Abstract
The objective of the study was to determine the diagnostic value for rheumatoid arthritis (RA) of anti-filaggrin autoantibodies (autoAb) recognizing citrullinated recombinant rat filaggrin (ACRF) in community cases of very early arthritis. To evaluate the diagnostic value of ACRF, were studied sera from patients with different classified rheumatic diseases and healthy subjects (group 1, n = 422) and 314 community cases of very early arthritis (group 2) that were classified as RA (n = 176), non-RA (n = 63) and undifferentiated (n = 75) arthritides after 1 years of follow-up. ACRF were measured using a new ELISA, with results expressed as the difference between the OD value obtained on citrullinated minus that on noncitrullinated rat filaggrin (differential ACRF; dACRF). For both groups, rheumatoid factors (RF), anti-keratin autoAb (AKA) and anti-perinuclear factor (APF) were tested; for group 2, anti-CCP autoAb were also tested. Different reactivity patterns against citrullinated and noncitrullinated filaggrin were observed. Almost all sera reacting with citrullinated but not noncitrullinated filaggrin were from RA patients. Among RA and non-RA sera that recognized both forms of filaggrin, a positive result was obtained only with RA sera. For groups 1 and 2, dACRF sensitivity was 58·4% and 30·7%, and specificity for RA was 99·5% and 98·4%, respectively. In group 2, dACRF specificity for RA was better than that of RF (92·1%), APF (95·2%), AKA (96·8%) and anti-CCP (95·2%). dACRF positive predictive value was high (98·2) and close to that given by the concomitant positivity of RF and anti-CCP autoAb. Despite a high positive correlation between AKA, APF, anti-CCP and dACRF test results, they were complementary since some sera were positive for only one test. Thus, in a community setting, anti-citrullinated rat filaggrin reactivity detected by a new ELISA, whose originality is based on the difference between serum's reactivities on the citrullinated and native forms of filaggrin, had a higher diagnostic value for RA than other autoAb.
Keywords: very early rheumatoid arthritis, anti-filaggrin antibodies, ELISA, anti-CCP, diagnostic value
INTRODUCTION
Several autoantibody (autoAb) populations are associated with rheumatoid arthritis (RA). Although rheumatoid factor (RF) is classically considered to lack RA specificity, certain studies conducted on population-based recruitments of recent-onset arthritis, have shown that RF may contribute to an early diagnosis of RA with a specificity around 90%[1,2]. However, since approximately half of RA patients are RF positive during the first year of the disease, this marker is not able to diagnose all very early RA, and seems to have prognostic rather than diagnostic value [3]. Two other autoAb populations, i.e. anti-Sa and anti-filaggrin (AFA), are markers highly specific to RA [4,5]. The latter autoAb population is particularly relevant because of its target antigens. AFA, which include anti-keratin Ab (AKA) and the anti-perinuclear factor (APF), recognize human epidermal filaggrin and other profilaggrin-related proteins of various epithelial tissues [6]. The tests used to detect AKA and APF have several technical disadvantages and have not yet been validated [7,8] Because of these technical difficulties, a more simple, rapid, specific and standardizable assay is needed to detect AFA. Recently, AFA were shown to bind to antigenic determinants partly formed by the amino-acid citrulline, generated by post-translational modification of arginine residues by the enzyme peptidyl arginine deiminase [9,10]. In light of those observations, ELISAs were developed using purified or recombinant human filaggrin or citrullinated filaggrin-derived synthetic peptides, that bear major immunogenic AFA epitopes [10–14]. Using different antigens, these ELISAs were shown to have sensitivities and specificities at least comparable to those of indirect immunofluorescence assays and, for one of them that used a modified peptide variant (cyclic citrullinated peptide (CCP)) [11], a higher sensitivity. However, their specificity does not reach 100% since anti-CCP autoAb have been detected in certain connective tissue diseases (CTD), with specificities ranging from 91% to 96% depending on the patients studied. More recently, a new ELISA based on a recombinant rat filaggrin deiminated in vitro was developed and assessed with 711 sera from patients with well-characterized rheumatic diseases. This test would provide the better diagnostic accuracy among those available for the detection of AFA, including anti-CCP autoAb [15].
In the present study, we determined the diagnostic value of autoAb recognizing citrullinated rat filaggrin (ACRF) using citrullinated and noncitrullinated recombinant rat filaggrin as the antigen in a solid-phase ELISA, especially in a cohort of community cases of very early arthritis.
