Abstract
Stress-associated immune responses were compared between young (8 weeks of age) and old (56 weeks) mice. Since stress suppresses the conventional immune system (i.e. T and B cells) but inversely activates the primordial immune system (i.e. extrathymic T cells, NKT cells, and granulocytes), these parameters were analysed after restraint stress for 24 h. The thymus became atrophic as a function of age, and an age-related increase in the number of lymphocytes was seen in the liver. Although the number of lymphocytes in both the thymus and liver decreased as the result of stress, the magnitude was much more prominent in the thymus. To determine stress-resistant lymphocyte subsets, two-colour immunofluorescence tests were conducted in the liver and spleen. NKT cells were found to be such cells in the liver of young mice. On the other hand, an infiltration of granulocytes due to stress was more prominent in the liver of old mice than in young mice. Liver injury as a result of stress was prominent in young mice. This age-related bias in the function of NKT cells and granulocytes seemed to be associated with a difference in the responses of catecholamines (high in old mice) and corticosterone (high in young mice) after stress. Indeed, an injection of adrenaline mainly induced the infiltration of granulocytes while that of cortisol activated NKT cells. The present results suggest the existence of age-related bias in the function of NKT cells and granulocytes after stress and that such bias might be produced by different responses of sympathetic nerves and steroid hormones between young and old mice.
Keywords: stress, natural killer T cells, granulocytes, steroid hormones, sympathetic nerves
INTRODUCTION
In a series of recent studies [1–5], we have revealed that stress induces the arrest of T-cell differentiation in the thymus, resulting in thymic atrophy. This pathway is termed the mainstream of T-cell differentiation in the thymus, by which conventional high TCR cells (TCRhigh cells)1 are generated [6,7]. The decreased level of T-cell differentiation in the thymus due to stress then results in immunosuppression in various peripheral immune organs. On the other hand, an alternative intrathymic pathway which produces natural killer T (NKT) cells [8–10] and an extrathymic, hepatic pathway which produces NK1·1– intermediate TCR cells (TCRint cells) [11,12] are inversely activated by stress. A similar phenomenon was also seen with ageing (i.e, thymic involution and the activation of extrathymic T cells) [6]. This reciprocal immune response after stress may be extremely important for adaptation of our body under emergency conditions. Abnormal self-cells which are generated by emergencies may be processed by autoreactive T cells with primordial properties (i.e. NKT cells and NK1·1–TCRint cells) [13,14] and auto antibody-producing B cells (i.e. B-1 cells) [15].
In light of these findings, we further investigated how the immune system was modulated by stress in young and old mice. This age-related examination is of interest because these different pathways of the immune system vary as a function of age apart from stress [16–18]. Our findings revealed that young and old mice had somewhat different immune responses to restraint stress. Namely, liver injury which was induced by the functional activation of NKT cells after stress was much more predominant in young mice than in old mice. This unique bias in the function of NKT cells seen in young mice seemed to be related to the higher production of steroid hormones after stress. In contrast, old mice rather deviated to sympathetic nerve activation after stress, which resulted mainly in an infiltration of granulocytes into tissues. Therefore, we report two different aspects of the immune system in this study, namely, age-related change of immune responses after stress and an intimate association of change with the sympathetic nervous system and steroid hormone secretion.
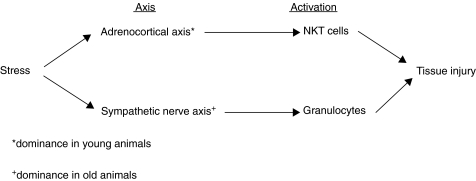
Finally, we propose the existence of two axes of immune responses after stress, including [1] an ‘adrenocortical axis’ which induces mainly NKT cells and [2] a ‘sympathetic nerve axis’ which induces granulocytes. These axes connect stress with subsequent tissue injury. In adult animals, these two axes are interwined. However, the ‘adrenocortical axis’ is dominant in young animals while the ‘sympathetic nerve axis’ becomes predominant in old animals.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) mice were used at the age of 8, 12 or 56 weeks. All mice were fed under specific pathogen-free conditions in the animal facility of Niigata University (Niigata, Japan).
