Abstract
Autoantibodies against C1q can be found in the circulation of patients with several autoimmune diseases including systemic lupus erythematosus (SLE). In SLE there is an association between the occurrence of these antibodies and renal involvement. How anti-C1q autoantibodies contribute to renal disease is currently unknown. Cohorts of MRL-lpr mice, which are known to develop age-dependent SLE-like disease, were used to study the relationship between levels of anti-C1q autoantibodies and renal disease. We collected serum, urine and renal tissue and analysed autoantibodies, complement levels and renal deposition as well as renal function. At 2 months of age all mice already had elevated levels of anti-C1q autoantibodies, and elution of kidneys revealed the presence of these antibodies in renal immune deposits in MRL-lpr mice and not in control MRL+/+ mice. In conclusion, anti-C1q antibodies are already present in serum and immune deposits of the kidney early in life and therefore can play a role in nephritis during experimental SLE-like disease in mice.
Keywords: C1q, complement, kidney, mouse, SLE
INTRODUCTION
The complement system plays a crucial role in innate defence [1,2] and in the generation of acquired immune responses [3]. Under normal circumstances the contribution of complement is beneficial to the host, but it may also amplify tissue injury. In autoimmune diseases, complement components can even be the target of an autoantibody response [4]. In systemic lupus erythematosus (SLE) C1q plays an important role, because on one hand C1q is involved in the classical pathway of complement and increases injury in tissues with immune complex deposits. On the other hand, a deficiency in C1q predisposes to the development of a SLE-like disease [5]. An explanation for this dual effect of C1q probably resides in its interactions with both immune complexes and apoptotic cells [6,7].
Anti-C1q autoantibodies are present in the serum of patients suffering from different autoimmune diseases such as SLE, rheumatoid arthritis (RA) and hypocomplementaemic urticarial vasculitis (HUVS) [8]. Approximately 30–50% of SLE patients have anti-C1q autoantibodies [9,10]. In SLE the presence of these autoantibodies is associated with hypocomplementaemia and nephritis [10]. Nephritis is a major cause of morbidity in SLE and a major determinant for the outcome of disease [11]. A rise in the titre of these autoantibodies seems to be predictive for a flare of nephritis [12,13]. Elution studies using post-mortem kidneys of patients suffering from lupus nephritis demonstrated the presence of anti-C1q reactivity in the immune deposits supporting a role for anti-C1q in renal injury [14].
Different mouse models of lupus are available [15], of which the MRL-lpr model is the best known [16]. These mice have a deficiency of the apoptosis-promoting Fas gene [17] and display most of the hallmarks of human lupus, including circulating immune complexes, autoantibodies, dermatitis and fatal nephritis [15]. Anti-C1q autoantibodies have been described in several strains of autoimmune mice [18], including MRL-lpr mice [18,19], although there has been some debate on the nature of the anti-C1q reactivity [18,20].
In the current paper we have studied in detail the production of mouse antimouse C1q autoantibodies. We have related these anti-C1q autoantibodies to titres of other autoantibodies, levels of C1q and C3 and renal injury parameters.
METHODS
Animals and experimental protocol
MRL/MpJ lpr mice (MRL-lpr), MRL/MpJ+/+ mice (MRL+/+) and BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) at 4 weeks of age; Rag 2−/− mice from Taconic (Taconic, Germantown, NY, USA) [21] and C1q−/− C57BL/6 mice [22] were maintained at our facility. All animals had free access to water and standard chow. Animal care and experimentation were performed in accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals.
Three female MRL-lpr mice were sacrificed immediately. Twenty-four mice were divided into groups of six mice and every month one group was sacrificed. Blood and urine were collected every month. We encountered some spontaneous deaths, therefore the number of samples for histology was five and three for months 4 and 5, respectively. We used three MRL+/+ mice for all experiments. Mice were anaesthetized with urethane (Sigma-Aldrich, Zwijndrecht, the Netherlands) and sacrificed by heart puncture.
Development of a novel antimouse C1q detection enzyme-linked immunosorbent assay (ELISA)
C1q binding peptide 2J and control peptide 5C [23] were coated at 12·5 µm in coating buffer for 2 h at 37°C. Plates were washed and incubated with purified mouse C1q [24] or NMS, immunoglobulin-deficient serum (Rag 2−/−) or C1q−/− serum. Bound C1q was detected using rabbit antimouse C1q-Dig and anti-Dig HRP.
