Abstract
We used a GAD65-specific human B–T cell line cognate system in vitro to investigate the modulation of GAD65 presentation by autoantibody, assessed in a proliferation assay. Generally, if the T cell determinant overlaps or resides within the antibody epitope, effects of presentation are blunted while if they are distant can lead to potent presentation. For three different autoreactive B–T cell line cognate pairs, the modulation of GAD65 presentation followed the mode of overlapping or distant epitopes with resultant potent or undetectable presentation. However, other cognate pairs elicited variability in this pattern of presentation. Notably, one B cell line, DPC, whose antibody epitope did not overlap with the T cell determinants, was consistently poor in presenting GAD65. Using the fluorescent dye Alexa Fluor 647 conjugated to GAD65 to study receptor-mediated antigen endocytosis showed that all the antigen-specific B cell clones were efficient in intracellular accumulation of the antigen. Additionally, multicolour immunofluorescence microscopy showed that the internalized GAD65/surface IgG complexes were rapidly targeted to a perinuclear compartment in all GAD-specific B cell clones. This analysis also demonstrated that HLA-DM expression was reduced strongly in DPC compared to the stimulatory B cell clones. Thus the capability of antigen-specific B cells to capture and present antigen to human T cell lines is dependent on the spatial relationship of B and T cell epitopes as well other factors which contribute to the efficiency of presentation.
Keywords: autoimmunity, antigen-specific B cells, GAD65, HLA-DM, T cells
INTRODUCTION
Type I diabetes is characterized by the presence of a lymphocytic infiltrate in the islets of Langerhans leading to the destruction of the insulin-producing β-cells. The disease is also accompanied by the presence of autoantibodies to glutamic acid decarboxylase 65 (GAD65) [1]. The antibodies to GAD65 in new onset type I diabetic patients are present at low titres and almost all recognize conformational determinants. A strong anti-GAD65 antibody response is also present in other autoimmune disorders, such as stiff man syndrome (SMS) and autoimmune polyglandular syndrome type 2 (APS 2), but the dominant epitopes differ from those in type I diabetes [2]. The susceptibility to develop type I diabetes is associated in Caucasian individuals with HLA-DR4 (DRB1*0401)/DQ8(DQB1*0302) and -DR3(DRB1*0301)/DQ2(DQB1*0201) haplotypes [3]. T cell lines to naturally processed determinants of GAD65 have been characterized from autoimmune disease patients [4–6] and HLA class II transgenic mice [7,8], leading to the identification of multiple DRB1*0401 restricted T cell determinants.
GAD65 is known to exist as a dimer [1]. Although the three-dimensional structure of GAD65 dimer is currently not known, homology-based models have been developed recently [9,10]. The modelling studies of each GAD65 monomer (of 585 amino acids) reveal that the middle (residues 201–460) and the carboxyl-terminal (residues 461–585) regions form independent folding domains [9,10]. Furthermore, although the amino-terminal region (residues 1–200) of GAD65 was not modelled, it was considered to form a separate independent domain, with the result that the predicted structure of GAD65 may comprise three independent folding domains [9]. The development of autoreactive B cell clones secreting human monoclonal IgG antibodies to GAD65 from a variety of patients with autoimmunity [11–14] has led to the fine mapping of the conformational determinants on the antigen [9,10,15].
While CD4 and CD8 subsets of T cells have been considered to be essential in the destruction of the islet β-cells, the role of autoantibodies in type I diabetes has not been clear. In the spontaneous animal model of type I diabetes in non-obese diabetic (NOD) mice, B cell-deficient animals are protected from developing islet cell destruction [16–18], whereas skewing of the B cell repertoire to islet cell autoimmunity in transgenic mice rapidly precipitates β-cell destruction [19]. In contrast, there is also evidence against the essential role of B cells in islet cell destruction in humans, where in one patient severe depletion of B cells was followed nevertheless by the development of type I diabetes [20]. Surface IgG on B cells can capture efficiently low doses of antigen present in inflammatory infiltrates which, after internalization by receptor-mediated uptake to late endosomal compartments, can influence proteolytic processing to facilitate the generation of subdominant or cryptic T cell epitopes responsible for triggering autoimmunity [21–24]. In the endosomal compartments, peptide loading to MHC class II is assisted by the molecular chaperone, HLA-DM, whose activity is modulated by HLA-DO [25].
It has been established that antibodies to GAD65 in sera from new onset type I diabetics, when complexed with antigen and presented by irradiated peripheral blood mononuclear cells (PBMCs), can enhance the presentation of the GAD65 p274–286 epitope to a DRB1*0401 restricted hybridoma [26]. More recent studies, using a panel of T cell hybridomas derived from DRB1*0401 transgenic mice together with autoreactive, EBV-immortalized B cell lines from type I diabetic patients, showed a remarkable relationship between the epitopes on GAD65 and T cell presentation of the antigen. T cell determinants distant from the antibody epitope led to potent presentation while those that overlapped with the antibody led to undectectable responses [27]. This effect was also seen when soluble immune complexes were presented by spleen cells as antigen-presenting cells (APCs) from humanized, MHC class II transgenic mice [27]. Using the above panel of GAD-specific B cells as APCs, we have examined antibody modulation of GAD65 presentation to DRB1*0401 restricted human T cell lines. We report that using human autoreactive B–T cell cognate pairs also leads to potent or undectectable presentation, depending on the topography of the epitopes on GAD65, but there are also variations. In particular, one autoreactive B cell clone was highly inefficient in presentating GAD65 to all the T cell lines, which may be related to differences in intracellular co-localization of internalized antigen/antibody complexes and HLA-DM-containing compartments.