MATERIALS AND METHODS
Patients
Group 1: patients studied to determine the specificity for RA of the citrullinated rat filaggrin ELISA.
From 1989 to 2002, sera from patients with classified rheumatic diseases referred to the Departments of Rheumatology and Immunology were collected and stored at –80°C. A total of 422 disease-associated and control sera were tested. This group of patients was divided into 3 subgroups (Table 1): (1) 101 with established RA (median duration: 10 years) fulfilling the 1987 ACR criteria [16] (subgroup 1a); (2) 225 patients with non-RA rheumatic diseases defined according to international criteria [17–22](subgroup 1b); (3) 96 blood donors as the healthy control group (subgroup 1c). This entire group was used to determine the characteristics of the citrullinated rat filaggrin ELISA.
Table 1.
Frequencies of autoantibodies recognizing citrullinated minus noncitrullinated recombinant rat filaggrin, expressed as differential ACRF (dACRF), in patients with various rheumatic diseases and healthy controls (group 1)
| dACRF | |||
|---|---|---|---|
| Disease | Patients n | n | % |
| Rheumatoid arthritis (subgroup 1a) | 101 | 59 | 58·4 |
| Non-RA rheumatic diseases (subgroup 1b) | 225 | 1 | 0·4 |
| Systemic lupus erythematosus | 32 | 0 | 0 |
| Primary Sjögren's syndrome | 26 | 1 | 3·8 |
| CREST syndrome; progressive systemic sclerosis | 18 | 0 | 0 |
| Mixed connective tissue disease | 10 | 0 | 0 |
| Dermatopolymyositis | 14 | 0 | 0 |
| Adult-onset Still's disease | 16 | 0 | 0 |
| Spondylarthropathies | 69 | 0 | 0 |
| Ankylosing spondylitis | 31 | 0 | 0 |
| Psoriatic arthritis/reactive arthritis | 38 | 0 | 0 |
| Osteoarthritis | 20 | 0 | 0 |
| Crystal-induced arthritis | 20 | 0 | 0 |
| Healthy controls (subgroup 1c) | 96 | 0 | 0 |
Group 2: community cases of very early arthritis (VErA cohort) to evaluate the diagnostic value of dACRF for RA.
This cohort included 314 patients (mean age: 51·7 ± 14·5 years, range: 19–84; F/M ratio = 2·17) recruited prospectively in two French regions, i.e. entire province of Upper Normandy (1 800 000 people) and metropolitan area of Amiens (300 000 people). They are primarily european Caucasians. New cases of inflammatory arthritis were recruited by general practitioners, private and hospital rheumatologists of these areas. Moreover, the entire population has been regularly informed of this study by medias. Patients were required to have swelling of at least 2 joints persisting ≥4 weeks, evolving for <6 months (median disease duration: 4·2 ± 1·75 months, range 0·9–6 months), and no local or sys-temic corticotherapy or disease-modifying anti-rheumatic drugs (DMARDs) before inclusion. Exclusion criteria were: inflammatory back pain, pregnant or nursing woman. At the end of follow-up, i.e. 1 years after the first symptoms, the diagnoses were established by a committee of 5 expert rheumatologists: RA (n = 176), non-RA (n = 63) and undifferentiated (n = 75) arthritis (Table 2). At entry into the study, the following markers were tested: RF, AKA, APF and anti-CCP. These early arthritis patients were used to determine the diagnostic value of dACRF for very early RA and to compare their frequency to those of AKA, APF and anti-CCP autoAb.
Table 2.