For further analysis we used the combination of peptide 2J and Rag 2-/- serum at 1 : 20 in phosphate buffered saline (PBS)-T-bovine serum albumin (BSA) for 1 h at 37°C. Serial dilutions of serum samples were incubated in PBS-T-BSA containing 0·5 m NaCl for 1 h at 37°C. Bound mouse IgG was detected using goat antimouse IgG HRP (Dako, Glostrup, Denmark). Data are expressed as OD415 or as arbitrary units relative to a standard serum arbitrarily set at 100 aU/ml.
Detection of anti-ds DNA and antihistone autoantibodies, C1q and C3 in serum
Anti-ds DNA and anti-histone autoantibodies were detected as described [25,26]. Data are expressed relative to a standard serum set arbitrarily at 1000 aU/ml.
Serum C1q was detected by both ELISA and Western blot as described [24]. For Western blot, sera of MRL-lpr and MRL+/+ mice were separated on a 10%, reducing SDS-PAGE blotted and stained for C1q. Serum C3 was detected by sandwich ELISA by coating goat antimouse C3 (Nordic, Tilburg, the Netherlands), and detecting bound C3 by rabbit antimouse C3-Dig (generated in our laboratory) and anti-Dig-HRP (Boehringer Mannheim). Data are expressed relative to a standard serum arbitrarily set at 1000 U/ml.
Renal function and pathology
To estimate renal function sera were tested in a blood urea nitrogen (BUN) assay kit (Sigma) expressed as mg/dl of urea nitrogen. Albuminuria was measured using an autoanalyser (Hitachi-911, Hitachi, Tokyo, Japan). Data are expressed as mg albumin per 24 h.
Histological studies
For light microscopy, renal tissue was fixed in methyl Carnoy's solution, embedded in paraffin and 3 µm sections were stained with haematoxylin–eosin (H&E) or periodic acid-Schiff (PAS). Evaluation of histopathological changes was performed on coded sections by a pathologist (HB) and expressed as an Activity Index (AI) and Chronicity Index (CI), as described by Austin et al. [27].
For immunohistochemistry, acetone-fixed, 3 µm cryostat sections of snap-frozen tissue were stained with Oregon green conjugated goat antimouse IgG (Molecular probes, Leiden, the Netherlands) or FITC-conjugated goat antimouse IgM (Nordic). Mouse C1q was detected using rabbit antimouse C1q and goat antirabbit FITC (Nordic). Mouse C3 was detected using goat antimouse C3 FITC (Nordic). Mouse C9 was detected using rabbit antirat C9 (a kind gift of Dr P. Morgan, Cardiff, Wales), which cross-reacts with mouse C9 and goat antirabbit FITC (Nordic).
Antibody elution from kidneys
Kidney tissue of three mice per group was weighed, pooled, minced and collected in 500 µl PBS containing protease inhibitor cocktail (Roche, Mannheim, Germany). The mixture was centrifuged at 3000 r.p.m. for 5 min, and supernatants were collected. Pellets were washed and resuspended in 250 µl elution buffer consisting of 0·1 m glycine HCl, 0·15 m NaCl, pH 2·5 and sonicated directly (Branson, Boom, Meppel, the Netherlands) on ice, three bursts of 30 s, followed by overnight rotation at 4°C. Samples were centrifuged for 10 min at 10 000 r.p.m.; supernatants were adjusted to pH 7·0. For further analysis all samples were standardized based on their initial weight. Samples were tested in anti-C1q and IgG ELISA.
Statistics
Statistical analysis was performed using GraphPad Prism 3·03 software. Correlation between parameters was tested for significance using the Spearman's rank correlation coefficient. P-values were considered statistically significant when P < 0·05.
RESULTS
Histopathological analysis
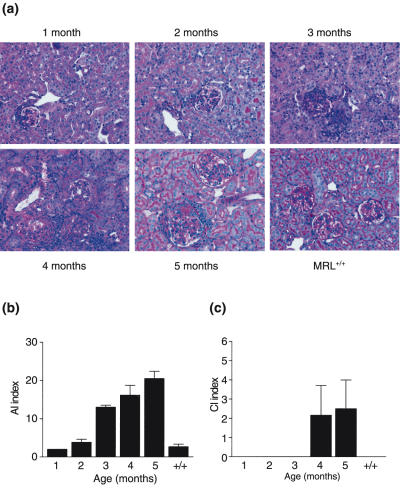
MRL-lpr mice aged 1 and 2 months exhibited normal renal architecture (Fig. 1a). Thereafter, a time-dependent increase of infiltrating cells was observed predominantly in perivascular and peritubular areas. The glomeruli showed increased cellularity with infiltrating cells and increased numbers of mesangial cells. At the latest time-point at 5 months of age the mice developed severe glomerulosclerosis, severe interstitial infiltrates, tubular atrophy and large protein casts (Fig. 1a). All mice exhibit this process of renal histological changes but with different kinetics. Control MRL+/+ mice do not exhibit significant renal abnormalities at the age of 5 months.