METHODS
Human autoreactive B cell lines
EBV immortalized B cell lines DPA (γ1, λ), DPC (γ1, λ) and DPD (γ1,κ) to GAD65 from a HLA-DR3, DR4 (DRB1*0301/0401-DRB3*0101-DRB4*0103-DQB1*0201/0302-DPB1*0401/0402) new onset (adult) type I diabetic patient specific for GAD65 have been described [12]. For control, another B cell line DS389 (γ1, λ) derived from a HLA-DR1, -DR4 (DRB1*0101/0401-DQB1*0501/0302) new onset (adolescent) type I diabetic patient, positive for antibodies to the protein tyrosine phosphatase antigen IA-2 but negative for antibodies to GAD65, were used. The IgG secreted by DS389 cells did not show any ICA activity nor any binding in immunoprecipitation assays to GAD65, GAD67, IA-2 or IA-2β (unpublished). All B cell lines were maintained in RPMI medium (Sigma-Aldrich, Dorset, UK) supplemented with 10% FCS (Sigma-Aldrich), 2 mmol/l glutamine, 100 µg/ml of penicillin G, 100 µg/ml of streptomycin sulphate and 0·25 µg/ml of amphotericin B as fungizone (Invitrogen, Inchinnan, UK). All the B cell clones grow as small clusters in suspension and tested free of mycoplasma infections.
Human T cell lines to GAD65
Three T cell lines (TCLs), termed TCL6/7, TCL15/1 and TCL 15/3, specific for DRB1*0401 restricted determinants of GAD65 were used. TCL 6/7 was derived from a HLA-DR3, -DR4 (DRB1*0301/0401, DQA1*0501/0301, DQB1*0201/0302) new onset type I diabetic patient, and specific for residues p270–283 of human GAD65 [4]. TCL 15/1 and TCL 15/3 were generated by a similar protocol [4] from a normal, healthy -DR4 control individual, homozygous for HLA-DRB1*0401, DQB1*0301/0302 and specific for residues p106–125 and p556–575 of GAD65, respectively (J. Endl, unpublished). All the TCLs were maintained in RPMI 1640 medium supplemented with 10% human pooled AB positive serum (Sigma-Aldrich) (RPMI/AB medium).
GAD65 antigen
Two different preparations of recombinant human (rh) GAD65 purified from insect cell cultures and suitable for T stimulatory assays were used [4,5,28]. One of these (tagged with histidine at the amino terminus) was purified by metal chelation chromatography and stabilized with pyridoxal phosphate as described [5]. The second was purchased commercially (Diamyd Medical, Sweden); the method of purification has not been disclosed. TCL 6/7 responds to both the preparations of GAD65 (but all studies reported for TCL 6/7 herein were conducted using the former preparation), while TCL15/1 and TCL 15/3 respond exclusively to the latter preparation [28]. Prior to use in T cell stimulatory cultures, the purified GAD65 was supplemented with 1% human serum albumin and dialysed overnight with cold RPMI-1640. Synthetic peptides of GAD65, p115–125, p270–283 and p556–575 [4] were used to stimulate the T cell lines.
Antigen presentation assays
These were set up in 96-well U-bottom plates (Nunc) as described [4]. As APCs, PBMC derived from unrelated HLA-DR4 (DRB1*0401, DQB1*0301) type I diabetic patients or healthy controls were used for restimulation and maintenance of the T cell lines. Prior to use in proliferation assays, the TCLs were stimulated with the appropriate source of dialysed GAD65 (5 µg/ml) and expanded in recombinant human IL-2 (10 U/ml) (Roche Diagnostics, Sussex, UK). Antigen-pulsed APC were used for stimulating the T cells by pulsing thawed and washed APCs with dialysed GAD65 (1–100 nm) for 3 h at 37°C, followed by irradiation at 3 Gy. For proliferation assays, rested T cells (1·5 × 104) were added to 7·5 × 104 prepulsed, irradiated PBMC as APCs (final ratio 5 : 1 of APCs : responder T cells) and incubated for 72 h at 37°C. For presentation by the B cell clones, 1·5 × 104 rested T cells were cultured with 1 × 104 prepulsed B cells irradiated at 4 Gy (final ratio 1 : 1·5 of APCs : responder T cells). After 72 h cells were pulsed with 1 µCi [3H]-thymidine and harvested 16–18 h on glass fibre filters and the radioactivity incorporated determined by direct beta plate counting in a Matrix 9600 counter (Packard Instruments, Pangbourne, UK).
For experiments dealing with soluble DPC antibody–GAD65 immune complexes, a suboptimal dose of GAD65 was determined first by stimulating TCL 6/7 with increasing concentrations of antigen alone. Subsequently, immune complexes were prepared by adding different amounts of purified DPC IgG to a constant predetermined, suboptimal concentration of GAD65 and the immune complexes formed by incubation for 1 h at 37°C. PBMC as APC were pulsed with each concentration of complexes; in parallel, APC were also pulsed with the same concentration of GAD65 alone and after incubation for 3 h at 37°C, the cells were irradiated, added to 1·5 × 104 T cells and proliferation measured after 72 h, as described above.
Endocytosis of GAD65 into B cells
Purified recombinant GAD65 (100 µg) was labelled with Alexa Fluor 647 NHS ester, according to the instructions in the commercial kits (Molecular Probes Europe BV, the Netherlands). For determination of binding of the conjugate to surface IgG, 107 B cells were resuspended in 1 ml complete RPMI medium containing different concentrations of Alexa Fluor-GAD65 (1 and 5 µg/ml) and incubated for 30 min at 4°C. After washing the cells in ice-cold phosphate buffered saline (PBS) containing 5% fetal calf serum (FCS), the cells were resuspended in 0·5 ml of cold PBS/FCS and the fluorescence measured in a FACSCalibur instrument. All studies were performed in duplicate. The results were expressed as mean fluorescence intensity (MFI) using CellQuest software. For assessing uptake via surface IgG, Alexa Fluor-GAD65 was added (1 µg/ml) to the medium containing the B cell lines followed by incubation of the cells at 37°C. At different time points, 100 µl aliquot of cells were removed, washed once in ice-cold PBS/FCS and after resupension, the fluorescence measured as described above.