Restraint stress
Mice were fixed in stainless steel mesh and kept for 24 h in a cage. As shown previously [5], these periods of stress are sufficient to induce granulocytosis in the gastric mucosa and result in gastric ulcers.
Cell preparations
Hepatic mononuclear cells (MNC) were isolated by a previously described method [19]. Briefly, the liver was removed, pressed through 200-gauge stainless steel mesh, and suspended in Eagle's MEM (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 5 mm HEPES and 2% heat-inactivated newborn calf serum. After being washed once with medium, the cells were fractionated by centrifugation in 15 ml of 35% Percoll solution (Amersham Pharmacia Biotech, Piscataway, NJ) for 15 min at 2000 r.p.m. The pellet was resuspended in erythrocyte lysing solution (155 mm NH4Cl, 10 mm KHCO3, 1 mm EDTA-Na, and 170 mm Tris, pH 7·3).
Thymocytes and splenic MNC were obtained by forcing the thymus and spleen, respectively, through 200-gauge stainless steel mesh. Splenic MNC were also used after treatment with the erythrocyte lysing soulution.
Immunofluorescence tests
FITC-, PE- or biotin-conjugated reagents of mAbs were used and biotin-conjugated reagents were developed with Tricolor-conjugated streptavidin (Caltag Laboratories, San Francisco, CA, USA) [20]. The mAbs used here were anti-CD3 (145–2C11), anti-NK1·1 (PK136), anti-CD4 (RM4-5), anti-CD8α (Lyt-2), anti-macrophage (M1/70), and anti-granulocyte (RB6–8C5) mAbs (BD PharMingen, San Diego, CA, USA). Cells were analysed by FACScan (BD Biosciences, Mountain View, CA, USA). To prevent nonspecific binding of mAbs, CD16/32 (2·4G2; BD PharMingen) was added before staining with labelled mAb. Dead cells were excluded by forward scatter, side scatter, and propidium iodide gating.
Measurement of transaminases
Liver injury was used to estimate the activity of asparate aminotransferase (AST) and alanine aminotransferase (ALT) in sera. The activity in each supernatant was quantified with an STA TEST KIT (Wako Pure Industries, Tokyo, Japan).
Cytotoxic assay
Using YAC-1 targets, NK-like cytotoxicity was examined by specific 51Cr-release assay with an incubation time of 4 h [21]. YAC-1 cells labelled with sodium chromate (51Cr) (Amersham Int., Arlington Heights, IL, USA) were used, and effector cells were identified as hepatic or splenic MNC. Percent cytotoxicity was determined using 104 YAC-1 cells at the indicated target-to-effector ratios in triplicate cultures. During a 4-h incubation assay, spontaneous chromium release of targets ranged from 10 to 15%. This release was eliminated by calculation.
Measurement of plasma concentration of catecholamines and corticosterone
Plasma pooled from 4 mice was used to measure the concentration of adrenaline, noradrenaline, dopamine, and corticosterone. The plasma levels of these catecholamines were analysed by the HPLC method. Plasma corticosterone of mice was also detected by radioimmunoassay with minor modifications. In the case of mice, corticosterone is the major steroid hormone.
Administration of adrenaline and hydrocortisone sodium succinate
To investigate the effect of catecholamines, 100 µg (per mouse) of adrenaline (Sigma Chemicals Co., St Louis, MO, USA) was intraperitoneally injected. To investigate the effects of steroid hormones, 10 mg (per mouse) of hydrocortisone sodium succinate (Pharmacia Co., Tokyo, Japan) was intraperitoneally injected. MNC were then examined at 24 h after administration. In earlier experiments [2,5], we decided the maximum responses of these reagents in terms of doses of reagents and evaluation time.
Statistical analysis
Differences among the data were analysed by two-factor anova test.