Fig. 1.
Histopathological changes in MRL-lpr and MRL+/+ mice of different ages. (a) Representative pictures of PAS-stained kidney sections of MRL-lpr mice of different ages and MRL+/+ control mice of 5 months of age. Ages of the mice are indicated above the pictures. 1 month, normal histology; 2 months, mild glomerulitis; 3 months, mild to moderate glomerulitis; 4 months, marked glomerular inflammation; with crescents and interstitial inflammation; 5 months, necrotizing glomerulonephritis; MRL+/+, 5 months, only mild glomerlitis. (b) Activity index of renal section of MRL-lpr mice of different ages and of control MRL+/+ mice of 5 months old. (c) Chronicity index of renal section of MRL-lpr mice of different ages and of MRL+/+ mice of 5 months old. Data are expressed as average index numbers and s.e.m.
Renal histological changes were quantified using the index system described by Austin et al. [27]. MRL-lpr mice develop progressive renal damage as expressed in both the AI (Fig. 1b) and the CI (Fig. 1c). Signs of chronicity are visible only in older mice.
Serum autoantibodies and glomerular deposition of IgG and IgM
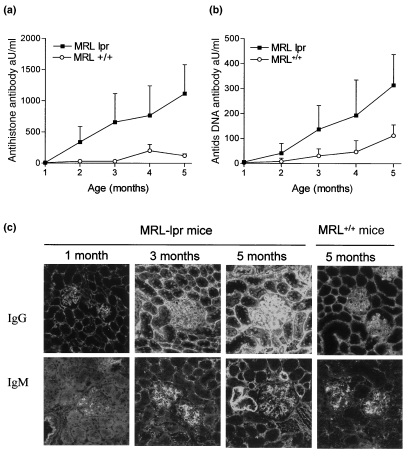
To study the presence and kinetics of autoantibody production we measured antihistone and anti-ds DNA autoantibodies. An age-dependent increase in serum levels of both autoantibodies was observed. Control MRL+/+ become only mildly positive and later in life (Fig. 2a,b). Because serum autoantibodies are involved directly in the formation of immune complexes, which may deposit in the glomerulus, we analysed renal IgG and IgM (Fig. 2c). As these mice age there was a pronounced IgG deposition both in glomeruli and in the peritubular space. IgM was absent at 1 month of age and as these mice age there was a clear increase in the intensities of deposited IgM.
Fig. 2.
Autoantibody titres and glomerular immunoglobulin deposition. (a) Anti-histone autoantibodies and (b) Anti-ds DNA autoantibodies in sera of MRL-lpr and MRL+/+ mice of different ages. Data are expressed relative to a standard serum, bars show average and s.e.m. (c) Representative pictures of renal sections of MRL-lpr and MRL+/+ mice of different ages for the deposition IgG and IgM.
Serum levels and glomerular deposition of complement components
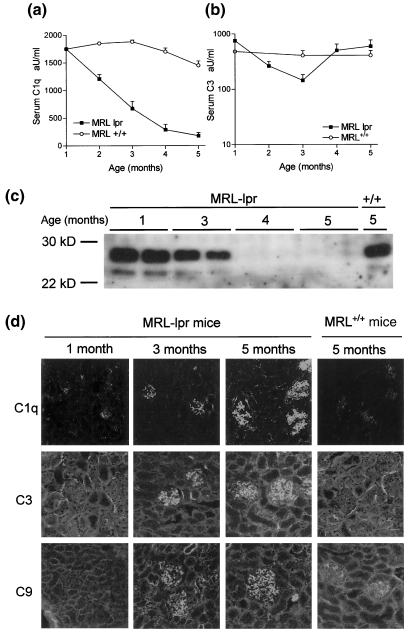
In order to find a relation between renal injury and changes in complement levels we measured serum levels of C1q and C3. C1q levels showed an age-dependent decrease in MRL-lpr mice starting at 2 months of age, resulting in a state of full depletion at 4 and 5 months (Fig. 3a). Western blot also revealed full depletion of C1q at the later time-points, excluding masking of epitopes on C1q by anti-C1q autoantibodies in ELISA (Fig. 3c). MRL+/+ mice exhibited only marginal depletion of C1q. In contrast, C3 levels showed some reduction around 3 months of age in MRL-lpr mice, but reached normal values again later in life (Fig. 3b). Control MRL+/+ mice did not exhibit significant changes in C3 levels.
