Abstract
The balance between Th1 and Th2 response determines the outcome of Helicobacter pylori infection. Interferon (IFN)-γ plays an inductive role in gastric inflammation, whereas interleukin (IL)-4 counterbalances Th1 response and suppresses the development of gastritis. Th cell response is regulated by co-stimulatory factors. A co-stimulatory molecule, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), plays an inhibitory role in IL-2-dependent cell growth and mediates an optimal inhibitory signal to Th1 and Th2 cells. We administered anti-CTLA-4 monoclonal antibody (MoAb), which blocks CTLA-4 signalling, to examine the relative role for this signalling during maturation of Th1 and Th2 cells in H. pylori infection in mice. Mice treated by anti-CTLA-4 MoAb within the first week of infection showed an inhibition of gastric inflammation, accompanied by an increasing ratio of H. pylori-specific IgG1/IgG2a in serum following infection. Furthermore, the treatment resulted in the higher ratio of IL-4/IFN-γ by splenocytes in response to H. pylori antigen at 6 weeks after infection, compared with untreated mice. These results suggest that the predominance of Th2 response by CTLA-4 blockade leads to an inhibition of the development of gastric inflammation. CTLA-4 signalling could contribute to the regulation of Th subsets and the development of gastric inflammation in H. pylori infection.
Keywords: CTLA-4, Helicobacter pylori, IFN-γ, IL-4
INTRODUCTION
Helicobacter pylori, a spiral microaerophilic bacterium, causes active gastritis [1]. Once H. pylori infection is established, the organisms colonize and inflame the gastric mucosa. Previous studies have demonstrated that long-lasting gastric inflammation may predispose toward low-grade gastric lymphoma and gastric carcinoma [2,3]. With respect to host immune response, induction of gastric inflammation is associated with the up-regulation of Th1 cytokine response (e.g. interferon (IFN)-γ), whereas anti-inflammatory immune response is associated with the predominance of Th2 cytokine response (e.g. interleukin (IL)-4). Neutralization of IFN-γ during an infection results in a significant reduction of gastric inflammation [4]. IFN-γ–/– animals have no mucosal inflammation when infected with H. pylori[5,6]. On the other hand, IL-4–/– mice infected with Helicobacter spp. show severe mucosal inflammation [5,7]. When Th2-directed immunity is stimulated by oral and systemic administration of whole bacteria preparations or purified antigens (e.g. urease), co-administered with strong adjuvants (e.g. cholera toxin), gastritis is inhibited [8–10]. Regulation of Th subsets determines whether or not inflammation occurs.
The co-stimulatory factors play an essential role in the regulation of T cell immune response [11,12]. CD28 is expressed on naive and activated T cells and engages its ligands, B7-1 (CD80) and B7-2 (CD86). The co-stimulatory signal mediated by CD28 is required for T cell activation and increases the production of cytokines [13]. On the other hand, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152), which is homologous to CD28 and also engages B7, is not expressed on resting T cells, but is induced after the initial steps of T cell activation. CTLA-4, expressing on activated T cells, plays an inhibitory role in T cell proliferation in murine and human lymphocytes [14,15]. Furthermore, CTLA-4 regulates the production of cytokines by Th1 and Th2 cells and has diverse effects on the progress of infectious diseases, which are dependent on pathogen and host factors. Administration of anti-CTLA-4 MoAb that blocks interaction between CTLA-4 and B7 enhances their susceptibility to Leishmania major and exacerbates tissue inflammation in BALB/c mice [16]. This response is attributed to an increased Th2 response by CTLA-4 blockade. However, there is no effect when the MoAb is administered to C57BL/6 mice that are Th1-biased during the parasite infection. In pulmonary mycobacterial infection, ad-ministration of anti-CTLA-4 MoAb enhances the antigen-specific expansion and differentiation of lymphocytes in the draining lymph node. Nevertheless, CTLA-4 blockade has no effect on IFN-γ mRNA expression of lymphocytes at the primary site of infection and fails to enhance protection and affect grauloma formation [17].