RESULTS
Age-related change in the number of lymphocytes in various immune organs and resistance to stress of liver lymphocytes
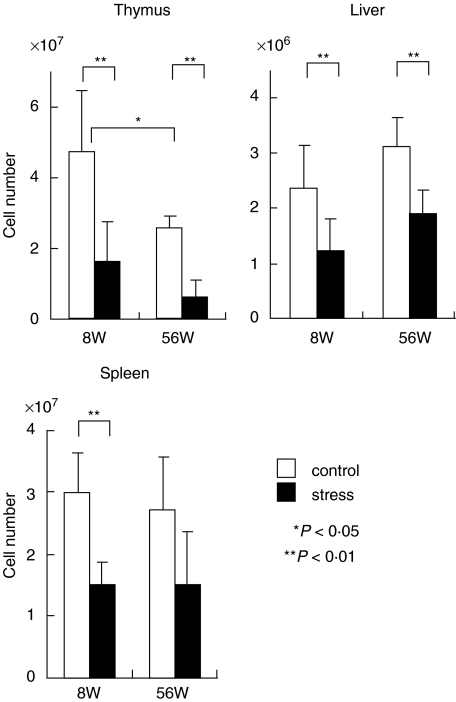
To examine age-dependence of stress in various lymphoid organs, the numbers of lymphocytes were first enumerated in the thymus, liver, and spleen of young and old mice (Fig. 1). Restraint stress was conducted for 24 h. The number of thymocytes showed an age-associated decrease, namely, the number in old mice decreased up to 50% of the number in young mice. On the other hand, the number of liver lymphocytes increased as a function of age while that of splenic lymphocytes remained almost unchanged. Stress-associated decreases in the number of thymocytes was seen in both young and old mice. As a result of restraint stress, a decrease in the number of lymphocytes in the liver and spleen was also seen in both young and old mice but the magnitude of the decrease in these organs was not as prominent as that in the thymus.
Fig. 1.
Age-related and stress-associated changes in the number of MNC yielded by the thymus, liver and spleen. Young mice at the age of 8 weeks and old mice at the age of 56 weeks were used. Restraint stress was conducted for 24 h. The number of MNC was enumerated in 4 mice and the mean and one SD were produced.
Identification of stress-resistant lymphocyte subsets in the liver
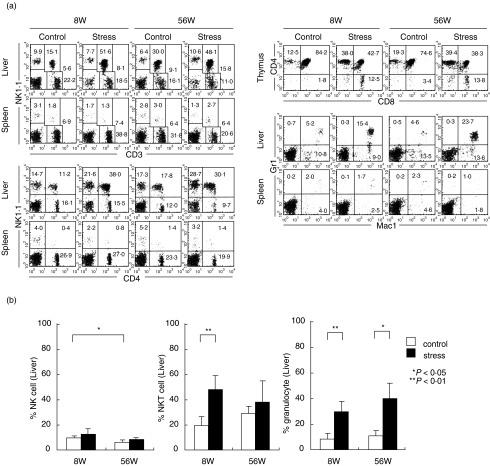
To examine the level of stress-resistance among various lymphocyte subsets, two-colour stainings of lymphocytes for various combinations were conducted in the liver and spleen (Fig. 2a). Two-colour staining for CD3 and NK1·1 showed the most stress-resistant lymphocyte subset to be CD3intNK1·1+ cells (i.e. NKT cells) in the liver. The majority of these cells were CD4+ NKT cells as estimated by the staining of CD4 and NK1·1. In the spleen, the change was minimal in all tested subsets.
Fig. 2.
Estimation of stress-resistant lymphocyte subsets in the liver and spleen. (a) Two-colour staining of lymphocytes for various combinations. (b) Increase in the proportion of NKT cells in young mice and of granulocytes in old mice. MNC were obtained before and after stress in young and old mice and the phenotype of cells was identified by immunofluorescence tests. Numbers in the figure indicate the percentages of fluorescence-positive cells in corresponding areas. The mean and one SD in the proportion of NK cells, NKT cells and granulocytes were produced by repeated experiments (n = 4).
It is known that thymic atrophy is always accompanied by a decrease in the proportion of double-positive (DP) CD4+8+ cells [2]. Reflecting this situation, the proportion of DP cells decreased significantly as shown by the staining of CD4 and CD8 (i.e. 85·6 to 42·7% in young mice and 74·6 to 38·3% in old mice).