Fig. 3.
Serum levels and glomerular deposition of complement components. (a) C1q levels and (b) C3 levels in sera of MRL-lpr and MRL+/+ mice of different ages. Data are expressed relative to a standard serum, bars show average and s.e.m. (c) Western blot analysis of mouse sera, of MRL-lpr mice of different ages and an MRL+/+ mouse at 5 months. Total mouse serum was separated on a 10% SDS-PAGE gel under reducing conditions, blotted onto nitrocellulose and stained with Dig-conjugated rabbit antimouse C1q, followed by goat anti-Dig-HRP. Shown are two mice per group for the MRL-lpr mice of ages, 1, 3, 4 and 5 months and one MRL+/+ mouse at 5 months. (d) Representative pictures of MRL-lpr and MRL+/+ mice of different ages stained for the deposition of C1q, C3, C9.
Because serum C1q becomes depleted, we investigated glomerular deposition of C1q and complement activation markers C3 and C9 in the kidney. Both for C1q, C3 and C9 we found an age-dependent increase of glomerular deposition (Fig. 3d, Table 1) indicating complement activation. At 1 month of age we found trace amounts IgG and C1q in the glomeruli of MRL-lpr mice, comparable to age-matched non-autoimmune BALB/c mice.
Table 1.
Summary of IF intensities of glomerular deposits of MRL-lpr mice and controls
| Age | Controls | ||||||
|---|---|---|---|---|---|---|---|
| Antigen | 1 | 2 | 3 | 4 | 5 | +/+ | BALB/c |
| C1q | +/– | + | + | + + | + + + | +/– | +/– |
| C3 | – | + | + | + + | + + + | – | – |
| C9 | – | + | + + | + + | + + + | – | – |
| IgG | + | + + | + + + | + + + | + + + | + | + |
| IgM | – | + | + + | + + | + + + | – | – |
Immune fluorescence intensities of glomerular deposition of complement components C1q, C3 and C9 and IgG and IgM. Intensities scored arbitrarily as −, negative; +/− positive above background; + positive; ++ strongly positive and +++ brightly positive.
Specific detection of mouse antimouse C1q autoantibodies
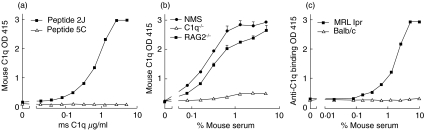
We developed a novel assay to detect anti-C1q autoantibodies reliably in mice utilizing C1q binding peptides to immobilize mouse C1q in ELISA. Immobilized peptide 2J [23] showed a dose-dependent binding of purified mouse C1q, whereas the control peptide 5C did not show binding (Fig. 4a). In addition, both normal mouse serum and serum from Rag 2-/- mice showed dose-dependent binding of C1q, whereas C1q-/- serum was negative (Fig. 4b). Using C1q from Rag 2-/- serum, which was completely devoid of any immunoglobulin, immobilized by peptide 2J, we detected mouse anti-C1q autoantibodies in serum of MRL-lpr mice but not in age-matched non-autoimmune BALB/c mice (Fig. 4c). C1q binding to 2J was not influenced by incubating in 0·5 m NaCl buffer (data not shown), which is necessary to exclude immune complex binding to C1q [28,29].
Fig. 4.
Development of a new ELISA for the detection of mouse antimouse C1q autoantibodies. (a) Purified mouse C1q binds dose-dependently to peptide 2J, whereas it does not bind to control peptide 5C. (b) Mouse serum was used as a source of mouse C1q. NMS, C1q−/− and Rag2−/− sera were tested in dose–response for its ability to deposit mouse C1q onto peptide 2J (c) Antimouse C1q reactivity was determined in sera of autoimmune mice and normal mice by incubation in dose–response using 0·5 m NaCl buffer on a plate coated with 2J and Rag2−/− serum. Data are expressed as OD 415 values.