To measure receptor-mediated endocytosis and fluid phase uptake simultaneously, Alexa Fluor Hydrazide 488 (1 µg/ml) and Alexa Fluor-GAD65 (1 µg/ml) were added to 107 B cells in 2 ml complete RPMI medium at 37°C. After washing, the fluorescence was measured in a FACSCalibur instrument using FL1 channel for Alexa Fluor Hydrazide 488 and FL4 channel for Alexa Fluor-GAD65. For inhibition experiments with unlabelled GAD65, the cells were resuspended at 0·5 × 106 cells/ml in 2 ml complete RPMI, followed by the addition of 1 µg/ml of Alexa Fluor-GAD65. At the same time, unlabelled, purified GAD65 was added at 0, 5, 10 and 20 µg/ml and incubated for 5 h at 37°C. After washing with ice-cold PBS/FCS, the fluorescence was measured as described. The results are expressed as percentage MFI, with 100% being represented by the binding of the Alexa Fluor-GAD65 in the absence of any competitor.
Anti-IgG inhibition
The effect of anti-IgG on receptor mediated uptake of GAD65 was evaluated by incubating washed B cell cultures at 0·5 × 106/ml in complete RPMI with varying concentrations of polyclonal anti-human Ig (IgA + IgG + IgM) (Jackson ImmunoResearch, Stratech, Luton, UK) at 0, 5, 10 and 20 µg/ml for 30 min at 4°C. To this was added Alexa Fluor 647-GAD65 (1 µg/ml) and Alexa Fluor Hydrazide 488 (1 µg/ml) for 2 h at 37°C. After washing, the fluorescence was measured as described. In other experiments, the effect of blocking surface IgG on T cell antigen presentation was performed as described previously [16,29]. The B cell lines were incubated with varying doses of affinity purified F(ab′)2 anti-human IgG F(ab′)2 fragment (Jackson ImmunoResearch) (0–240 µg/ml) in ice-cold RPMI/AB medium and incubated on ice for 30 min, before being prepulsed with suboptimal dose of 1 µg/ml GAD65 or 1 µm synthetic peptide for 3 h at 37°C. After fixation in 0·025% glutaraldehyde for 2 min at room temperature, the cells were washed three times in warm complete medium and used immediately as APCs for presentation to TCL 6/7.
Multicolour immunofluorescence to determine GAD65 uptake into endosomal compartments
Purified GAD65 was labelled with Alexa Fluor 555 Hydrazide NHS according to the instructions in the labelling kits (Molecular Probes) and used in fluorescence microscopy for detection of the internalized antigen. Log phase B cell cultures were washed in ice-cold PBS/FCS and resuspended in complete RPMI at 0·4 × 106/ml, followed by addition of Alexa Fluor 555-GAD65 (1 µg/ml) and cultured at 37°C from 5 to 120 min. Aliquots of samples were removed at the indicated time periods, washed once in ice-cold PBS/FCS, resuspended in 100 µl cold PBS and cytocentrifuged onto poly-l-lysine-coated slides. The slides were fixed subsequently in 4% paraformaldehyde in PBS at 37°C for 20 min, washed with PBS and the cells permeabilized in 1% NP40/PBS for 30 min at room temperature. After washing, the cells were stained with monoclonal anti-HLA-DM antibody (BD Biosciences Pharmingen) at 10 µg/ml for 45 min at room temperature. After washing once in PBS, the cells were stained with Cy 5-labelled polyclonal anti-mouse IgG Fab (BD Biosciences Pharmingen) at a final dilution of 1 : 70. At the same time, the slides were also stained with Alexa Fluor 488 labelled polyclonal anti-human IgG (Molecular Probes) at 1 : 100 dilution to identify IgG in the B cells. After washing once in PBS at room temperature, the slides were stained for nuclear DNA with Dapi (1 µg/ml) (Sigma-Aldrich) for 2 min, washed and mounted for examination using a Zeiss Axiophot Fluorescence microscope and appropriate filters. The fluorescent images were recorded using a digital camera and Metamorph software.
Flow cytometry analysis
The expression of sIgG, MHC class II and co-stimulatory molecules CD80 and CD86 was assessed by FACScan flow cytometry using CellQuest software. Cell suspensions (2–3 × 105 cells) were incubated with appropriately diluted monoclonal antibodies (MoAbs) to HLA-DR4 (NFLD.D1), CD19 (BU12) and CD86 (BU63), washed and stained with FITC anti-mouse IgG (Serotec, Oxford, UK). Cells were then washed, fixed in CellFIX (BD Biosciences Pharmingen, Cowley, UK) and analysed on the flow cytometer. In the analysis, dead cells and debris were excluded from live cells on the basis of forward- and side-scatter parameters. Staining for CD80 was performed by direct staining using FITC-anti CD80 (BD Biosciences Pharmingen). For determining expression levels of sIgG, the washed B cell clones were incubated with biotin-conjugated, affinity-purified goat anti-human Ig (IgA + IgG + IgM) (0·2 µg) (Southern Biotechnology, Cambridge Bioscience, Cambridge, UK) on ice and washed as above, and then incubated with strepavidin–phycoerythrin conjugate (1 : 10) (BD Biosciences Pharmingen) for analysis.
Measurement of antibody avidity
The affinity constants (KD) of DPA, DPC and DPD human MoAbs was determined by inhibition enzyme-linked immunosorbent assay (ELISA) test method [30], using the same preparation of the purified GAD65 used for the T cell presentation studies. The dimer molecular size of GAD65 (120 000 Da) was used in the calculations [1].
RESULTS
Epitopes recognized by B cell lines on GAD65 and overlap with T cell determinants
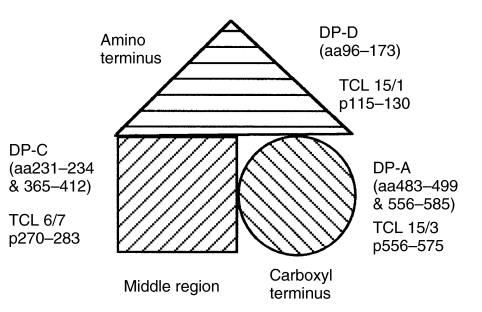
Although the three-dimensional structure of GAD65 is not known, homology-based models have been recently developed, which reveal that the middle (residues 201–460) and the carboxyl-terminal (residues 461–585) regions form independent folding domains [9,10]. The EBV-immortalized, GAD65-specific monoclonal B cell clones DPA, DPC and DPD recognize three independent, conformational determinants on GAD65 [9]. Detailed fine mapping shows that the epitopes for DPA were dependent on residues 483–499 and 556–585, DPC on residues 231–234 and 365–412 and DPD on residues 96–173 of GAD65 [9] (Fig. 1). Interestingly, the epitope of DPA overlaps with that recognized by TCL 15/3 (p556–575), DPD overlaps with TCL 15/1 (p115–130), while the epitope for DPC does not overlap with any of the TCLs (Fig. 1). Antigen presentation by the non-GAD specific control cell line DS389 cells would occur only by non-specific, fluid phase uptake, which requires high concentrations of GAD65. In contrast, in the GAD-specific B cell clones, a highly efficient internalization of antigen by receptor-mediated endocytosis would lead to increased uptake of small doses of antigen for T cell presentation. Depending on whether the antibody epitope was distant or overlapped with the T cell determinant, that could lead to either potent or undetectable presentation, respectively, of the receptor internalized antigen.
