (17A2, cychrome-conjugated), CD19 (HIB19, cychrome-conjugated), CD14 (M5E2, FITC-or phycoerythrin (PE)-conjugated), IL-1α (364–3B3-14, FITC-conjugated), IL-6 (MQ2–13A5, FITC-conjugated), IL-8 (G265-8, FITC-conjugated), TNF-α (MAb11, FITC-conjugated), IL-10 (JES3–19F1, PE-conjugated) (Pharmingen, Heidelberg, Germany) and TGF-β (TB21, PE-conjugated) (IQ Products, Groningen, the Netherlands). IL-10 could only be detected in CD14-positive monocytes and TGF-β in CD3-positive T-lymphocytes. Preincubation with a surplus of unconjugated anticytokine MAbs (5 µg/10 µl; Pharmingen) served as a reliable negative control for intracellular staining to each sample [26]. Isotype-specific antibodies were used to detect irrelevant specificity for surface molecule staining. Flow cytometric analysis was performed as previously described [26,28].
Elisa
IL-10 was analysed in culture supernatant using a commercial enzyme-linked immunoassay according to the manufacturers's instructions (Coulter/Immunotech, Krefeld, Germany). All samples were analysed in duplicate. The optical density was determined photometrically at 405 nm using the ELISA reader Spectra Classic (SLT Labinstruments GmbH, Crailsheim, Germany) and plotted against a standard curve. The intra-assay coefficient of variation was <4% for all tests. The assay detects values as low as 5 pg/ml. Cytokine levels were within the assay's detection limit in all stimulated samples.
Statistical analysis
Statistical differences between groups were tested for unpaired data by the nonparametric Mann–Whitney U-test and for paired data by the Wilcoxon test. Both tests were used as two-tailed tests. The level of significance was defined as P < 0·05 in single comparisons. It was corrected according to Bonferroni in multiple comparisons between groups (α′: 0·05/2 = 0·025). Data were expressed as median and range in parentheses. Statistical analyses were performed using SPSS® 9·0 statistical software (SPSS Inc., Chicago, USA).
RESULTS
Kinetics of IL-10 production in neonates and adults
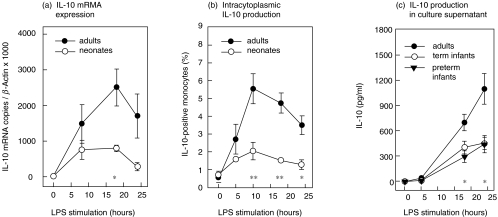
The peak of intracytoplasmic IL-10 was achieved after 10 h and of IL-10 mRNA after 18 h of stimulation with a decline thereafter (Fig. 1a,b). The amount of IL-10 in culture supernatant was steadily increasing with a maximum after 24 h in all groups investigated (Fig. 1c). Without stimulation IL-10 could not be detected.
Fig. 1.
Kinetics of IL-10 production a, IL-10 mRNA expression determined by QRT-PCR The relative amount of IL-10 mRNA/β-Actin was significantly lower in neonates compared with adults (n = 6, respectively) after stimulation with LPS for 18 h. Differences are significant at a level of *P < 0·01. b, Intracytoplasmic IL-10 detection by flow cytometry The percentage of IL-10/CD14-positive monocytes was considerably lower in neonates (n = 5) than adults (n = 5) after stimulation with LPS for 10, 18 and 24 h. Differences are significant at a level of *P < 0·05 or **P < 0·01. c, Determination of IL-10 in culture supernatant by enzyme-linked immunoassay The amount of IL-10 in culture supernatant was strikingly lower in both preterm infants and term infants than adults (n = 6, respectively) after stimulation with LPS for 18 and 24 h. Differences are significant at a level of *P <0·02.