In this study, we administered anti-CTLA-4 MoAb to mice infected with H. pylori to assess the role of CTLA-4 co-stimulatory pathway in the regulation of Th subset expression during development of the immune response to this organism. The induction of H. pylori-associated gastritis was inhibited by blocking CTLA-4 signalling, even though the bacterial colonization was not affected. CTLA-4 blockade at the early stage of infection gradually increased the serum levels of H. pylori-specific IgG1 relative to IgG2a and enhanced the production of IL-4 relative to IFN-γ by splenic cells at 6 weeks after infection. These indicated that Th2-directed response by CTLA-4 blockade appeared to be the major factor responsible for the suppressed inflammatory state.
MATERIALS AND METHODS
Animals and bacteria
Pathogen-free 6-week-old female C57BL/6 mice were obtained from Seac Yoshitomi (Fukuoka, Japan). Mice were housed in a specific-pathogen-free environment and provided with food and water ad libitum. Experiments were performed according to the guidelines of the Ethical Committee for Animal Experiments at Oita Medical University, Oita, Japan. Sydney strain (SS1) of H. pylori (kindly provided by Dr A. Lee; School of Microbiology and Immunology, University of New South Wales, Sydney, NSW, Australia) was grown in brucella broth containing 10% horse serum under microaerobic conditions (5% O2, 10% CO2, 85% N2) at 37°C. Mice were inoculated by gastric intubation with 0·5 ml of live SS1 [5 × 107 colony-forming units (CFU)/ml] on day 1.
In vivo treatment with anti-CTLA-4 MoAb
Hamster antimurine CTLA-4 [UC10–4F10-11] MoAb was kindly provided by Dr Jeffrey A. Bluestone (University of Chicago, Chicago, IL, USA). The mice were injected intraperitoneally with either 100 µg of anti-CTLA-4 MoAb or hamster IgG (Cappel, West Chester, PA, USA) on day 0, and then daily until day 7 post-infection. Spleen and stomach samples were obtained at 0, 1, 2, 4 and 6 weeks after infection. Previous studies have shown that this dose of anti-CTLA-4 MoAb will block CTLA-4 function [17,18].
Evaluation of H. pylori infection and mucosal inflammation
Half the stomach, divided longitudinally along the greater and lesser curvatures, was fixed with neutral buffered 15% formalin and embedded in paraffin for haematoxylin–eosin (H&E) and Giemsa staining. A quarter of the anal section of the stomach was sampled to evaluate bacterial colonization and urease activity. The specimen was homogenized with a sterile Dounce tissue grinder containing 500 µl of saline. The homogenate was diluted serially for culture and was used directly for urease activity analysis. Urease activity was assessed 4-h and 24-h postmortem in duplicate by measuring the absorbance at 550 nm [19]. The fixed sections were examined blindly by two independent examiners. The intensity of inflammatory cells was classified into four grades according to the updated Sydney system (0: no infiltration, 1: rare, 2: moderate and 3: marked) [20]. The extent of inflammation was scored using a five-point scale (0: none, 1: <25%, 2: 25–50%, 3: 50–75% and 4: >75% over the length of the entire section) [4]. The inflammatory cells included mononuclear cells and neutrophils.
Serum levels of IgG1 and IgG2a
Determination of IgG1 and IgG2a in blood samples was performed by enzyme-linked immunosorbent assay (ELISA) as described previously [9,10]. Briefly, microtitre plates (Nunc, Roskilde, Denmark) were coated with H. pylori whole cell sonicated antigen (HpAg) and sera at a dilution of 1 : 25 were added. After washing the wells were incubated with peroxidase-conjugated goat-antimouse IgG1 or goat-antimouse IgG2a (Cappel). 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt (ABTS) was used for colour development, and the absorbance was measured with a microplate reader (Thermo max, Molecular Devices) set at 414 nm. Each sample was tested in duplicate. The concentrations of H. pylori-specific serum IgG1 and IgG2a expressed as U/ml were calculated using standard curves generated by titrating pooled high-titre sera. Data were analysed using softmax (Molecular Devices, Sunnyvale, CA, USA).