In a final portion of these experiments, two-colour staining for Mac-1 and Gr-1 was conducted to identify granulocytes (Gr-1+Mac-1+) and macrophages (Gr-1–Mac-1+). The proportion of granulocytes was also found to increase in the liver as the result of restraint stress. This response was prominent in old mice.
All these experiments were repeated (n = 4) and the mean and one SD in the populations of lymphocytes subsets and granulocytes were determined (Fig. 2b). The change of NK cells was minimal, but that of NKT cells and granulocytes was statistically significant (P < 0·05 or < 0·01). The increase in the proportion of NKT cells was prominent in young mice, whereas that of granulocytes was prominent in old mice.
Cytotoxic activity and liver injury
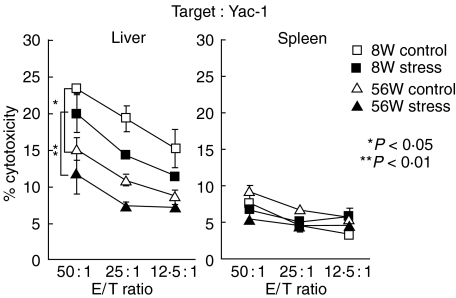
It is known that NKT cells mediate NK-like cytotoxicity when they are activated. In this experiment, such NK-like activity against YAC-1 cells was examined by using lymphocytes in the liver and spleen (Fig. 3). In the liver of both young and old mice, a prominent inactivation of NK-like cytotoxicity was demonstrated after stress. Although the proportion of NK and NKT cells was higher in old mice than that in young mice, the cytotoxicity was inversely high in young mice before stress. These results suggested that NKT cells were initially activated in number but that long-lasting stress finally induced functional inactivation.
Fig. 3.
NK-like cytotoxicity before and after stress in young and old mice. Lymphocytes were isolated from the liver and spleen, and NK-like cytotoxicity against YAC-1 cells was determined by 4 h-incubation at the indicated E/T ratios. The mean and one SD were produced in triplicate cultures.
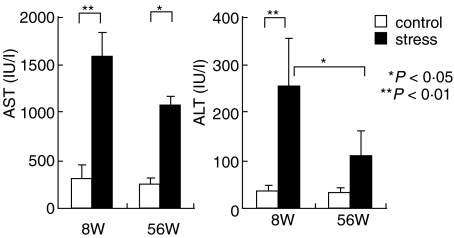
The possibility that NKT cells stimulated by stress mediated liver injury was then investigated (Fig. 4). The serum levels of transaminases (AST and ALT) were examined in mice before and after stress. The highest levels of AST and ALT were always seen in young mice after stress. In other words, the activation of NKT cells in number (see Fig. 2) and the level of liver injury seemed to be correlated, especially in young mice.
Fig. 4.
A comparison of the magnitude of liver injury between young and old mice after stress. To determine the magnitude of liver injury, serum levels of AST and ALT were measured. The mean and one SD were produced from 4 mice.
Mechanisms underlying the activation of NKT cells and granulocytes
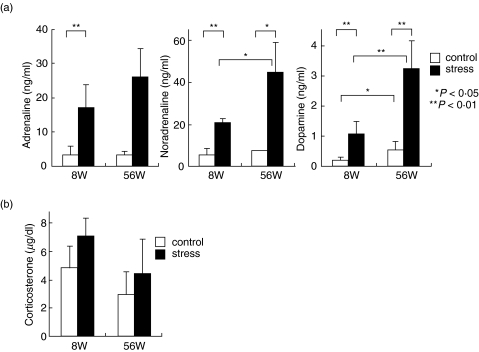
It is well established that stress induces the activation of the sympathetic nervous system and the secretion of steroid hormones [2,3]. In this experiment, the serum levels of catecholamines (i.e, adrenaline, noradrenaline and dopamine) and corticosterone were examined in mice before and after restraint stress (Fig. 5). As expected, a prominent elevation in the concentration of catecholamines and corticosterone was observed. In the case of catecholamines, old mice tended to show an elevated level, especially in the levels of noradrenaline and dopamine. In contrast, the increased level of corticosterone was moderate in both young and old mice, although the baseline of corticosterone was higher in young mice than in old mice.