After 18 h of stimulation neonates produced lower amounts of IL-10 mRNA copies compared to adults (800 copies (488–1052) versus 2405 copies (977–4000); P < 0·01) (Fig. 1a). The percentage of IL-10/CD14-positive monocytes was strikingly lower in neonates than in adults after 10 h stimulation (1·8% (0·9–3·5%) versus 4·8% (3·7–8·6%); P < 0·01); 18 h stimulation (1·6% (1·2–1·8%) versus 4·4% (3·1–6·6%); P < 0·01) and 24 h stimulation (1·5% (0·5–2%) versus 3·5% (1·8–4·8%); P < 0·05; n = 5) (Fig. 1b). In addition, the amount of IL-10 in culture supernatant was profoundly lower in preterm infants than in adults after 18 h (269 pg/ml (121–617 pg/ml) versus 643 pg/ml (477–981 pg/ml); P < 0·02) and in both preterm and term infants versus adults after 24 h stimulation (441 pg/ml (167–893 pg/ml), 424 pg/ml (220–757 pg/ml), 970 pg/ml (654–1777 pg/ml); P < 0·02; n = 6, respectively,). There was no difference between the amount of IL-10 in culture supernatant in preterm and term infants (Fig. 1c).
TGF-β production in neonatal and adult lymphocytes
TGF-β is a constitutively expressed cytokine which could only be detected in relevant amounts in the cytoplasm and at the cell surface by flow cytometry compared to nonstimulated cultures. TGF-β expression at the surface accounts for 25·9% (range 23·4–30·0%) of TGF-β production, analysed in the absence of permeabilization. The kinetics of intracytoplasmic TGF-β levels showed a maximum in CD3-positive lymphocytes after 24 h of stimulation with PMA and ionomycin (not shown). The amount of TGF-β/CD3-positive lymphocytes was significantly lower in neonates (n = 11) than in adults (n = 25) (6·7% (1·1–11·7%) versus 12·8% (4·9–42·3%); P <0·0001) (Fig. 2). In CD19-positive lymphocytes and CD14-positive monocytes no TGF-β could be detected.
Fig. 2.
Intracytoplasmic TGF-β detection in neonatal and adult CD3-positive lymphocytes by flow cytometry. After 24 h of stimulation with PMA and ionomycin the amount of TGF-β-positive lymphocytes was significantly lower in neonates (n = 11) than adults (n = 25) (P < 0·0001). Data are presented as a vertical point plot, the median is indicated by a line.
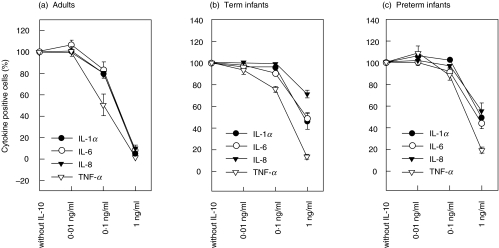
Inhibition of proinflammatory cytokines by recombinant IL-10 and TGF-β
Whole blood was incubated with different concentrations of recombinant IL-10 or TGF-β for 1 h before stimulation with LPS. Data are expressed as percentage of cytokine-producing monocytes which incubated without recombinant IL-10 or TGF-β and were set as 100%. IL-10 displayed a dose-related inhibition of cytokine-producing monocytes in all investigated groups (n = 6, respectively) (Fig. 3). However, there was a lower inhibition of IL-1α- and TNF-α-production in monocytes in preterm and term infants compared with adults using 0·1 ng/ml IL-10 (Fig. 4). The inhibition of production of IL-1α (42% (37–79%) and 49% (20–70%) versus 4·7% (0·6–12%); P < 0·005), IL-6 (45% (28–65%) and 49% (36–64%) versus 9% (2·6–15%); P < 0·005), IL-8 (53% (38–76%) and 73% (57–82%) versus 7·8% (0·9–21%); P < 0·005) and TNF-α (18% (13–30%) and 12% (8–22%) versus 1·1% (0·5–3%); P < 0·005) in monocytes was considerably lower in preterm and term infants compared with adults using 1 ng/ml IL-10, respectively (Fig. 4).
Fig. 3.
Inhibition of cytokine synthesis by interleukin-10. (a) Adults; (b) Term infants; (c) Preterm infants. Cells were incubated with recombinant IL-10 in whole blood culture one hour before stimulation with LPS. Stimulated cells which were incubated without recombinant IL-10 were set as 100%. There was a dose-related inhibition of cytokine-positive cells in all investigated groups. However, the inhibition of IL-1α-, IL-6-, IL-8- and TNF-α-positive monocytes was significantly lower in term infants and preterm infants compared with adults (n = 6, respectively) using 0·1 ng/ml or 1 ng/ml IL-10 (data are given in result section).