Cytokine response to H. pylori
Specimens of spleen were pressed gently and ground in a coarse glass grinder with chilled RPMI-1640 medium (Sigma Chemical Co., St Louis, MO, USA) supplemented with 10% fetal calf serum (FCS). Cell suspensions were depleted of erythrocytes by TRIS-ammonium chloride lysis then washed twice in medium. After filtration through 150-µm and 50-µm nylon meshes, the cells were centrifuged at 400 g for 10 min at 4°C and the supernatant was discarded. The cells were resuspended in complete medium (sterile RPMI-1640 containing 10% FCS with 200 U/ml penicillin and 100 µg/ml streptomycin) and then counted in a haemocytometer by the trypan blue dye exclusion method. Single cell suspensions of splenic cells (2·0 × 106 cell/ml) in six-well plates (Nunc-Immuno Plate, Roskilde, Denmark) were incubated for 48 h at 37°C in the presence of 10 µg/ml of HpAg in complete medium. Immunoassay kits for IL-4 and IFN-γ were purchased from BioSource International, Inc. (Camarillo, CA, USA) and cytokine analysis was performed using the instructions provided by the supplier. After colour development, cytokine concentrations were determined with a microplate reader.
Statistical analysis
Differences between groups were examined for statistical significance using the Mann–Whitney U-test. A P < 0·05 denotes a statistically significant difference.
RESULTS
Treatment with anti-CTLA-4 MoAb suppresses the development of gastric inflammation
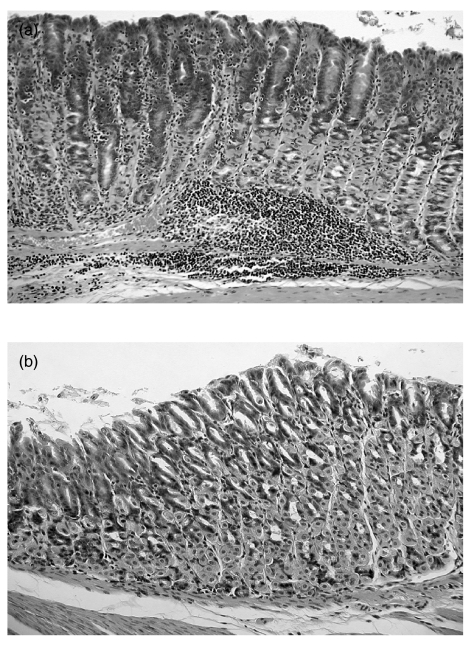
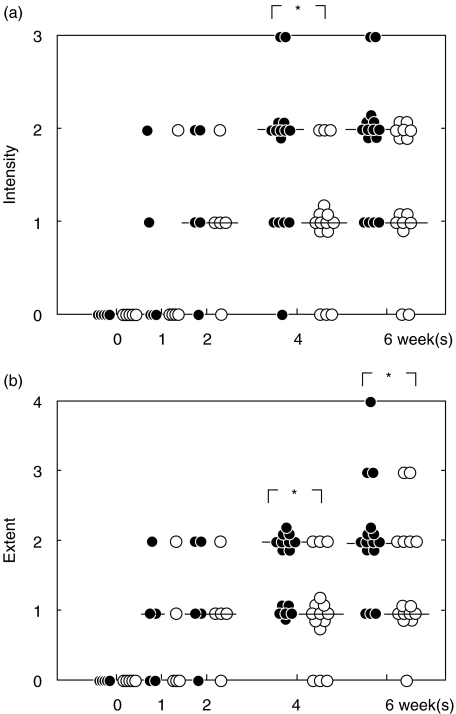
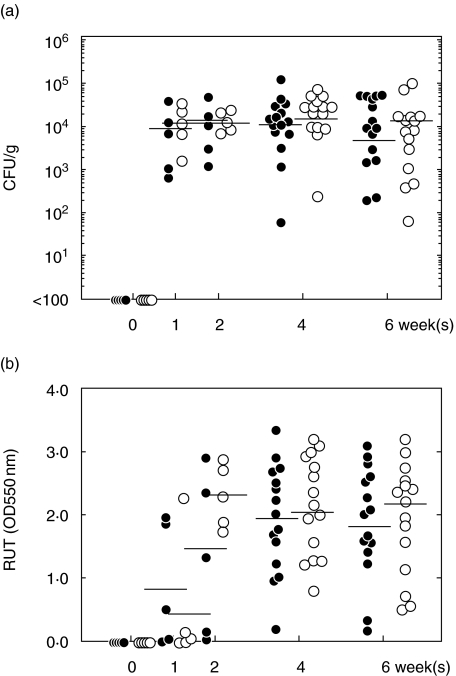
To investigate the role of CTLA-4 during an acute H. pylori infection, we administered purified anti-CTLA-4 MoAb to infected mice between day 0 and day 7. H. pylori-infected mice without the MoAb treatment (control mice) developed moderate to severe and extensive gastric inflammation, dominated by mononuclear cells, infiltrating into the lamina propria and the submucosal layer and penetrating the lamina muscularis (Fig. 1a). On the other hand, anti-CTLA-4 MoAb-treated mice showed mild and focal inflammation following infection (Fig. 1b). Figure 2 shows significant differences between groups of mice for the intensity of inflammation at 4 weeks and for the extent of inflammation on and after 4 weeks after infection. The presence of H. pylori was confirmed in all mice used. Treatment with anti-CTLA-4 MoAb had no significant effect on bacterial dosage and urease activity (Fig. 3). The bacterial number reached a steady state within the first week of infection, irrespective of treatment.