Fig. 5.
Comparisons of serum levels of catecholamines and corticosterone between young and old mice after stress. Sera were obtained from 4 mice at each column to produce the mean and one SD.
Induction of granulocytes by the administration of adrenaline but induction of NKT cells by the administration of steriod hormone
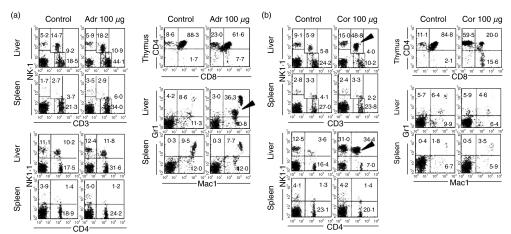
The experiments thus far described indicated corticosterone to be related to the induction of NKT cells while catecholamines seemed to be related to the induction of granulocytes after stress. This hypothesis was confirmed by in vivo injections (Fig. 6). Adrenaline (100 µg/mouse) or hydrocortisone (10 mg/mouse) was intraperitoneally injected into adult mice (12 weeks of age) and the phenotype of cells was examined 24 h after the injection. When adrenaline was injected, the proportion of NKT cells remained almost unchanged (Fig. 6a). Thymic atrophy was not prominent, namely, there was only a moderate decrease of DP CD4+8+ cells in the thymus (88·3 to 61·6%). Interestingly, the proportion of granulocytes (Mac-1+Gr-1+) increased prominently, especially in the liver (8·6 to 36·3%, indicated by an arrowhead).
Fig. 6.
In vivo injection of adult mice (12 weeks of age) with (a) adrenaline (Adr) or (b) hydrocrotisone (Cor). MNC were isolated from the liver, spleen and thymus before and after each injection (24 h). Two-colour stainings for the indicated combinations were conducted. Representative results of three experiments are depicted.
When hydrocortisone was injected, the proportion of NKT cells increased prominently in the liver (Fig. 6b). CD3intNK1·1+ cells belonged to the CD4+ subset (indicated by arrowheads, respectively). At this time, the proportion of DP CD4+8+ cells decreased (84·8 to 20·0%) in the thymus. Interestingly, the proportion of granulocytes remained unchanged by this injection.
DISCUSSION
Findings of the present study indicate the existence of two axes which induce immune responses and subsequent tissue damage after stress (Fig. 7). One axis was the ‘adrenocortical axis’ which connected stress with the secretion of corticosterone (i.e. steroid homones) and the activation of NKT cells. At such time, thymic atrophy, which suggested an arrest of the mainstream of T-cell differentiation in the thymus, occurred. Another axis was the ‘sympathetic nerve axis’ which connected stress with sympathetic nerve stimulation (i.e. the secretion of catecholamines) and the activation of granulocytes. It is conceivable that NKT cells may induce tissue damage in vivo by their cytotoxicity while granulocytes may induce tissue damage through the production of superoxides.
Fig. 7.
Hypothesis on two axes which connect stress with tissue injury, including (1) the adrenocortical axis with the activation of NKT cells and (2) the sympathetic nerve axis with the activation of granulocytes.
Primarily, these two axes of stress-associated immune responses seemed to be intertwined under usual conditions. However, these axes were found to be biased in one direction in young and old mice, respectively. When young mice were exposed to restraint stress, thymic atrophy and the activation of NKT cells in the liver became prominent. When old mice were exposed to the same stress, granulocytosis became prominent, especially in the liver. In parallel with these responses seen in leucocytes, the elevation in the serum level of corticosterone was mainly seen in young mice while the elevation in the serum level of catecholamines was mainly seen in old mice. In other words, although both young and old mice showed liver injury after stress, the associated mechanisms appeared to deviate toward one direction of the axes.