Frequencies of autoantibodies recognizing citrullinated minus noncitrullinated recombinant rat filaggrin, expressed as differential ACRF (dACRF) and anti-CCP antibodies in the VErA cohort (group 2)
| Anti-CCP | dACRF | ||||
|---|---|---|---|---|---|
| Disease | Patients n | n | % | n | % |
| RA | 176 | 69 | 39·2 | 54 | 30·7 |
| Non-RA rheumatic diseases | 63 | 3 | 4·8 | 1 | 1·6 |
| Spondylarthropathies | 22 | 2 | 9 | 1 | 4·5 |
| Psoriatic arthritis | 12 | 1 | 8·3 | 0 | 0 |
| Others | 10 | 1 | 10 | 1 | 10 |
| Osteoarthritis | 15 | 0 | 0 | 0 | 0 |
| Crystal-induced arthritis | 6 | 0 | 0 | 0 | 0 |
| Paramalignant arhtritis | 3 | 0 | 0 | 0 | 0 |
| Parvovirus B19 arthritis | 3 | 0 | 0 | 0 | 0 |
| Sarcoidosis | 2 | 0 | 0 | 0 | 0 |
| Connective tissue disease | 12 | 1 | 8·3 | 0 | 0 |
| Primary Sjögren's syndrome | 4 | 1 | 25 | 0 | 0 |
| Systemic lupus erythematosus | 1 | 0 | 0 | 0 | 0 |
| Mixed connective tissue disease | 2 | 0 | 0 | 0 | 0 |
| Behçet's disease | 1 | 0 | 0 | 0 | 0 |
| Wegener's granulomatosis | 1 | 0 | 0 | 0 | 0 |
| Unclassified | 3 | 0 | 0 | 0 | 0 |
| Undifferentiated arthritis | 75 | 2 | 2·7 | 3 | 4 |
The local Ethics Committee approved the studies to be conducted. Prior to entry into the protocol, each patient was informed of the nature, duration and purpose of the study and gave his/her written consent.
Cloning, expression, protein purification and citrullination
Based on published data [23], selected oligonucleotides were synthesized and used to amplify by PCR a sequence encoding a complete filaggrin subunit from a Wistar rat genomic DNA. The PCR product was ligated to a pCAPS vector (Roche Diagnostics, Meylan, France) according to the manufacturer's instructions, and the sequence of the resulting construct was verified. For expression of recombinant filaggrin in Escherichia coli, the plasmid pMR78 [24], which contains a hexa-histidine box upstream from the multiple cloning site, was used. The recombinant protein was expressed in E. coli strain DH5α. Transfected bacteria were grown at 37°C until the OD600 nm reached 1 and then incubated for a further 3 h prior to harvesting. Bacteria were lysed with 100 mm sodium phosphate-300 mm NaCl (pH 7·5) containing 1 mg/ml of lysozyme and protease inhibitors and sonicated on ice (4 × 30 s). After centrifugation of the lysate, the recombinant protein was purified under native conditions by metal-chelate chromatography, as previously described [25] followed by gel filtration. The recombinant rat filaggrin was citrullinated using peptidylarginine deiminase (PAD) (P1584, Sigma Inc, St Louis, MO, USA) as described [26]. PAD (4 U/mg) was added and after 4 h of incubation at 37°C, the reaction was stopped by addition of EDTA. The purified protein was analysed by SDS-PAGE, Western-blotting with an anti-penta-histidine antibody (Qiagen, Courtaboeuf, France), amino-acid analysis and mass spectrometry. Quantification of citrulline in the recombinant rat filaggrin was made by an amino-acid analysis of the hydrolysed filaggrin after the enzymatic reaction of citrullination. Hydrolysis was carried out by heating the protein in contact of HCl at 150°C for 1 h. Then, the hydrolysed protein was derivatized on an automatic analyser Abl 420 A and eluted on a RP-HPLC. Amino-acids were quantified by UV detection. Each hydrolysed protein was analysed 3 times and the final results were an average of these triplicated with a good reproducibility (<5%). The proportion of the arginine residues converted into citrulline by the deimination process was 40%.
ELISA for detection of autoAb recognizing citrullinated rat filaggrin (dACRF)
Microtitre plates (96-wells, Nunc Maxisorp, Polylabo, Strasbourg, France) were coated with noncitrullinated or citrullinated rat filaggrin at 0·25 µg/well for 2 h at 37°C. Free sites were blocked with PBS containing 1% (w/v) BSA for 1 h at 37°C. After 3 washes with PBS containing 0·05% Tween 20 (PBST), 100 µl of human serum samples diluted 1 : 100 in Tris-NaCl containing 1% (w/v) BSA (Tris-BSA) were added to each well. The plates were incubated for 1 h at 37°C and then washed 3 times with PBST. Bound human IgG were detected by using an anti-human IgG horseradish peroxidase-labelled conjugate (814407, Roche Diagnostics) diluted 1 : 20 000 in Tris-BSA. After a 1-h incubation at 37°C, the plates were washed 3 times with PBST. The enzymatic reaction was detected with the o-phenylenediamine dihydrochloride colour-development reagent (bioMérieux, Marcy l’Etoile, France) for 10 min at room temperature. The reaction was stopped by adding 100 µl of 0·9 m sulphuric acid/well and optical density (OD) was read at 492 nm. Each serum sample was assayed in duplicate on citrullinated and noncitrullinated filaggrin-coated plates; OD were averaged. A positive sample was tested on each plate in order to validate the procedure. For each sample, the difference between OD obtained on citrullinated and noncitrullinated filaggrin-coated plates was calculated, defining differential ACRF (dACRF). The threshold of positivity was an OD difference >0·2.