Fig. 1.
Histological findings. (a) Section of the stomach from a representative control mouse (H. pylori-infected mouse without anti-CTLA-4 MoAb treatment) at 6 weeks after the challenge. (b) Section of the stomach from a representative treated mouse (H. pylori-infected mouse with anti-CTLA-4 MoAb treatment) at 6 weeks after the challenge. (a) Control mice developed moderate to severe and extensive gastric inflammation, dominated by mononuclear cells, infiltrating into the lamina propria and the submucosal layer and penetrating the lamina muscularis. Magnification × 160. (b) Anti-CTLA-4 MoAb-treated mice did not show more than mild focal inflammation. Magnification × 200.
Fig. 2.
Intensity score (a) and extent score (b). Gastric inflammation is evaluated as the intensity and extent score. Closed circles: control mice; open circles: treated mice. Data points are given in blots and a horizontal line shows the median for each group each point. *P < 0·05 control versus treated mice. Anti-CTLA-4 MoAb was administered within the first week of infection. The treatment led to the state of controlled inflammation in H. pylori-infected mice.
Fig. 3.
Bacterial dose (a) and urease activity (b). Closed circles: control mice; open circles: treated mice. The homogenized specimen was used for culture and for urease activity analysis. Urease activity was assessed in duplicate by measuring the absorbance at 550 nm. Data points are given in blots and a horizontal line shows the mean for each group at each point. *P < 0·05 control versus treated mice. The MoAb treatment had no significant effect on bacterial dosage and urease activity. Bacterial number reached a steady state level within the first week of infection, irrespective of treatment.
In vivo CTLA-4 blockade enhances serum levels of H. pylori-specific IgG1
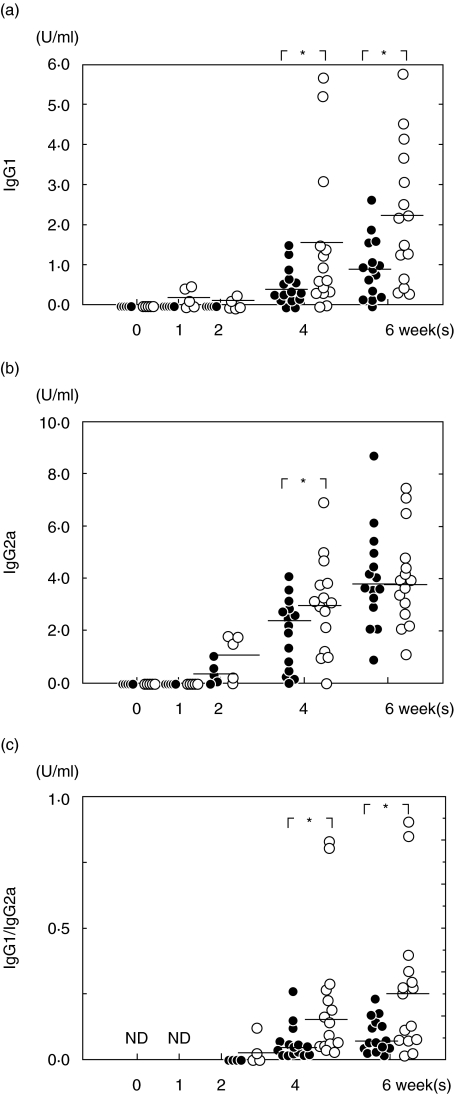
To determine the phenotypes of Th cells induced by anti-CTLA-4 MoAb treatment, serum levels of H. pylori-specific IgG1 and IgG2a were measured by ELISA. As shown in Fig. 4, specific IgG1 in control mice was undetectable until 4 weeks post-infection, while IgG1 response in treated mice increased at the first week of infection, and was then sustained significantly above that in control mice even after discontinuation of treatment. On the other hand, IgG2a response increased gradually following infection and was not affected notably by in vivo treatment with anti-CTLA-4 MoAb. Consequently, a higher ratio of IgG1/IgG2a in treated mice was observed during infection, compared with control mice.