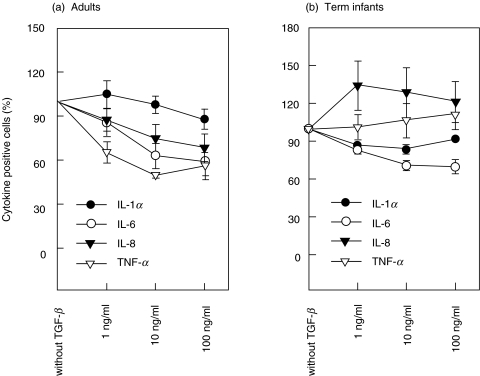
Fig. 4.
Inhibition of cytokine synthesis by TGF-β. Cells were incubated with recombinant TGF-β in whole blood culture one hour before stimulation with LPS. Stimulated cells which were incubated without recombinant TGF-β were set as 100%. (a) Recombinant TGF-β induced only a modest dose-related inhibition of IL-6, IL-8 and TNF-α-producing monocytes in adults (n = 5). (b) There was no response to TGF-β in IL-8- and TNF-α-producing monocytes and only a slight decrease in IL-1α- and IL-6-producing monocytes in neonates (n = 5; P < 0·05).
In contrast, recombinant TGF-β induced only a modest dose-related inhibition of IL-6, IL-8 and TNF-α-producing monocytes in adults compared to recombinant IL-10 (Figs 3 and 4). However, neonates showed a diminished response to recombinant TGF-β compared to adults (n = 5, respectively). There was no response to TGF-β in IL-8- and TNF-α-producing monocytes and only a slight decrease in IL-1α- and IL-6-producing monocytes in neonates (P < 0·05).
DISCUSSION
The advances in neonatal intensive care enable survival of very low birth weight infants who are extremely prone to infections and their complications. Neonatal sepsis and its sequelae are one of the leading causes of death and remain a critical determinant of outcome in infants of very low birth weight [21–25]. Older children and adults are usually able to restrict bacterial infections, whereas neonates often develop a systemic inflammatory response syndrome (SIRS) with detrimental effects. There is growing evidence that this process is crucially mediated by the action of distinct inflammatory cytokines [11,16,18]. The inflammatory response to infection or injury is a highly conserved and regulated reaction of the organism. Proinflammatory cytokines are produced that activate cellular defenses followed by a production of anti-inflammatory cytokines to attenuate and control the proinflammatory response. This anti-inflammatory response referred as compensatory anti-inflammatory response syndrome (CARS) soon ensures that the effects of the proinflammatory cytokines do not become destructive. If balance cannot be established and homeostasis is not restored, a massive proinflammatory reaction with severe organ dysfunction will ensue [15,19]. In an ex vivo modell of sepsis we could demonstrate an enhanced inflammatory response in term and preterm infants [13]. Even fetuses between 22 and 27 weeks of gestation were able to produce substantial amounts of proinflammatory cytokines [14]. From these data we hypothesized that an imbalance between pro- and anti-inflammatory cytokines is responsible for the enhanced cytokine response demonstrated previously and the high prevalence of organ sequelae during neonatal infection in preterm infants [13,16]. To test this hypothesis, we analysed both the capability of neonates to produce and to respond to anti-inflammatory cytokines. IL-10 and TGF-β were analysed as the predominant immunoregulatory and anti-inflammatory cytokines [29–31]. Although, it has been demonstrated that neonates and children were able to produce IL-10 during septic shock [32–35], data on IL-10 production in comparison to adults are controversial. After T cell stimulation, both similar [36,37] and diminished IL-10 levels in neonates compared to adults were reported [38]. However, significantly lower amounts of IL-10 were seen in neonates after stimulation with LPS or TNF-α[39]. Because we were especially interested in sepsis-related mortality we used LPS for cell stimulation to simulate a sepsis-like situation. In contrast to previous studies we determined cytokine synthesis at different levels of production using QRT-PCR [27,40], intracytoplasmic cytokine detection by flow cytometry [28], as well as enzyme-linked immunoassay.