In recent studies [2,3], we have reported that the mainstream of T-cell differentiation in the thymus and the extrathymic pathway of T-cell differentiation in the liver are reciprocally regulated, especially by steroid hormones. In addition to ageing [16–18], an activation of the extrathymic pathway is induced by stress [1–5], malignancy [22,23], autoimmune diseases [24–26], pregnancy [27,28], and chronic GVH disease [29,30]. When ageing and stress were both present, a reciprocal response was prominently seen in the present study (see Fig. 1). NKT cells are primarily generated by an alternative intrathymic pathway and then home to the liver [8–10]. However, it has been found that the population of NKT cells in the liver is maintained without a continuous supply from the thymus during adult life [31]. In other words, their precusors home to the liver at the neonatal stage and are renewed in situ thereafter.
The present results also revealed that the axis of sympathetic nerves and granulocytes became prominent as a function of age. The connection between sympathetic nerves and granulocytes is due to the presence of surface adrenergic receptors on granulocytes [32]. Many adrenergic stimulations eventually activate the function of granulocytes in the periphery [33,34] and the number of granulocytes in the bone marrow [35].
In the present study, it was found that the increase in the proportion of NKT cells was prominent or that the increase in the proportion of NK cells was not so prominent in the liver after stress. Since NKT cells mediate NK-like cytotoxicity when they are activated [11,36], the level of such cytoxicity was examined in this study. A prominent inactivation of NK-like cytotoxicity was demonstrated in the liver, but not in the spleen. This cytotoxicity might be associated with liver injury after stress, especially in young mice. However, long-lasting stress finally suppressed the cytotoxicity of NKT cells. We previously reported that NK-like cytotoxicity by NKT cells is mediated through both the Fas ligand/Fas system [21] and the perforin system [37]. It is well established that NKT cells activated by α-galactosilceramide and other stimuli can mediate tissue damage due to their autoreactivity [34,38,39]. Even in these cases, the function of activated NKT cells are finally exhausted in vivo.
On the other hand, liver injury induced by granulocytes might be mediated by superoxides. It is well established that granulocytes are the main source of cells for superoxide production [40,41]. It is also known that activated granulocytes or neutrophils are intimately related to tissue damage, including that of the liver, lung and other organs [42–44].
Finally, to confirm our hypothesis (i.e. the existence of two axes of stress-associated responses), we conducted in vivo injections of adult mice with adrenaline and glucocorticoid. By the injection of adrenaline, thymic atrophy was not so prominent, but the induction of granulocytes was. By the injection of glucocorticoid, thymic atrophy and the activation of NKT cells were prominent, but the induction of granulocytes was minimal. These results support our speculation about stress-associated immune responses. The concept of two axes of stress-associated immune responses seems to be extremely important for properly understanding the physiological responses after stress and subsequent tissue injury. Age-associated bias in this phenomenon is also interesting.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan. We wish to thank Mrs Masako Watanabe for preparation of the manuscript.