Fig. 1.
Schematic diagram of a simplified, structural domain model of GAD65 (from [9,10]), showing the epitopes for the human MoAbs DPA, DPC and DPD on the carboxyl, middle and amino terminal regions, respectively. The location of the DRB1*0401-restricted T cell determinants for TCL 15/3, TCL 6/7 and TCL 15/1 are also indicated.
Presentation of GAD65 by antigen-specific EBV- B cell clones
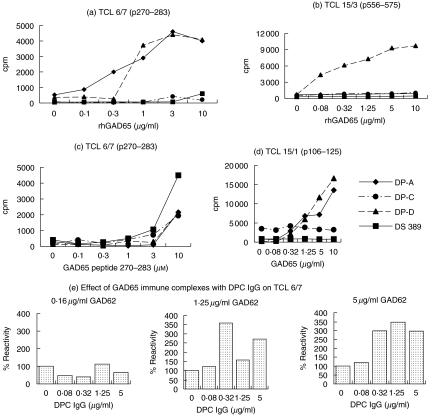
Presentation of GAD65 by the GAD-specific B cell clones to the TCLs gave striking differences (Fig. 2). With TCL 6/7 and TCL 15/3, B cells recognizing distant regions of GAD were the most potent (Fig. 2a,b). Presentation of peptides was broadly similar for all the GAD-specific B cell clones, indicating similar costimilatory activity (Fig. 2c). Moreover, with TCL 15/1, the DPD cells specific for the same N-terminal domain also presented very well (Fig. 2d). Presentation was consistently undetectable by the B cell clone (DS389) without GAD-specificity (Fig. 2).
Fig. 2.
Differential presentation by antigen-specific B cell clones, DPA, DPC and DPD of GAD65 to DRB1*0401-restricted T cell lines, measured in a proliferation assay by [3H]-thymidine incorporation. As control, a non-antigen-specific B cell clone, DS389 developed from a DRB1*0401 type I diabetic patient (with no islet cell reactivity) was used. (a) Presentation of the p270–283 epitope of TCL 6/7 by the B cell clones. In greater than five experiments with presentation of GAD65 by DPA and DPD cells, there has been a consistent enhancement of T cell stimulation in comparison to DPC and DS389 cells. (b) Presentation of the p556–575 epitope of TCL 15/3 by the B cell clones. (c) Presentation of the peptide p270–283 by DPA, DPC, DPD and DS389 cells. The experiment with all the four B cell clones was repeated twice with similar results. (d) Presentation of the p106–125 epitope of TCL 15/1 by the B cell clones. The T cell presentation experiments by the autoreactive and DS389 cell lines were repeated three times with similar results. All proliferation assays were conducted in triplicate cultures, but for simplicity the s.e.m. bars are not shown. The symbols of each of the B cell clones are shown in (d). (e) Soluble immune complexes at suboptimal concentrations of GAD65 and purified DPC antibody led to potentiation of presentation at 1·25 and 5·0 µg/ml GAD65 to TCL 6/7 using PBMC as APCs. The proliferative response (average cpm of triplicate cultures) in the presence of the antigen GAD65 and no DPC antibody (100% values) were 597cpm at 0·16 µg/ml GAD65, 1262cpm at 1·25 µg/ml GAD65 and 2092cpm at 5 µg/ml GAD65. The experiment was performed twice with broadly similar results.
Interestingly, the DPC B cell clones failed consistently to present GAD65 to any of the T cells, even though its epitope does not overlap theirs (Fig. 2). In stark contrast, the DPD clones presented very well to all three T cells, although its 96–173 epitope does overlap that of TCL 15/1 (106–125).
The results indicate that there is considerably more complexity in the presentation of GAD65 by the autoreactive B cells as APCs than predicted from the mapping of the antibody and T cell epitopes on the structural model of the antigen [9,10]. To explore whether the poor presentation by DPC cells was due to effect of the antibody complexed to GAD65 on processing of all the three determinants, preformed soluble immune complexes of GAD65 and purified DPC antibody were prepared with suboptimal antigen concentrations in different ratios of DPC antibody and used to pulse APCs (macrophages and dendritic cells in PBMC) from a DRB1*0401 donor. The pulsed APCs were added to TCLs for assessing proliferative responses. Representative experiments of presentation of soluble complexes to TCL 6/7, at 0·16, 1·25 and 5 µg/ml GAD65 immunocomplexed with varying concentrations of purified DPC antibody are shown in Fig. 2e. Immune complexes of GAD65 with purified DPC IgG enhanced presentation of TCL 6/7 (by 300–350%) compared to antigen alone (Fig. 2e). Thus, at least in professional APCs such as dendritic cells and macrophages, binding of DPC antibody to GAD65 did not interfere with antigen-processing via FcR.