IL-10 could be detected at all levels of production whereas reliable amounts of TGF-β were only observed in the cytoplasm and at the cell surface. We could demonstrate a diminished production of IL-10 mRNA and IL-10 protein in neonates compared to adults. Even after prolonged stimulation for 24 h the amount of IL-10 positive monocytes as well as IL-10 in culture supernatant were strikingly lower than in adults. The amount of TGF-β-positive lymphocytes was significantly lower in neonates compared to adults, as well. Taken together, these data strongly support a reduced anti-inflammatory potential in term and preterm infants during the inflammatory cascade. Furthermore, we could demonstrate a diminished response to IL-10 in term and especially preterm infants compared to adults. After preincubation with recombinant human IL-10 the amount of IL-1α-, IL-6-, IL-8- and TNF-α-positive monocytes in stimulated cultures was strikingly higher in both preterm infants and term infants compared to adults. In addition, neonates displayed a minor response to recombinant TGF-β compared to adults. These results not only indicate a diminished anti-inflammatory capacity but also a reduced response to anti-inflammatory stimuli in preterm and term infants. Whether a reduced expression of the IL-10 receptor or a diminished intracytoplasmic signal transduction are responsible for the diminished response to IL-10 in neonates is the topic of ongoing studies.
From these data we conclude that the CARS in term and preterm infants fails to control inflammation which results in an exaggerated cytokine release with detrimental consequences [41–44]. This is of special importance in preterm infants who are exceedingly prone to these destructive effects due to the vulnerability of developing organ systems. In several animal models proinflammatory cytokines were able to affect the integrity of the blood–brain barrier and to induce brain injury [43,44]. In preterm infants a breakdown of a still immature blood–brain barrier and incomplete myelination may be important in the pathophysiology of the dreaded complications of intraventricular haemorrhage and cerebral white matter damage [16,17]. Furthermore, proinflammatory cytokines were found in high amounts in brain white matter lesions of preterm infants [45], suggesting a key role of these mediators in the pathogenesis of brain damage of preterm infants. Moreover, we could demonstrate that prolonged mechanical ventilation induces pulmonary inflammation but fails to stimulate the anti-inflammatory cytokine IL-10 adequately in preterm infants [46]. Blahnik et al. [47] could also show a comparable TNF-α production but a trend toward a reduced IL-10 production in preterm versus term lung macrophages. An immature CARS offers a basis for persistent inflammation in the lungs of preterm infants with hyaline membrane disease and predisposes these infants toward the development of chronic lung disease.
To counterbalance this exaggerated inflammatory reaction a treatment with IL-10 seems promising. Although there was a diminished response to IL-10 in term and especially preterm infants in our study, all investigated groups showed a dose-dependent decrease of proinflammatory cytokines using recombinant human IL-10. The efficacy of IL-10 could be demonstrated by reversing detrimental effects of endotoxin-induced inflammation on perinatal cerebral hypoxia-ischemia [48]. In addition, IL-10 treatment of humans was effective, safe and without significant adverse effects [49,50]. Since selective blocking of diverse proinflammatory cytokines was disappointing in the past [18,51], the broad anti-inflammatory potential of IL-10 seems to be promising in the prevention of inflammatory-triggered organ damage in high risk infants during infection.
In conclusion, we could demonstrate that neonates display an immature CARS during infection which may predispose preterm infants with incomplete organ development to harmful effects of proinflammatory cytokines. The presented data have major implications in the understanding of neonatal diseases and may offer a basis for preventive strategies in preterm infants.
Acknowledgments
The study was supported in part by ‘Friedrich Bluhme and Else Jebsen Stiftung’ as well as by ‘Scientific support of the Medical University of Lübeck’, Grant no. 3601.