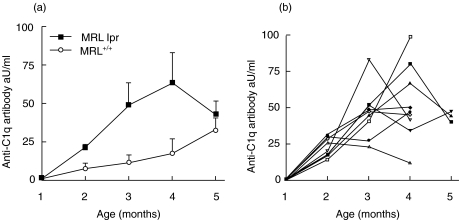
In our experiments, MRL-lpr mice showed an age-dependent increase in anti-C1q autoantibodies (Fig. 5a). Starting at 2 months of age, these mice have elevated anti-C1q. Anti-C1q autoantibodies also occur in MRL+/+ mice but the titre increased later in life. Although all mice showed an initial rise in titre at later time-points, individual mice showed fluctuating anti-C1q titres (Fig. 5b).
Fig. 5.
Anti-C1q autoantibody titres in MRL-lpr and MRL+/+ mice. (a) Anti-mouse C1q reactivity in sera of MRL-lpr and MRL+/+ mice of different ages. (b) Anti-mouse C1q titres in individual mice over time. Data are plotted for individual MRL-lpr mice from the groups that lived up to 4 and 5 months of age. Data are expressed relative to a positive standard serum arbitrarily set at 100 aU/ml.
Correlation between anti-C1q autoantibodies and renal damage
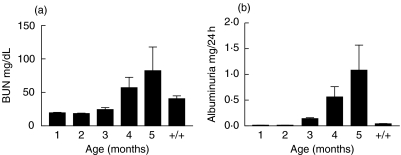
From 3 months of age MRL-lpr mice display progressive renal damage as assessed by measuring albuminuria and loss of renal function as assessed by BUN levels (Fig. 6a,b). MRL+/+ mice had normal renal function and no albuminuria up to 5 months of age. Several studies in patients suggested a correlation between the occurrence of anti-C1q autoantibodies and lupus-nephritis, a rise in the anti-C1q autoantibody titre was suggested to be predictive for a flare in nephritis [12,13]. Therefore we investigated the correlation between levels of anti-C1q autoantibodies and albuminuria as a measure for renal injury in individual mice. Despite the fact that starting from 2 months of age all mice have anti-C1q autoantibodies and all mice develop progressive renal damage we could not detect any correlation between these parameters at any time-point (P = 0·80, n = 18). Also the relative increase in the level of anti-C1q autoantibodies between months 2 and 4 was not correlated with albuminuria (P = 0·61). Finally, albuminuria was not correlated to the levels of anti-DNA and antihistone autoantibodies (P = 0·55 and P = 0·491, respectively), nor a combination of all three antibodies (P = 0·889).
Fig. 6.
Time course of renal function and renal damage in MRL-lpr and MRL+/+ mice. (a) Blood urea nitrogen (BUN) level in sera of MRL-lpr and MRL+/+ mice of different ages. MRL+/+ serum of mice 5 months of age were used as a control. Data are expressed as mg BUN/dl serum, bars show average and s.e.m. (b) Albuminuria measured in urine of MRL-lpr and MRL+/+ mice of different ages. MRL+/+ serum of mice 5 months of age were used as a control, bars show average and s.e.m.
However, we did observe a significant inverse correlation between autoantibody titres and serum C1q for anti-C1q autoantibodies (P = 0·03, n = 24).
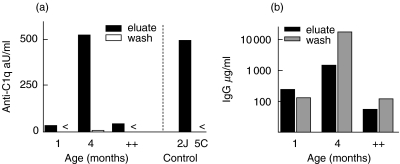
Anti-C1q autoantibodies can be eluted from renal tissue
Kidney cortex of 1 and 4 months old MRL-lpr and MRL+/+ mice were eluted to investigate the presence of anti-C1q autoantibodies in renal tissue. Anti-C1q autoantibodies were present in the eluates, and not in the wash (Fig. 7a). Anti-C1q reactivity was already present in eluates of renal cortex at 1 month of age which was absent in normal BALB/c mice (data not shown). The reactivity markedly increased with age, and exceeded the amount in age-matched MRL+/+ mice. The reactivity was specific for C1q as no binding to an irrelevant coating with peptide 5C and Rag2−/− serum was observed (Fig. 7a, right-hand side). Both wash and eluate contained substantial amounts of IgG (Fig. 7b), but only the eluate contained anti-C1q reactivity, suggesting accumulation of these anti-C1q autoantibodies in the kidney in immune deposits.