Fig. 4.
Serum levels of IgG1 (a), IgG2a (b) and the ratio of IgG1/IgG2a (c). Closed circles: control mice; open circles: treated mice. Data points are given in blots and a horizontal line shows the mean for each group at each point. *P < 0·05 control versus treated mice; ND: no data. H. pylori-specific IgG1 in treated mice increased at the first week of infection, and was then sustained significantly above those of control mice after discontinuation of treatment. The ratio of IgG1/IgG2a in treated mice was higher than that in control mice during infection.
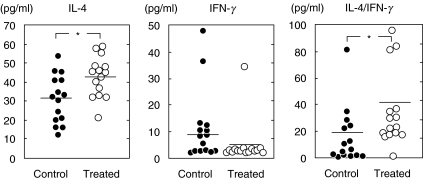
CTLA-4 blockade increased the ratio of IL-4/IFN-γ at 6 weeks after infection
Figure 5 shows cytokine response to H. pylori at 6 weeks after infection. Production of IL-4 and IFN-γ by splenocytes in response to HpAg was measured by ELISA. Mice treated with anti-CTLA-4 MoAb showed a significant increase of IL-4 production (P < 0·05), but are impaired in the ability to produce IFN-γ, resulting in a higher ratio of IL-4/IFN-γ compared with control mice (P < 0·05). The ratio of IL-4/IFN-γ was nearly twofold higher than that of control mice.
Fig. 5.
Production of IL-4 and IFN-γ by splenic cells at 6 weeks after infection. Cells were obtained from control and treated mice (15 mice per group) at 6 weeks post-challenge. Closed circles: control mice; open circles: treated mice. Data points are given in blots and the horizontal line shows the mean for each group at each point. *P < 0·05 control versus treated mice. At 6 weeks after infection, splenocytes from treated mice showed an increase of IL-4 response and an increase of IL-4/IFN-γ ratio.
DISCUSSION
CTLA-4-induced inhibition of T cell response occurs 24–72 h after initiation of T cell activation. This delay correlates with the expression of detectable CTLA-4 on the activated T cell surface following stimulation [21,22]. The inhibitory effect of CTLA-4 has been attributed to a direct inhibition of IL-2-dependent cell growth and a blockade of intracellular signals required for T cell activation, resulting in long-term unresponsiveness [23]. Therefore, the induction of CTLA-4 in early immune response can affect disease outcomes. CTLA-4 blockade at the time of immunization enhances protective immune response to cryptococcal infection [18]. A soluble form, CTLA-4Ig, administered at the time of bacterial infection, suppresses abscess formation [24]. When treatment with anti-CTLA-4 MoAb or CTLA-4Ig is delayed, disease outcome is not affected [18,24]. In murine H. pylori infection, transcription of CTLA-4 mRNA increases transiently to high levels on the second day after infection, returning to baseline level by day 4 (data not shown). In assessing CTLA-4 activity during the acute H. pylori infection, it is important that anti-CTLA-4 MoAb is administered within the first week of infection.