Indirect immunofluorescence detection of AKA/APF
APF was tested as previously described [8]. Briefly, buccal mucosa cells from a positive donor were used as the substrate. Sera were deposited on fresh buccal cell smears for 90 min. A fluorescein isothiocyanate-conjugated anti-human IgG serum (Dakopatts, Copenhagen, Denmark) was then added for 30 min The test was considered positive when serum diluted 1 : 80 bound to at least 10% of the cells giving rise to the typical perinuclear immunofluorescence pattern. AKA were detected using a semiquantitative indirect immunofluorescence assay on sections of the middle third of a rat oesophagus, as previously described [27]. Sera were incubated at a dilution of 1 : 10. Only sera that gave an intense, regular, linear, laminated labelling restricted to the stratum corneum were considered positive for RA. Labeling intensity was estimated on a semiquantitative scale from 0 to 4 and were considered positive when >2.
ELISA detection of anti-CCP antibodies
Anti-CCP autoAb were detected using a commercial kit (Euroimmun, Groβ Grönau, Germany), according to the recommendations of the manufacturer. A titre >5 arbitrary units (AU) was considered positive.
ELISA detection of rheumatoid factors
RF of the IgM isotype were detected using an in-house ELISA as already described (1). Briefly, flat-bottomed 96-well microtiter plates (Virion, Roche, France) were coated with a 10-µg/ml solution of purified rabbit IgG (Jackson Immunoresearch Laboratories, Westgrove, USA) in 0·1 m carbonate-bicarbonate, pH 9·6, at 4°C overnight. After coating, the plates were incubated for 2 h at 37°C with test serum samples and with a serial dilution of a standard serum for each isotype. Bound RF were detected with F(ab)′2 goat anti-human IgM conjugated to alkaline-phosphatase (Sigma Immunochemicals, Munich, Germany). Finally, a 1-mg/ml solution of p-nitrophenol phosphate was added to the wells and the OD generated by the enzymatic reaction was read at 405 nm in a microplate reader. The upper limit of normal Ab values was considered to be 3 SD above the mean level in serum samples from 105 healthy blood donors. This threshold of positivity was previously determined by using receiver operating characteristic (ROC) curve to discriminate RA from other rheumatic diseases as described elsewhere [28]. The RF titre (16 IU/ml) at which the accuracy of this test was greatest in terms of sensitivity and specificity was chosen as optimal cut-off titre. The frequency of positive results among healthy blood donors was 1% (1/105).
Statistics
To assess the diagnostic value of dACRF and other tests, sensitivity, specificity and positive- and negative- predictive values were calculated from results obtained for cohort I. Potential relationship between two autoAb populations were examined using Fischer's exact test and the kappa reliability test. The Mac Nemar test was used to compare frequencies of 2 autoAb populations. A P < 0·05 was considered significant.
RESULTS
Characteristics of the noncitrullinated/citrullinated rat filaggrin ELISA
Sera from patients with classified rheumatic diseases and healthy blood donors (group 1) were tested on citrullinated- and noncitrullinated rat filaggrin-coated plates. Four different reactivity patterns, whose distributions differed dramatically among RA and non-RA patients or controls, were observed as shown in Table 3. Among sera reacting with citrullinated but not noncitrullinated filaggrin, almost all were from RA patients. Indeed, only 1 serum from a patient with primary Sjögren's syndrome had such reactivity. A second pattern was obtained with sera that recognized both forms of filaggrin. For these sera, when the OD measured in noncitrullinated filaggrin-coated wells was subtracted from that observed in citrullinated filaggrin-coated wells (dACRF), a positive result was obtained only with RA sera. The other two patterns were obtained with sera that did not bind to citrullinated rat filaggrin and reacted or not with noncitrullinated filaggrin. Table 3 and Fig. 1 show that many sera obtained from patients and controls could bind to noncitrullinated filaggrin.
Table 3.