REFERENCES
- 1.Kawamura T, Toyabe S, Moroda T, et al. Neonatal granulocytosis is a postpartum event which is seen in the liver as well as in the blood. Hepatology. 1997;26:1567–72. doi: 10.1053/jhep.1997.v26.pm0009397999. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama S, Tsukahara A, Suzuki S, Tada T, Minagawa M, Watanabe H, Hatakeyama K, Abo T. Quick recovery in the generation of self-reactive CD4low natural killer (NK) T cells by an alternative intrathymic pathway when restored from acute thymic atrophy. Clin Exp Immunol. 1999;117:587–95. doi: 10.1046/j.1365-2249.1999.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu T, Kawamura T, Miyaji C, et al. Resistance of extrathymic T cells to stress and a role of endogenous glucocorticoids in stress-associated immunosuppression. Scand J Immunol. 2000;51:285–92. doi: 10.1046/j.1365-3083.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- 4.Oya H, Kawamura T, Shimizu T, et al. The differential effect of stress on natural killer T (NKT) and NK cell function. Clin Exp Immunol. 2000;121:384–90. doi: 10.1046/j.1365-2249.2000.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura T, Miyaji C, Toyabe S, Fukuda M, Watanabe H, Abo T. Suppressive effect of anti-ulcer agents on granulocytes – A role for granulocytes in gastric ulcer formation. Dig Dis Sci. 2000;45:1786–91. doi: 10.1023/a:1005526126694. [DOI] [PubMed] [Google Scholar]
- 6.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 7.Abo T. Extrathymic pathways of T-cell differentiation and immunomodulation. Int Immunopharmacol. 2001;1:1261–73. doi: 10.1016/s1567-5769(01)00057-1. [DOI] [PubMed] [Google Scholar]
- 8.Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- 9.Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant Va14+ natural killer-T cell development. Eur J Immunol. 1999;29:3313–8. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–8. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 11.Halder RC, Kawamura T, Bannai M, Watanabe H, Kawamura H, Mannoor MDK, Morshed SRM, Abo T. Intensive generation of NK1.1- extrathymic T cells in the liver by injection of bone marrow cells isolated from mice with a mutation of polymorphic major histocompatibility complex antigens. Immunology. 2001;102:450–9. doi: 10.1046/j.1365-2567.2001.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyakawa R, Miyaji C, Watanabe H, Yokoyama H, Tsukada C, Asakura H, Abo T. Unconventional NK1.1- intermediate TCR cells as major T lymphocytes expanding in chronic graft-versus-host disease. Eur J Immunol. 2002;32:2521–31. doi: 10.1002/1521-4141(200209)32:9<2521::AID-IMMU2521>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Kawachi Y, Watanabe H, Moroda T, Haga M, Iiai T, Hatakeyama K, Abo T. Self-reactive T cell clones in a restricted population of interleukin-2 receptor β+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995;25:2272–8. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- 14.Naito T, Kawamura T, Bannai M, et al. Simultaneous activation of natural killer T cells and autoantibody production in mice injected with denatured syngeneic liver tissue. Clin Exp Immunol. 2002;129:397–404. doi: 10.1046/j.1365-2249.2002.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune diseases in (NZB∼NZW) F1 mice. Eur J Immunol. 2002;32:2551–61. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Iiai T, Watanabe H, Seki S, et al. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992;77:556–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukahara A, Seki S, Iiai T, et al. Mouse liver T cells. Their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 18.Miyaji C, Watanabe H, Minagawa M, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 20.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moroda T, Iiai T, Suzuki S, et al. Autologous killing by a population of intermediate T cell receptor cells and its NK1.1+ and NK1.1– subsets, using Fas ligand/Fas molecules. Immunology. 1997;91:219–26. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura T, Kawachi Y, Moroda T, et al. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996;89:68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura T, Seki S, Takeda K, Narita J, Ebe Y, Naito M, Hiraide H, Abo T. Protective effect of NK1.1+ T cells as well as NK cells against intraperitoneal tumors in mice. Cell Immunol. 1999;193:219–25. doi: 10.1006/cimm.1999.1477. [DOI] [PubMed] [Google Scholar]
- 24.Yamagiwa S, Kuwano Y, Hasegawa K, et al. Existence of a small population of IL-2Rβhi TCRint cells in SCG and MRL-lpr/lpr mice which produce normal Fas mRNA and Fas molecules from the lpr gene. Eur J Immunol. 1996;26:1409–16. doi: 10.1002/eji.1830260702. [DOI] [PubMed] [Google Scholar]