Fig. 7.
Renal elution study. (a) Anti-C1q reactivity in both eluate and first washing step of renal cortex of MRL lpr mice of different ages and control MRL+/+ mice of 5 months old. Data are expressed relative to a standard serum arbitrarily set at 100 aU/ml. Specificity controls are shown, the sample of 4 months was retested on a specific coating, 2J and an aspecific coating 5C, both together with Rag2-/- serum. < indicates below the detection limit. (b) Total mouse IgG in both eluate and first washing step of renal material of MRL-lpr mice of different ages and MRL+/+ mice of 5 months old. Data are expressed as µg/ml.
DISCUSSION
Studies in patients with SLE have shown a strong association between the occurrence of anti-C1q autoantibodies and renal involvement and a possible relationship with flares of disease activity [9,10,12,13]. To obtain insight into the relationship of anti-C1q, other autoantibodies and occurrence of renal injury, longitudinal studies were performed in cohorts of mice. Our results indicate that (a) anti-C1q autoantibodies are elevated in MRL-lpr mice at 2 months of age and that the rise in titre parallels that of other autoantibodies such as antihistone and anti-dsDNA; (b) serum C1q declines strongly as the anti-C1q titre rises; (c) both immunoglobulins and complement accumulate in glomeruli; and (d) that anti-C1q autoantibodies already accumulate in immune deposits in the kidney early in life.
To analyse anti-C1q autoantibodies reliably high NaCl conditions [28] should be used to prevent binding of immune complexes to the globular heads [29]. Ideally, homologous C1q should be used, but purification of mouse C1q is costly, results in low yields of around 10% and is often contaminated with mouse IgG, resulting in high background values. This IgG is difficult to remove because it seems to be bound covalently to C1q [30]. Therefore, in the present study we used a peptide, 2J [23], which binds C1q of different species tightly. This peptide, in combination with immunoglobulin-deficient Rag2−/− serum, is an ideal detection method for mouse antimouse C1q autoantibodies.
We found that levels of anti-C1q autoantibodies are already elevated significantly in MRL-lpr mice at 2 months of age. Furthermore, the titre increased over time and was paralleled by general signs of autoimmunity such as antihistone and anti-ds DNA reactivity. Our finding is in line with the observations made in SLE patients that there is a correlation between the presence of anti-C1q autoantibodies and involvement of the kidney [10]; all mice develop anti-C1q autoantibodies and nephritis. Surprisingly, neither anti-C1q nor anti-ds DNA or antihistone antibody titres, or a combination of these three autoantibodies, were correlated with albuminuria at the level of individual mice. Therefore, we propose that one antibody specificity alone is not sufficient to induce disease and that a combination of autoantibodies with different specificities and presumably also non-humoral factors are involved.
Analysis of eluted immune deposits from renal cortex revealed age-dependent accumulation of anti-C1q autoantibodies in the kidneys of MRL-lpr mice at 1 month of age. These observations are in line with the finding of anti-C1q autoantibodies in post-mortem material of end-stage kidneys from patients with lupus nephritis [14]. Because the total IgG content of the immune deposits exceeds the amount of anti-C1q autoantibodies, other autoantibodies may also be present in these glomerular immune deposits. However, the quantity of eluate that we had at our disposal made it impossible to analyse other autoantibodies as well. Although the presence of these autoantibodies does not prove that they are pathogenic, it indicates that they may contribute to initiation of inflammation.
Three possible pathogenic effects of anti-C1q autoantibodies can be envisioned. First, anti-C1q autoantibodies may interfere with solubilization of immune complexes due to reduced levels of C1q in the circulation. This seems unlikely, as C1q−/− MRL-lpr mice develop disease equal to WT controls [31]. Secondly, anti-C1q autoantibodies may react with immune complexes containing C1q, either in the circulation or already present in the kidney. Such a process has been shown before, demonstrating that anti-C1q antibodies can deposit on C1q present in immune complexes or on C1q having a direct interaction with the GBM [32,33]. However, these studies employed human C1q and antihuman C1q antibodies. Thirdly, anti-C1q autoantibodies may interact with C1q directly and target it to the kidney. Indeed, we showed recently that injection of rabbit antimouse C1q antibodies in healthy mice, in the absence of immune complexes, resulted in the glomerular deposition of mouse C1q and antimouse C1q antibodies [24].