Flow cytometry analysis of surface molecules on B cell lines
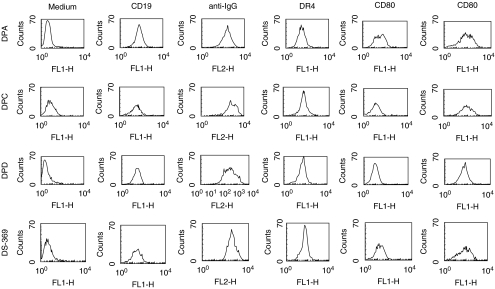
Although the GAD65-specific DP clones presented the respective synthetic peptides of GAD65 efficiently to the TCLs, to eliminate the possibility that the differences in presentation of GAD65 between DPC cells and that observed with DPA and DPD cells was related to differences in expression of surface IgG, MHC class II or the B7 family of co-stimulatory molecules, we examined these parameters by flow cytometry. All the GAD65-specific B cell lines showed positive staining with the B cell marker, CD19 together with similar levels of expression of surface IgG, parallel to that of the non-antigen-specific DS389 cell line (Fig. 3). Similarly, there were no dramatic differences in the overall surface expression of HLA-DR4, CD80 and CD86 between the different B cell lines.
Fig. 3.
Quantification of cell surface marker expression by flow cytometry in the autoreactive DPA, DPC, DPD and the control DS389 B cell lines. The phenotypic markers tested include CD19, sIgG, HLA-DR4 and the co-stimulatory molecules CD80 and CD86. The experiment was repeated more than three times in two different laboratories (JPB and JE) with similar profiles.
Affinity constants (KD) of anti-GAD65 human monoclonal antibodies
To explore whether the differences in the T cell presentation were related to the antigen-capture on the membrane surface, the avidity of the secreted IgG from the autoreactive B cell lines for GAD65 was measured by inhibition ELISA test [30]. Both DPA and DPC human monoclonal antibodies showed similar high affinity for the antigen, with affinity constants of 0.11 nM and 0.31 nM respectively, whilst DPD antibody showed a lower affinity constant of 2.79 nM (not shown). These results are similar to the reported affinities for these human DP antibodies determined by surface plasmon resonance on BIAcore [27].
Binding and internalization kinetics of GAD65 in B cell clones
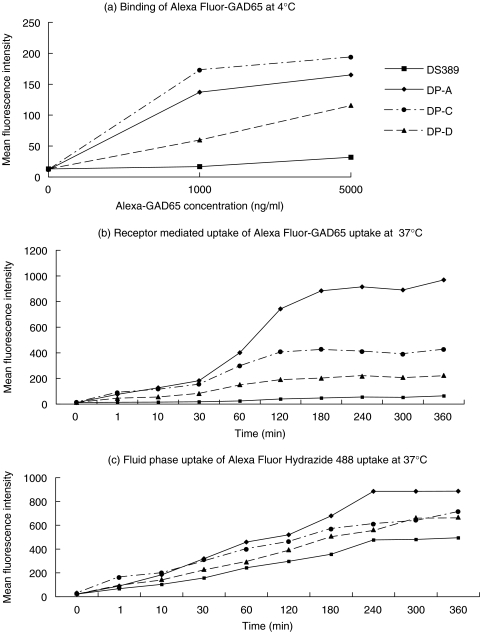
Although all three GAD-specific B cell clones showed similar levels of expression of surface IgG (sIgG), we determined their ability to bind and internalize Alexa Fluor-GAD65 by receptor-mediated endocytosis. At 4°C, DPA and DPC cells showed a simi-lar dose-dependent binding pattern, while DPD cells displayed reduced binding, probably as a result of lower avidity even though it presented GAD65 very well to all three T cells. In contrast, the DS389 cells showed negligible binding (Fig. 4a). To exclude the possibility that conjugation of Alexa Fluor 647 to GAD65 may lead to inactivation of some of the antibody binding sites, we also performed the experiments using unlabelled GAD65, followed by rabbit polyclonal anti-GAD65 antiserum and PE-labelled antirabbit Ig, which showed identical data as above (not shown).
Fig. 4.
Receptor-mediated endocytosis and fluid phase uptake by the autoreactive B cell lines. (a) Binding of Alexa Fluor-GAD65 at 1 µg/ml to the GAD-specific B cell clones at 4°C. (b) Internalization of the Alexa Fluor-GAD65 (1 µg/ml) at 37°C. (c) Assessment of fluid phase uptake by the B cell lines using the fluorescent dye, Alexa Fluor hydrazide 488 (1 µg/ml; this corresponds to a 200-fold molar excess compared to the GAD65 concentration used in (b).
To study receptor-mediated internalization, the B cell clones were incubated with Alexa Fluor-GAD65 at 37°C. At different time-points, aliquots were washed briefly at 4°C and the fluorescence measured. All three GAD-specific B cell clones internalized Alexa Fluor-GAD65, but at different efficiencies (Fig. 4b). In particular, DPA and DPC cells showed a twofold difference in their uptake of antigen, although the antibody affinities are similar (Fig. 4b). Uptake was lower still with DPD cells, which were the most potent presenters, and almost undectable with the control DS389 cells (Fig. 4b).
We also studied fluid phase uptake by the B cell clones using the fluorescent label, Alexa Fluor Hydrazide 488. Using this low molecular weight endocytosis marker at equimolar quantities to labelled GAD65, no uptake could be measured (not shown). Only when the concentration of the Alexa Fluor Hydrazide 488 was increased 200-fold could we measure uptake of the fluorescence label (Fig. 4c). This underlines the extreme efficiency of sIgG receptor-mediated uptake compared to fluid phase endocytosis. In sum, the results show that all antigen-specific B cells can internalize native antigen by receptor-mediated endocytosis. In particular, DPC is more efficient in receptor-mediated uptake than DPD, which presented GAD65 far better to all the three T cells, making it unlikely that differences in uptake are the explanation for the poor T cell presentation of GAD65 by DPC cells.