- 25.Arai K, Yamamura S, Hanyu T, Takahashi HE, Umezu H, Watanabe H, Abo T. Extrathymic differentiation of resident T cells in the joints of mice with collagen-induced arthritis. J Immunol. 1996;157:5170–7. [PubMed] [Google Scholar]
- 26.Moroda T, Iiai T, Kawachi Y, Kawamura T, Hatakeyama K, Abo T. Restricted appearance of self-reactive clones into T cell receptor intermediate cells in neonatally thymectomized mice with autoimmune disease. Eur J Immunol. 1996;26:3084–91. doi: 10.1002/eji.1830261239. [DOI] [PubMed] [Google Scholar]
- 27.Kimura M, Watanabe H, Sato S, Abo T. Female predominance of extrathymic T cells in mice: Statistical analysis. Immunol Lett. 1994;39:259–67. doi: 10.1016/0165-2478(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 28.Minagawa M, Narita J, Tada T, Maruyama S, Shimizu T, Bannai M, Oya H, Hatakeyama K, Abo T. Mechanisms underlying immunologic states during pregnancy. possible association of the sympathetic nervous system. Cell Immunol. 1999;196:1–13. doi: 10.1006/cimm.1999.1541. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Kawamura T, Kawamura H, Haga M, Shirai K, Watanabe H, Eguchi S, Abo T. Intermediate TCR cells in mouse lung. Their effector function to induce pneumonitis in mice with autoimmune-like graft-versus-host disease. J Immunol. 1997;158:5805–14. [PubMed] [Google Scholar]
- 30.Weerasinghe A, Kawamura T, Moroda T, Seki S, Watanabe H, Abo T. Intermediate TCR cells can induce graft-versus-host disease after allogeneic bone marrow transplantation. Cell Immunol. 1998;185:14–29. doi: 10.1006/cimm.1998.1263. [DOI] [PubMed] [Google Scholar]
- 31.Kameyama H, Kawamura T, Naito T, Bannai M, Shimamura K, Hatakeyama K, Abo T. Size of the population of CD4+ natural killer T cells in the liver is maintained without supply by the thymus during adult life. Immunology. 2001;104:135–41. doi: 10.1046/j.0019-2805.2001.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki SS, Toyabe T, Moroda T, et al. Abo. Circadian rhythm of leukocytes and lymphocytes subsets and its possible correlation with the function of autonomic nervous system. Clin Exp Immunol. 1997;110:500–8. doi: 10.1046/j.1365-2249.1997.4411460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruyama S, Minagawa M, Shimizu T, et al. Administration of glucocorticoids markedly increases the numbers of granulocytes and extrathymic T cells in the bone marrow. Cell Immunol. 1999;194:28–35. doi: 10.1006/cimm.1999.1492. [DOI] [PubMed] [Google Scholar]
- 34.Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–15. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 35.Yamamura S, Arai K, Toyabe S, Takahashi HE, Abo T. Simultaneous activation of granulocytes and extrathymic T cells in number and function by excessive administration of nonsteroidal anti-inflammatory drugs. Cell Immunol. 1996;173:303–11. doi: 10.1006/cimm.1996.0282. [DOI] [PubMed] [Google Scholar]
- 36.Weerasinghe A, Sekikawa H, Watanabe H, et al. Association of intermediate T cell receptor cells, mainly their NK1.1- subset, with protection from malaria. Cell Immunol. 2001;207:28–35. doi: 10.1006/cimm.2000.1737. [DOI] [PubMed] [Google Scholar]
- 37.Bannai MH, Oya T, Kawamura T, et al. Disparate effect of beige mutation on cytotoxic function between natural killer and natural killer T cells. Immunology. 2000;100:165–9. doi: 10.1046/j.1365-2567.2000.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3e- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–53. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 39.Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of a-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Miura S, Mori M, Kai A, Suzuki H, Fukumura D, Suematsu M, Tsuchiya M. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–8. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-α. Int Immunol. 1993;5:691–4. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 42.Brown AP, Schultze AE, Holdan WL, Buchueitz JP, Rot RA, Ganey PE. Lipopolysaccharide-induced hepatic injury is enhanced by polychlorinated biphenyls. Environ Health Perspect. 1996;104:634–40. doi: 10.1289/ehp.96104634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Partrick DA, Moore FA, Moore EE, Jr, Barnett CC, Silliman CC. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996;4:194–210. [PubMed] [Google Scholar]
- 44.Srinivasan RJP, Buchweitz PE. Ganey. Alteration by flutamide of neutrophil response to stimulation. Implications for tissue injury. Biochem Pharmacol. 1997;53:1179–85. doi: 10.1016/s0006-2952(97)00099-3. [DOI] [PubMed] [Google Scholar]