REFERENCES
- 1.Bagchi A, Viscardi RM, Taciak V, Ensor JE, McCrea KA, Hasday JD. Increased activity of interleukin-6 but not tumor necrosis factor-a in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatr Res. 1994;36:244–52. doi: 10.1203/00006450-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Jones CA, Cayabyab RG, Kwong KYC, Stotts C, Wong B, Hamdan H, Minoo P, DeLemos RA. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–75. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick L, Harris MC. Cytokines and the inflammatory response. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia: W.B. Saunders; 1997. pp. 1967–78. [Google Scholar]
- 5.Pillay V, Savage N, Laburn H. Circulating cytokine concentrations and cytokine production by monocytes from newborn babies and adults. Pflugers Arch. 1994;428:197–201. doi: 10.1007/BF00724497. [DOI] [PubMed] [Google Scholar]
- 6.Rowen JL, Smith CW, Edwards MS. Group B Streptococci elicit Leukotriene B4 and Interleukin-8 from human monocytes: neonates exhibit a diminished response. J Infect Dis. 1995;172:420–6. doi: 10.1093/infdis/172.2.420. [DOI] [PubMed] [Google Scholar]
- 7.Schibler KR, Liechty KW, White WL, Rothstein G, Christensen RD. Defective production of interleukin-6 by monocytes: a possible mechanism underlying several host defense deficiencies of neonates. Pediatr Res. 1992;31:18–21. doi: 10.1203/00006450-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Schibler KR, Trautman MS, Liechty KW, White WL, Rothstein G, Christensen RD. Diminished transcription of interleukin-8 by monocytes from preterm neonates. J Leukoc Biol. 1993;53:399–403. doi: 10.1002/jlb.53.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Yachie A, Takano N, Ohta K, Uehara T, Fujita SI, Miyawaki T, Taniguchi N. Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun. 1992;60:749–53. doi: 10.1128/iai.60.3.749-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berner R, Niemeyer CM, Leititis JU, Funke A, Schwab C, Rau U, Richter K, Tawfeek MS, Clad A, Brandis M. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–77. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 12.Kashlan F, Smulian J, Shen-Schwarz S, Anwar M, Hiatt M, Hegyi T. Umbilical vein interleukin-6 and tumor necrosis factor alpha plasma concentrations in the very preterm infant. Pediatr Infect Dis J. 2000;19:238–43. doi: 10.1097/00006454-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Schultz C, Rott C, Temming P, Schlenke P, Möller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–22. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Schultz C, Temming P, Gembruch U, Strunk T, Bucsky P. Polyclonal intravenous immunoglobulin to prevent brain injury in preterm infants. Lancet. 2002;359:1522–4. doi: 10.1016/S0140-6736(02)08447-7. [DOI] [PubMed] [Google Scholar]
- 15.Bone RC, Grodzin CJ, Balk RA. Sepsis. a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 16.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Dammann O, Leviton A. Brain damage in preterm newborns. might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104:541–50. doi: 10.1542/peds.104.3.541. [DOI] [PubMed] [Google Scholar]
- 18.Sáez-Llorens X, McCracken GH. Sepsis syndrome and septic shock in pediatrics: current concepts of terminology, pathophysiology, and management. J Pediatr. 1993;123:497–508. doi: 10.1016/s0022-3476(05)80942-4. [DOI] [PubMed] [Google Scholar]
- 19.Powell KR. Sepsis and shock. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. Philadelphia: W.B. Saunders Company; 2000. pp. 747–51. [Google Scholar]
- 20.Duggan PJ, Maalouf EF, Watts TL, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358:1699–700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 21.Klein JO. Bacterial sepsis and meningitis. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia: W.B. Saunders Company; 2001. pp. 943–98. [Google Scholar]
- 22.LaGamma EF, Drusin LM, Mackles AW, Machalek S, Auld PAM. Neonatal infections: an important determinant of late NICU mortality in infants <1000 g at birth. Am J Dis Child. 1983;137:838–41. doi: 10.1001/archpedi.1983.02140350016005. [DOI] [PubMed] [Google Scholar]
- 23.Martinot A, Leclerc F, Cremer R, Leteurtre S, Fourier C, Hue V. Sepsis in neonates and children: definitions, epidemiology, and outcome. Pediatr Emerg Care. 1997;13:277–81. doi: 10.1097/00006565-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Stoll BJ, Gordon T, Korones SB, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:63–71. doi: 10.1016/s0022-3476(96)70191-9. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Gordon T, Korones SB, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:72–80. doi: 10.1016/s0022-3476(96)70192-0. [DOI] [PubMed] [Google Scholar]