Blocking early expression of CTLA-4 is thought to influence the fate of T cells, halting cell cycle progression and regulating proinflammatory and anti-inflammatory responses present at the time when T cells encounter an antigen [25]. Early events of the immune response stimulate the primary cytokine production that regulates the subsequent development of T cell response [26]. The polarized Th1 or Th2 pattern of cytokine production, once acquired, appears stable without a reversion from one subset to another [27,28]. In this study, despite the short treatment with anti-CTLA-4 MoAb, treated mice showed a gradual increase of IgG1 in serum following infection and higher levels of IL-4 production by splenocytes at 6 weeks after infection, compared with control mice. Cytokine response in spleens reflects not only systemic but also local response to H. pylori, because lymphocytes from gastric epithelia are recruited from circulation and are similar in cytokine profiles of splenocytes in H. pylori infection [29,30]. CTLA-4 signalling during the initial priming of T cells is required for differentiation into mature Th2 population in response to H. pylori infection.
CD4+CD25+ regulatory T (Treg) cells, expressing CTLA-4 constitutively, suppress the intestinal inflammation in models of inflammatory bowel disease and the injection of anti-CTLA-4 MoAb for 6 weeks abolishes the protective effect of Treg cells and leads to intestinal inflammation [31]. In H. pylori infection, Treg cells play an inhibitory role on the immunopathology in vivo and the activation of H. pylori-reactive T cells in vitro[32,33]. CTLA-4 is expressed on CD25+ T cells immediately after H. pylori infection and then CD25+ subsets increase gradually during infection [34]. The frequency of CD25+ cells in duodenal ulcer patients infected with H. pylori and asymptomatic carriers is considerably higher, compared with uninfected individuals. Interestingly, higher expression of CTLA-4 is observed in duodenal ulcer patients than asymptomatic carriers [35]. Taken together, these reports suggest that CTLA-4 signalling reduces immune activation and pathology after H. pylori infection. Therefore, the treatment of anti-CTLA-4 MoAb was expected to enhance the inflammatory immune response in H. pylori infection.
However, our study using anti-CTLA-4 MoAb injection for 1 week after infection shows the opposite effect, resulting in a reduction of the intensity and extent of inflammation throughout the gastric mucosa. Thus, the inflammation was controlled but not abrogated. The predominance of Th2 immunity leads to controlled inflammation in H. pylori infection [5,7,10]. CTLA-4 is expressed at a higher level in Th2 than Th1 cells but functions similarly in both Th1 and Th2 cells [36]. The higher expression of CTLA-4 on Th2 cells means that a Th2-related immune response would be much more sensitive to CTLA-4-dependent inhibition than Th1 cells. Indeed, previous studies have demonstrated that T cells from mice treated with anti-CTLA-4 MoAb and deficient in CTLA-4 gene are Th2-biased [16,37,38]. Treatment with anti-CTLA-4 MoAb in H. pylori infection resulted in an up-regulation of IgG1/IgG2a ratio in serum and IL-4/IFN-γ ratio in spleens. CTLA-4 blockade induced Th2-directed immunity, which might suppress H. pylori gastritis.
The failure of bacterial elimination in anti-CTLA-4 MoAb-treated mice seems to contradict the notion that Th2 polarized immune response induced by vaccinations reduces gastric inflammation and bacterial burden in H. pylori infection [8,10]. Recent studies have shown that vaccination with complete Freund's adjuvant, a Th1-biased adjuvant, leads to clearance of H. pylori[39]. Mice protected against H. pylori infection by immunization produce high levels of IFN-γ to recall antigen [40]. IFN-γ–/– mice show higher levels of bacterial colonization with some strains of H. pylori, compared to wild-type infected mice [6]. In IL-4–/– mice infected with Helicobacter spp. gastric inflammation decreases, but bacterial growth is variable based on the experimental models used [5,7,40]. Th1 immune response and other host factors would interplay in the elimination of H. pylori. In this study, no significant difference of IFN-γ response was observed between groups of mice. Previous studies have demonstrated that severity of gastritis in H. pylori infection is not correlated with IFN-γ secretion by gastric mucosa [4,41]. IFN-γ plays a critical role in disease induction but is not required for the progress of inflammation after Helicobacter infection [42].
We have shown that CTLA-4 blockade inhibited the development of H. pylori-associated gastritis coinciding with the induction of Th2 immune response. CTLA-4 signalling during acute H. pylori infection may play an inhibitory role in Th2 response, resulting in an induction of active gastritis.