C1q-deficient MRL-lpr mice display a renal disease similar to WT MRL-lpr mice [31]. We have tested the presence of anti-C1q autoantibodies in these C1q−/− MRL mice and WT controls and observed anti-C1q reactivity only in WT MRL mice (data not shown). This may be interpreted as an indication that anti-C1q autoantibodies are of limited relevance for the development of nephritis. However, we feel that anti-C1q can exert injury only when it deposits together with C1q in the kidney. Recently we have reported that anti-C1q antibodies deposit in the kidney only if C1q is present in the glomerulus [34]. A similar situation has been observed in C3−/− MRL-lpr mice. Although C3−/− MRL-lpr mice have a renal disease similar to WT MRL-lpr mice [35], systemic complement inhibition, at the level of C3, using Crry-Ig in MRL-lpr mice was shown to protect against renal disease [36–38].
The origin of anti-C1q reactivity is currently unknown. Anti-C1q reactivity is paralleled by antihistone and anti-ds DNA reactivity, suggesting that these autoantigens might elicit a similar immune response or may be presented to the immune system simultaneously. Because C1q binds to apoptotic cells and blebs [6,7], and these blebs are known to contain most autoantigens [39], a general autoimmune response against these apoptotic blebs may also induce anti-C1q reactivity.
Taken together, the present study shows a relation between anti-C1q reactivity and lupus nephritis and demonstrates the accumulation of anti-C1q autoantibodies in glomeruli of MRL mice. Considering the possible pathogenic pathways, we conclude that anti-C1q autoantibodies may be involved in the increased deposition of immune complexes and subsequent amplification of inflammation as observed in lupus nephritis.
Acknowledgments
This work was supported by a grant from the Dutch Kidney Foundation (grant 98·1763). The authors wish to thank Vanessa van Ham, Leiden, the Netherlands, for excellent technical support and Dr J. W. Drijfhout, Leiden the Netherlands, for providing valuable reagents for the present study. Dr J. Cortes-Hernandez, London, UK, is greatly acknowledged for providing reagents.
REFERENCES
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 4.Trouw LA, Roos A, Daha MR. Autoantibodies to complement components. Mol Immunol. 2001;38:199–206. doi: 10.1016/s0161-5890(01)00043-8. [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ, Davies KA, Morley BJ, Botto M. Complement deficiency and autoimmunity. Ann NY Acad Sci. 1997;815:267–81. doi: 10.1111/j.1749-6632.1997.tb52069.x. [DOI] [PubMed] [Google Scholar]
- 6.Nauta AJ, Trouw LA, Daha MR, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–36. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–9. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 8.Siegert CE, Daha MR, van der Halma CV, Breedveld FC. IgG and IgA autoantibodies to C1q in systemic and renal diseases. Clin Exp Rheumatol. 1992;10:19–23. [PubMed] [Google Scholar]
- 9.Monova D, Monov S, Rosenova K, Argirova T. Autoantibodies against C1q: view on association between systemic lupus erythematosus disease manifestation and C1q autoantibodies. Ann Rheum Dis. 2002;61:563–4. doi: 10.1136/ard.61.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegert C, Daha M, van der Westedt MLV, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 11.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–24. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 12.Coremans IE, Spronk PE, Bootsma H, et al. Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis. 1995;26:595–601. doi: 10.1016/0272-6386(95)90595-2. [DOI] [PubMed] [Google Scholar]
- 13.Moroni G, Trendelenburg M, Del Papa N, et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am J Kidney Dis. 2001;37:490–8. doi: 10.1053/ajkd.2001.22071. [DOI] [PubMed] [Google Scholar]
- 14.Mannik M, Wener MH. Deposition of antibodies to the collagen-like region of C1q in renal glomeruli of patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 1997;40:1504–11. doi: 10.1002/art.1780400819. [DOI] [PubMed] [Google Scholar]
- 15.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 16.Theofilopoulos AN, Dixon FJ. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 18.Hogarth MB, Norsworthy PJ, Allen PJ, et al. Autoantibodies to the collagenous region of C1q occur in three strains of lupus-prone mice. Clin Exp Immunol. 1996;104:241–6. doi: 10.1046/j.1365-2249.1996.19725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinder PK, Maeurer MJ, Schorlemmer HU, Loos M. Autoreactivity to mouse C1q in a murine model of SLE. Rheumatol Int. 1995;15:117–20. doi: 10.1007/BF00302128. [DOI] [PubMed] [Google Scholar]