Proof of sIgG-mediated uptake of GAD65 by GAD-specific B cell clones
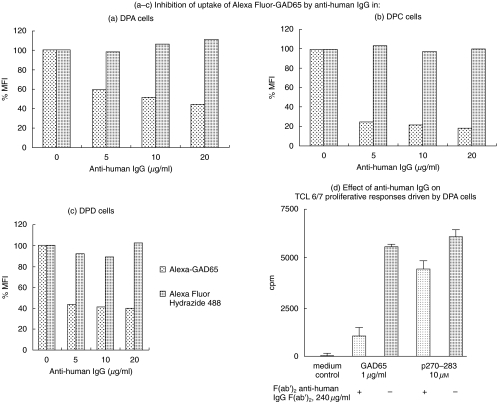
We next confirmed that the sIgG specific for GAD65 in DPA, DPC and DPD cells was responsible for binding and internalization of the labelled antigen. Polyclonal anti-human IgG antibody blocked, in a dose-dependent manner, the binding and uptake of the antigen (Fig. 5a–c). Moreover, it also inhibited T cell stimulation [16,29] when anti-Ig blocked DPA cells were prepulsed with a suboptimal dose of GAD65 or the stimulatory GAD65 peptide p270–283 for 3 h at 37°C and then fixed in glutaraldehyde. There was a dramatic reduction in the proliferative response of TCL 6/7 (>80%) when anti-Ig blocked DPA cells pulsed with GAD65 were used as APCs (Fig. 5d). In contrast, the inhibition observed when peptide was presented by anti-Ig blocked DPA cells was less (<30%). Similar blocking was seen with DPD cells, but had no effect on presentation by DS389 cells (not shown). These results demonstrate clearly the crucial role of sIgG in DPA and DPD cells in mediating the enhanced presentation of GAD65.
Fig. 5.
(a–c) Surface IgG on the autoreactive B cell clones is responsible for binding and internalization of GAD65 and (d) efficient presentation of the endocytosed antigen to TCLs. Inhibition of binding of Alexa Fluor-GAD65 (1 µg/ml) with polyclonal anti-human IgG antibody in (a) DPA cells, (b) DPC cells and (c) DPD cells. The results are expressed as percentage mean fluorescence intensity (MFI), with 100% being represented by the binding of the Alexa Fluor-GAD65 in the absence of any competitor. (d) Inhibition by DPA cells as APC for presentation of GAD65 to TCL 6/7 by antihuman IgG F(ab′)2, demonstrating the crucial importance of surface IgG in binding the antigen for internalization and processing. The data represent the results of three experiments. The error bars show the standard error mean (s.e.m.) of triplicate cultures.
Co-localization of IgG, GAD65 and HLA-DM in the B cell clones
Immunofluorescence microscopy of fixed, antigen-specific DPA, DPC and DPD cells and the control cells, DS389 loaded with Alexa Fluor 555-GAD65 and stained for IgG and HLA-DM, was performed to follow the fate of GAD65 internalized by receptor-mediated endocytosis. For this purpose, B cell clones were incubated for 5–120 min at 37°C with Alexa Fluor 555-GAD65; uptake was terminated by washing the cells in ice-cold PBS. The cells were then fixed and permeabilized followed by staining for surface and intracellular IgG (depicted in green), HLA-DM (in blue) and GAD65 (in red) (Fig. 6).
Fig. 6.
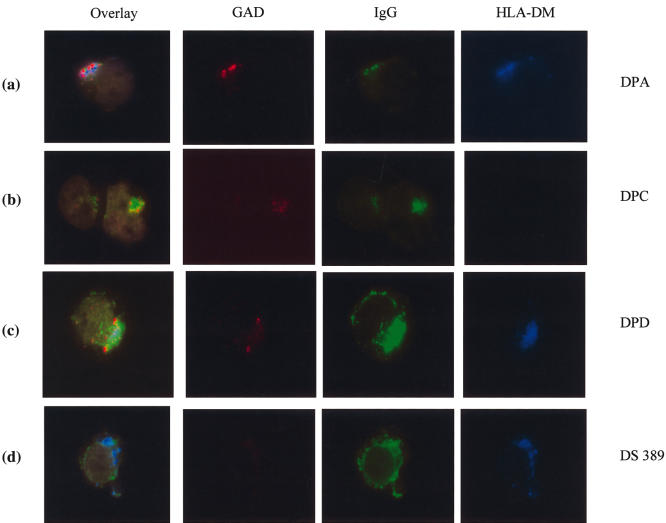
Co-localization of GAD65, IgG and HLA-DM in antigen-specific B cell clones by fluorescent microscopy, to follow the fate of GAD65 internalized by receptor-mediated endocytosis. This was performed by continuous incubation of the B cell clones with Alexa Fluor 555-GAD65 for various periods (5–120 min) at 37°C, with the uptake terminated by washing the cells in ice-cold PBS. The cytospin preparations of the cells were fixed and permeabilized followed by staining for GAD65 (depicted in red), IgG (in green), and HLA-DM (in blue). Represented are the staining patterns obtained after an incubation of 120 min. DPA [row (a)] and DPD cells [row (c)] showed a strong perinuclear clustering of Alexa Fluor 555-GAD65, together with IgG and HLA-DM, while DPC cells also accumulated GAD65/IgG complexes in this compartment which was, however, devoid of HLA-DM [row (b)].
After 5 min loading with Alexa Fluor 555-GAD65 at 37°C, all three antigen-specific B cell clones showed binding to sIgG, which was absent in the control DS389 cells (data not shown). At the same time, there was a weaker staining to sIgG of DPD cells, due presumably to the lower avidity of the antibody for GAD65 (not shown). After a longer incubation of 120 min, DPA, DPC and DPD cells showed a strong perinuclear clustering of GAD65. Moreover, merging the optical images showed that GAD65, IgG and HLA-DM co-localized in perinuclear patches in DPA and DPD cells [Fig. 6, rows (a) and (c), respectively]. In contrast, in DPC cells only GAD65 and IgG could be detected in perinuclear patches, but no HLA-DM [Fig. 6, row (b)]. The control DS389 cells showed a similar perinuclear clustering of HLA-DM to the antigen-specific DPA and DPD cells, but there was no detectable uptake of GAD65 in these cells at 120 min incubation [Fig. 6, row (d)]. An examination of HLA-DM staining in a larger number of cells revealed much weaker or undetectable HLA-DM staining in DPC than in DPA, DPD and the control DS389 cells (Fig. 7). Taken together, this analysis shows strongly reduced expression levels of HLA-DM in DPC cells compared to the other B cell clones used in this study.
Fig. 7.