- 26.Schultz C, Rott C, Temming P, von Puttkamer J, Bucsky P. Influence of specimen age and use of different negative controls in determination of intracytoplasmic cytokine levels after whole-blood culture assay. Clin Diagn Laboratory Immunol. 2002;9:295–8. doi: 10.1128/CDLI.9.2.295-298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Härtel C, Bein G, Kirchner H, Klüter H. A human whole-blood assay for analysis of T-cell function by quantification of cytokine mRNA. Scand J Immunol. 1999;49:649–54. doi: 10.1046/j.1365-3083.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 28.Schultz C. Intracytoplasmic detection of proinflammatory cytokines and chemokines in monocytes by flow cytometry. In: Körholz D, Kiess W, editors. Methods in Molecular Biology. New Jersey: Humana Press; 2002. pp. 29–39. Cytokines and Colony Stimulating Factors, Methods and Protocols – Part II. Detection Assays for Cytokines and Growth Factors. [DOI] [PubMed] [Google Scholar]
- 29.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann NY Acad Sci. 1993;685:713–39. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 30.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 32.Derkx B, Marchant A, Goldman M, Bijlmer R, van Deventer S. High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis. 1995;171:229–32. doi: 10.1093/infdis/171.1.229. [DOI] [PubMed] [Google Scholar]
- 33.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin-10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113:1625–31. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 34.Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28:2591–4. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 35.Romagnoli C, Frezza S, Cingolani A, et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–50. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 36.Rainsford E, Reen DJ. Interleukin-10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116:702–9. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi HN, HayGlass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 38.Chheda S, Palkowetz KH, Garofalo R, Rassin DK, Goldman AS. Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-α and its receptors. Pediatr Res. 1996;40:475–83. doi: 10.1203/00006450-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Kotiranta-Ainamo A, Rautonen J, Rautonen N. Interleukin-10 production by cord blood mononuclear cells. Pediatr Res. 1997;41:110–3. doi: 10.1203/00006450-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Härtel C, Bein G, Müller-Steinhardt M, Klüter H. Ex vivo induction of cytokine mRNA expression in human blood samples. J Immunol Meth. 2001;249:63–71. doi: 10.1016/s0022-1759(00)00334-3. [DOI] [PubMed] [Google Scholar]
- 41.Andrews T, Zhang P, Bhat NR. TNF-α potentiates IFN-γ-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–83. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–56. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brett FM, Mizisin AP, Powell HC, Campbell IL. Evolution of neuropathologic abnormalities associated with blood–brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropathol Exp Neurol. 1995;54:766–75. doi: 10.1097/00005072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 44.DeVries HE, Blom-Roosemalen MC, VanOsten M, DeBoer AG, VanBerkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood–brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-α and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 46.Schultz C, Tautz J, Reiss I, Möller JC. Prolonged mechanical ventilation induces pulmonary inflammation in preterm infants. Biol Neonate. 2003;84:64–6. doi: 10.1159/000071446. [DOI] [PubMed] [Google Scholar]
- 47.Blahnik MJ, Ramanathan R, Riley CR, Minoo P. Lipopolysaccharide-induced tumor necrosis factor-α and IL-10 production by lung macrophages from preterm and term neonates. Pediatr Res. 2001;50:726–31. doi: 10.1203/00006450-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Froen JF, Munkeby BH, Stray-Pedersen B, Saugstad OD. Interleukin-10 reverses acute detrimental effects of endotoxin-induced inflammation on perinatal cerebral hypoxia-ischemia. Pediatr Res. 2002;52:790. doi: 10.1016/s0006-8993(02)02700-2. (Abstract. 70) [DOI] [PubMed] [Google Scholar]
- 49.Chernoff AE, Granowitz EV, Shapiro L, et al. A randomised, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995;154:5492–9. [PubMed] [Google Scholar]
- 50.Kumar A, Creery WD. The therapeutic potential of interleukin-10 in infection and inflammation. Arch Immunol Ther Exp. 2000;48:529–38. [PubMed] [Google Scholar]
- 51.Fisher CJ, Dhainaut JA, Opal SM, et al. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis. J Am Med Assoc. 1994;271:1836–43. [PubMed] [Google Scholar]