REFERENCES
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;341:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–7. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 3.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–71. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–38. [PubMed] [Google Scholar]
- 5.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–9. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 6.Sawai N, Kita M, Kodama T, et al. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–85. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection. TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–57. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 8.Guy B, Hessler C, Fourage S, et al. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–6. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 9.Ikewaki J, Nishizono A, Goto T, Fujioka T, Mifune K. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol Immunol. 2000;44:29–39. doi: 10.1111/j.1348-0421.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 10.Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531–9. doi: 10.1128/iai.67.5.2531-2539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krummel MF, Sullivan TJ, Allison J. Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Immunol. 1996;8:519–23. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 12.Linsley PS, Greene JL, Tan P, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 co-stimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 14.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzel FP, Maier RA., Jr Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA4 antibody. Infect Immun. 1999;67:6454–60. doi: 10.1128/iai.67.12.6454-6460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirman J, McCoy K, Hook S, et al. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–92. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGaha T, Murphy JW. CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response. Infect Immun. 2000;68:4624–30. doi: 10.1128/iai.68.8.4624-4630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldie J, Veldhuyzen van Zanten SJ, Jalali S, et al. Optimization of a medium for the rapid urease test for detection of Campylobacter pylori in gastric antral biopsies. J Clin Microbiol. 1989;27:2080–2. doi: 10.1128/jcm.27.9.2080-2082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston, 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nakata Y, Uzawa A, Suzuki G. Control of CD4 T cell fate by antigen re-stimulation with or without CTLA-4 engagement 24 h after priming. Int Immunol. 2000;12:459–66. doi: 10.1093/intimm/12.4.459. [DOI] [PubMed] [Google Scholar]
- 22.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 23.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance? J Immunol. 1997;158:1989–93. [PubMed] [Google Scholar]
- 24.Tzianabos AO, Chandraker A, Kalka-Moll W, et al. Bacterial pathogens induce abscess formation by CD4 (+) T-cell activation via the CD28-B7-2 co-stimulatory pathway. Infect Immun. 2000;68:6650–5. doi: 10.1128/iai.68.12.6650-6655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–66. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 27.Abehsira-Amar O, Gibert M, Joliy M, Theze J, Jankovic DL. IL-4 plays a dominant role in the differential development of Th0 into Th1 and Th2 cells. J Immunol. 1992;148:3820–9. [PubMed] [Google Scholar]
- 28.Hsieh CS, Heimberger AB, Gold JSO, Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibraghimov A, Kabok Z, Garcia G, Castriotta L, Torrey D, Pappo J. Gastric CD4+ intraepithelial lymphocytes: a novel mucosa-associated lymphoid population induced in mice by Helicobacter pylori infection. Mucosal Immunol Update. 2000;8:9–12. [Google Scholar]
- 31.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25 (+) CD4 (+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin Exp Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe K, Murakami K, Maeda K, Fujioka T, Nasu M, Nishizono A. Intraperitoneal immunization led to T cell hyporesponsiveness to Helicobacter pylori infection in mice. Microbiol Immunol. 2002;46:441–7. doi: 10.1111/j.1348-0421.2002.tb02718.x. [DOI] [PubMed] [Google Scholar]
- 35.Stromberg E, Lundgren A, Edebo A, Lundin S, Svennerholm AM, Lindholm C. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol Med Microbiol. 2003;36:159–68. doi: 10.1016/S0928-8244(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 36.Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161:3347–56. [PubMed] [Google Scholar]
- 37.Hellings PW, Vandenberghe P, Kasran A, et al. Blockade of CTLA-4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur J Immunol. 2002;32:585–94. doi: 10.1002/1521-4141(200202)32:2<585::AID-IMMU585>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162:5784–91. [PubMed] [Google Scholar]
- 39.Gottwein JM, Blanchard TG, Taroni OS, et al. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Infect Dis. 2001;184:308–14. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 40.Akhiani AA, Pappo J, Kabok Z, et al. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002;169:6977–84. doi: 10.4049/jimmunol.169.12.6977. [DOI] [PubMed] [Google Scholar]
- 41.Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullberg MC, Rothfuchs AG, Jankovic D, et al. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;69:4232–41. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]