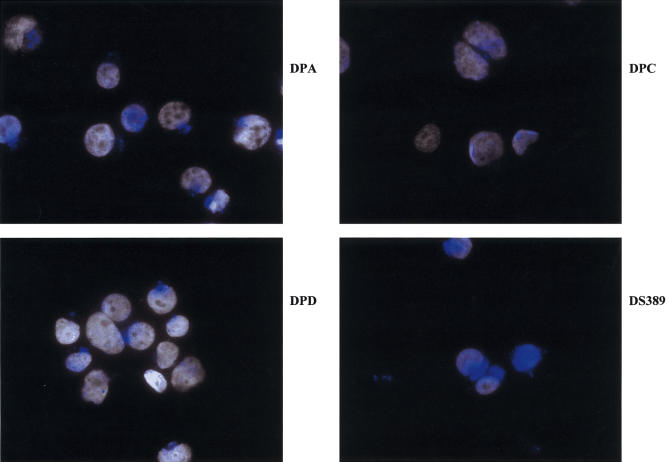
Unique intracellular distribution of HLA-DM in DPC cells in comparison to DPA, DPC and the control DS389 cells after incubation with GAD65 for 60 min. The nuclei are stained with Dapi and HLA-DM staining is shown in blue. While in the GAD-specific B cell clones, DPA and DPD the HLA-DM is enriched strongly in small perinuclear clusters, in DPC it is expressed diffusely in numerous small vesicles spread over the whole cytoplasm. Note also the perinuclear localization of HLA-DM in the control B cell clone, DS389, but the vesicles are less compact than in DPA and DPD cells.
DISCUSSION
We have used a human in vitro cognate system to study autoantigen presentation by GAD65-specific, EBV-immortalized B cell clones established from type I diabetic patients to specific T cells. The studies show that presentation of GAD65 by some B cell–T cell pairs followed the topographical relationship of overlapping and distant antibody and T cell determinants on the structural model of the antigen to modulate T cell presentation, but great variability was also apparent in other pairs. In some combinations, presentation of GAD65 was ∼100-fold more potent than of GAD peptides, especially when the antibody and T cell epitopes did not overlap. In other combinations presentation was undetectable; in two of these cases, the epitopes did overlap but, in a third (TCL 15/1), similar overlap did not prevent potent presentation by DPD. These results suggest that either the antibody epitope of DPD excludes amino acids 106–125, or that its approximate 10-fold lower affinity [27] leads to rapid dissociation of the GAD65/antibody complexes in the acidic endosomal compartments and efficient processing of the released GAD65.
These data are consistent in part with the recent report using the same B cells as APCs, but paired with murine hybridomas [27]. The major difference is the undetectable presentation of GAD65 by DPC cells, even though its antibody epitope did not overlap with those of any of the T cell lines we tested. This was not related to antibody avidity or differences in co-stimulation, as presentation of the synthetic peptide p270–283 at high doses was comparable to DPA and DPD cells. Nor were the differences related to the epitope specificity of the DPC antibody, as soluble immune complexes of DPC and GAD65 led to enhancement of T cell presentation when using PBMCs as APC. Differences in receptor-mediated endocytosis and internalization of the captured antigen were also excluded. All three antigen-specific B cell clones were derived from the same patient, thus making any possible genetic differences in the B cell lines regulating their APC function unlikely [31].
The only obvious percularity we noted in the DPC cells was its much lower content of HLA-DM molecules and their failure to co-localize with endocytosed GAD65. Antigens internalized via receptor-mediated endocytosis reach the late endosomal/lysosomal compartment (MIIC), where the loading of antigen peptides on the nascent MHC class II polypeptides is catalysed by HLA-DM [23,32,33]. In contrast, exogenous peptide antigens can bind directly to surface HLA-DR molecules or, after endocytosis, to HLA-DR molecules in early endosomes [33]. This pathway was functional in all B cell lines investigated, as proliferation was driven by antigenic peptides. Thus our finding that expression of intracellular HLA-DM was reduced strongly in DPC cells could provide a link to its very poor capacity to present native antigen to all the human T cell lines tested in our study.
The reasons for the differences in antigen presentation by DPC cells between the study by Jaume and colleagues [27] and our own are not completely clear. One explanation may be that the earlier study relied on presentation by human B cells to murine T cell hybridomas [27]. In addition, there may be differences in the fine specificities or avidities of T cell lines used in the two studies. Also, the methods used to assess T cell stimulation were different, because activation of the murine T cell hybridomas was measured on the basis of cytokine secretion, which is known to have less stringent stimulation requirements than those used in this study based upon cell proliferation [34].
Our study suggests that, despite highly facilitated internalization mediated by antigen-specific sIgG, additional factors determine whether B cells can process and present autoantigenic peptides successfully to T cells. If B and T cell epitopes are spatially distant antigen presentation can be boosted. A close overlap between B and T cell epitopes can limit the presentation of antigens internalized by high affinity sIgG. Finally, some antigen-specific B cells may be inefficient in driving a sustained T cell proliferation, which might be correlated with lower expression of HLA-DM.
Acknowledgments
We thank Ms Eleanor Oatham for assistance with the T cell presentation experiments with immune complexes and Ms Aikatarini Fragou for the affinity constant determination of the human MoAbs. The gifts of various MoAbs from Professor Ian Maclennan, Professor Peter Beverley and Dr Shiela Drover are gratefully acknowledged.