- 20.Uwatoko S, Mannik M, Oppliger IR, et al. C1q-binding immunoglobulin G in MRL/1 mice consists of immune complexes containing antibodies to DNA. Clin Immunol Immunopathol. 1995;75:140–6. doi: 10.1006/clin.1995.1063. [DOI] [PubMed] [Google Scholar]
- 21.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 22.Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 23.Roos A, Nauta AJ, Broers D, et al. Specific inhibition of the classical complement pathway by C1q-binding peptides. J Immunol. 2001;167:7052–9. doi: 10.4049/jimmunol.167.12.7052. [DOI] [PubMed] [Google Scholar]
- 24.Trouw LA, Seelen MA, Duijs JM, Benediktsson H, Van Kooten C, Daha MR. Glomerular deposition of C1q and anti-C1q antibodies in mice following injection of antimouse C1q antibodies. Clin Exp Immunol. 2003;132:32–9. doi: 10.1046/j.1365-2249.2003.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eivazova ER, McDonnell JM, Sutton BJ, Staines NA. Cross-reactivity of antiidiotypic antibodies with DNA in systemic lupus erythematosus. Arthritis Rheum. 2000;43:429–39. doi: 10.1002/1529-0131(200002)43:2<429::AID-ANR25>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 26.Termaat RM, Assmann KJ, van Son JP, Dijkman HB, Koene RA, Berden JH. Antigen-specificity of antibodies bound to glomeruli of mice with systemic lupus erythematosus-like syndromes. Lab Invest. 1993;68:164–73. [PubMed] [Google Scholar]
- 27.Austin HA, III, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–95. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 28.Kohro-Kawata J, Wener MH, Mannik M. The effect of high salt concentration on detection of serum immune complexes and autoantibodies to C1q in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:84–9. [PubMed] [Google Scholar]
- 29.Siegert CE, Daha MR, van der Voort EA, Breedveld FC. IgG and IgA antibodies to the collagen-like region of C1q in rheumatoid vasculitis. Arthritis Rheum. 1990;33:1646–54. doi: 10.1002/art.1780331107. [DOI] [PubMed] [Google Scholar]
- 30.Kaul M, Loos M. Dissection of C1q capability of interacting with IgG. Time-dependent formation of a tight and only partly reversible association. J Biol Chem. 1997;272:33234–44. doi: 10.1074/jbc.272.52.33234. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell DA, Pickering MC, Warren J, et al. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol. 2002;168:2538–43. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 32.Uwatoko S, Gauthier VJ, Mannik M. Autoantibodies to the collagen-like region of C1Q deposit in glomeruli via C1Q in immune deposits. Clin Immunol Immunopathol. 1991;61:268–73. doi: 10.1016/s0090-1229(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 33.Coremans IE, Bruijn JA, de Heer E, van der Voort EAM, Breedveld FC, Daha M. Stabilization of glomerular deposits of C1q by antibodies against C1q in mice. J Clin Laboratory Immunol. 1995;44:47–61. [Google Scholar]
- 34.Trouw LA, Duijs JMGJ, Van Kooten C, Daha MR. Immune deposition of C1q and anti-C1q antibodies in the kidney is dependent on the presence of glomerular IgG. Mol Immunol. 2003;40:595–602. doi: 10.1016/j.molimm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Sekine H, Reilly CM, Molano ID, et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol. 2001;166:6444–51. doi: 10.4049/jimmunol.166.10.6444. [DOI] [PubMed] [Google Scholar]
- 36.Bao L, Haas M, Boackle SA, et al. Transgenic expression of a soluble complement inhibitor protects against renal disease and promotes survival in MRL/lpr mice. J Immunol. 2002;168:3601–7. doi: 10.4049/jimmunol.168.7.3601. [DOI] [PubMed] [Google Scholar]
- 37.Bao L, Haas M, Kraus DM, et al. Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. J Am Soc Nephrol. 2003;14:670–9. doi: 10.1097/01.asn.0000051597.27127.a1. [DOI] [PubMed] [Google Scholar]
- 38.Bao L, Zhou J, Holers VM, Quigg RJ. Excessive matrix accumulation in the kidneys of MRL/lpr lupus mice is dependent on complement activation. J Am Soc Nephrol. 2003;14:2516–25. doi: 10.1097/01.asn.0000089831.96794.0b. [DOI] [PubMed] [Google Scholar]
- 39.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]