REFERENCES
- 1.Lernmark A. Glutamic acid decarboxylase − gene to antigen to disease. J Int Med. 1996;240:259–77. doi: 10.1046/j.1365-2796.1996.27859000.x. [DOI] [PubMed] [Google Scholar]
- 2.Powers AC, Bavik K, Tremble J, Daw K, Scherbaum WA, Banga JP. Comparative analysis of epitope recognition of glutamic acid decarboxylase by autoantibodies from different autoimmune disorders. Clin Exp Immunol. 1999;118:349–56. doi: 10.1046/j.1365-2249.1999.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nepom GT. Class II antigens and disease susceptibility. Ann Rev Med. 1995;46:17–25. doi: 10.1146/annurev.med.46.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Endl J, Otto H, Jung G, et al. Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J Clin Invest. 1997;99:2405–15. doi: 10.1172/JCI119423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach JM, Otto H, Nepom GT, et al. High affinity presentation of an autoantigenic peptide in type 1 diabetes by an HLA class II protein encoded in a haplotype protecting from disease. J Autoimmun. 1997;10:375–86. doi: 10.1006/jaut.1997.0143. [DOI] [PubMed] [Google Scholar]
- 6.Schloot NC, Batstra MC, Duinkerken G, et al. GAD65 reactive T cells in a non-diabetic stiff-man syndrome patient. J Autoimmun. 1999;12:289–96. doi: 10.1006/jaut.1999.0280. [DOI] [PubMed] [Google Scholar]
- 7.Wicker LS, Chen SL, Nepom GT, et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SD, Cope AP, Cognia M, et al. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR (alpha1*0101,beta1*0401) transgenic mice. Proc Natl Acad Sci USA. 1997;94:8082–7. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz H, Chandonia JM, Kash S, et al. High-resolution autoreactive epitope mapping and structural modeling of the 65 kDa form of human glutamic acid decarboxylase. J Mol Biol. 1999;287:983–99. doi: 10.1006/jmbi.1999.2655. [DOI] [PubMed] [Google Scholar]
- 10.Myers MA, Davies JM, Tong JC, et al. Conformational epitopes on the diabetes autoantigen GAD65 identified by peptide phage display and molecular modeling. J Immunol. 2000;165:3830–8. doi: 10.4049/jimmunol.165.7.3830. [DOI] [PubMed] [Google Scholar]
- 11.Richter W, Endl J, Eiermann TH, et al. Human monoclonal islet cell antibodies from a patient with insulin-dependent diabetes mellitus reveal glutamate decarboxylase as the target antigen. Proc Natl Acad Sci USA. 1992;89:8467–71. doi: 10.1073/pnas.89.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madec AM, Rousset F, Ho S, et al. Four IgG anti-islet human monoclonal antibodies isolated from a type 1 diabetes patient recognize distinct epitopes of glutamic acid decarboxylase 65 and are somatically mutated. J Immunol. 1996;156:3541–9. [PubMed] [Google Scholar]
- 13.Syren K, Lindsay L, Stoehrer B, et al. Immune reactivity of diabetes-associated human monoclonal autoantibodies defines multiple epitopes and detects two domain boundaries in glutamate decarboxylase. J Immunol. 1996;157:5208–14. [PubMed] [Google Scholar]
- 14.Tremble J, Morgenthaler NG, Vlug A, et al. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase-65 from a non-diabetic patient with multiple autoantibodies and Graves’ disease: a comparison with those present in Type I diabetes. J Clin Endocrinol Metab. 1997;82:2664–70. doi: 10.1210/jcem.82.8.4171. [DOI] [PubMed] [Google Scholar]
- 15.Sohnlein P, Muller M, Syren K, et al. Epitope spreading and a variety but not disease specific GAD65 antibody response in type 1 diabetes. Diabetologia. 2000;43:210–7. doi: 10.1007/s001250050031. [DOI] [PubMed] [Google Scholar]
- 16.Fallone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–8. [PubMed] [Google Scholar]
- 17.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen presenting cells for the initiation of T cell mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1999;151:3912–8. [PubMed] [Google Scholar]
- 18.Noorchasm H, Lieu YK, Noorchasm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:143–750. [PubMed] [Google Scholar]
- 19.Hulbert CB, Riseili M, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol. 2001;167:5535–8. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 20.Martin S, Wolf-Eichbaum D, Duikerken G, et al. Development of type 1 diabetes despite severe hereditary B cell deficiency. New Engl J Med. 2001;345:1036–40. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity. J Exp Med. 1995;181:1945–8. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amigorena S, Bonnerot C. Role of B cell and Fc receptors in the selection of T cell epitopes. Curr Opin Immunol. 1998;10:88–92. doi: 10.1016/s0952-7915(98)80037-x. [DOI] [PubMed] [Google Scholar]
- 23.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts C, Antoniou A, Manoury B, et al. Modulation by epitope specific antibodies of class II MHC restricted presentation of the tetanus toxin antigen. Immunol Rev. 1998;164:11–6. doi: 10.1111/j.1600-065x.1998.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 25.Brocke P, Garbi N, Momburg F, Hammerling GJ. HLA-DM, HLA-DO and tapasin: functional similarities and differences. Curr Opin Immunol. 2002;14:22–9. doi: 10.1016/s0952-7915(01)00294-1. [DOI] [PubMed] [Google Scholar]
- 26.Reijonen H, Daniels TL, Lernmark Å, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T cell epitope from GAD65. Diabetes. 2000;49:1621–6. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 27.Jaume JC, Parry SL, Madec AM, Sonderstrup G, Baekkeskov S. Suppressive effect of glutamic acid decarboxylase 65-specific autoimmune B lymphocytes on processing of T cell determinants located within the antibody epitope. J Immunol. 2002;169:665–72. doi: 10.4049/jimmunol.169.2.665. [DOI] [PubMed] [Google Scholar]
- 28.Roep BO, Atkinson MA, van Endert PM, et al. Autoreactive T cell responses in insulin dependent (type 1) diabetes mellitus. J Autoimmun. 1999;13:267–82. doi: 10.1006/jaut.1999.0312. Report of the First International Workshop for Standardization of T cell Assays. [DOI] [PubMed] [Google Scholar]
- 29.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand F, Chafforte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant solution of antigen–antibody complexes by enzyme linked immunosorbent assay. J Immunol Meth. 1984;77:305–19. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 31.Reijonen H, Elliot JF, van Endert PM, Nepom GT. Differential presentation of glutamic acid decarboxylase 65 (GAD 65) T cell epitopes among HLA-DRB1*0401 positive individuals. J Immunol. 1999;163:1674–81. [PubMed] [Google Scholar]
- 32.Chen X, Laur O, Kambayashi T, et al. Regulated expression of human histocompatibility leukocyte antigen (HLA)-DO during antigen-dependent and antigen-independent phases of B cell development. J Exp Med. 2002;195:1053–62. doi: 10.1084/jem.20012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arndt SO, Vogt AB, Markovic-Plese S, et al. Functional HLA-DM on the surface of B cells and immature dendritic cells. EMBO J. 2000;19:1241–51. doi: 10.1093/emboj/19.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evavold BD, Allen PM. Separation of IL-4 production from proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–10. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]