Patterns of reactivity with citrullinated and/or noncitrullinated rat filaggrin of sera obtained from patients with different rheumatic diseases and healthy individuals (group 1)
| ACRF+ | ACRF– | ACRF+and ANCRF+ | |||||
|---|---|---|---|---|---|---|---|
| Group 1 Subgroup | ANCRF– | ANCRF+ | ANCRF– | ACNRF+ | ANCRF < ACRF* | ANCRF = ACRF* | ANCRF > ACRF* |
| 1a: | |||||||
| RA (n = 101) | 51 (50·4) | 13 (12·9) | 35 (34·6) | 2 (2) | 10 (9·9) | 1 (1) | 2 (2) |
| 1b: | |||||||
| SLE (n = 32) | 0 | 12 (37·5) | 14 (43·8) | 6 (18·8) | 0 | 0 | 12 (37·5) |
| SS (n = 26) | 1 (3·8) | 13 (50) | 10 (38·5) | 2 (7·7) | 0 | 2 (7·7) | 11 (42·3) |
| SPA (n = 69) | 0 | 34 (49·3) | 29 (42) | 6 (8·7) | 0 | 15 (21·7) | 19 (27·5) |
| 1c: | |||||||
| Controls (n = 96) | 0 | 18 (18·8) | 63 (65·6) | 15 (15·6) | 0 | 4 (4·2) | 14 (14·6) |
Results are expressed in number of patients (%). ACRF, anti-citrullinated rat filaggrin; ANCRF, anti-non-citrullinated rat filaggrin; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, primary Sjögren's syndrome; SPA, spondylarthropathy.
OD comparisons.
Fig. 1.
Scattergrams showing OD values to native (ANCRF) and deiminated (ACRF) filaggrin, and the OD difference (dACRF) given by sera from RA patients (subgroup 1a) and controls (subgroup 1b) in the rat filaggrin ELISA. RA, rheumatoid arthritis; ANCRF, anti-non-citrullinated rat filaggrin; ACRF, anti-citrullinated rat filaggrin; dACRF, autoAb recognizing citrullinated minus noncitrullinated rat filaggrin; OD, optical density.
To determine the reproducibility of the assay, we selected several RA sera with low (n = 10), mild (n = 5) and high (n = 5) dACRF reactivities and retested them at least 10 times, in the same microplates and at different times. The intra-assay variation coefficient was consistently <5%, regardless of the degree of reactivity. The interassay variation coefficient was ≤10%; mean (range) = 6·4% (2–10%).
Although only RA sera and one non-RA patient serum gave a positive OD difference in our assay, an OD difference >0·2 was considered as the positive threshold. Using this cut-off value, the sensitivity of dACRF in well established RA was 58·4%; compared to normal controls and non-RA rheumatic diseases, the specificity of dACRF for RA was 100% and 99·5%, respectively.
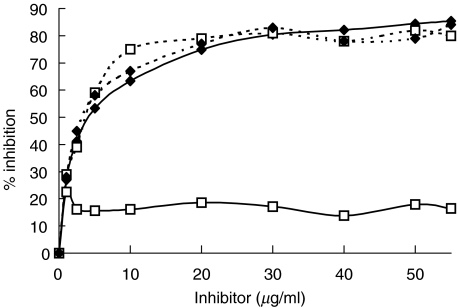
To verify that the rat-filaggrin ELISA was specific to filaggrin, we ran several tests. First, we conducted inhibition experiments. The anti-citrullinated filaggrin specificity of 4 sera, 2 from RA patients that reacted exclusively with citrullinated filaggrin and 2 from non-RA patients that bound to both citrullinated-and noncitrullinated filaggrin, was studied on citrullinated rat filaggrin-coated microplates by preincubating these sera with increasing concentrations of citrullinated- or noncitrullinated filaggrin (v/v) and then were tested on citrullinated- rat filaggrin coated-plates. As shown in Fig. 2, preincubation of the non-RA sera with increasing concentrations of citrullinated- or noncitrullinated-rat filaggrin was able to inhibit its binding to citrullinated-filaggrin-coated plates whereas the anti-citrullinated filaggrin activity of the RA sera was inhibited only by citrullinated filaggrin. Second, the anti-filaggrin reactivity detected in our ELISA was confirmed in an immunoblotting assay using noncitrullinated and citrullinated recombinant rat filaggrin as the substrates (data not shown).
Fig. 2.
Inhibition of anti-citrullinated rat filaggrin activity in an ELISA of sera from one RA ( ) and one non-RA (
) and one non-RA ( ) patients by increasing concentrations of citrullinated (⋄) or noncitrullinated (□) filaggrin. Diluted sera were incubated with soluble citrullinated- or non citrullinated filaggrin for 2 h at 37°C and then tested on citrullinated-rat filaggrin coated plates.
) patients by increasing concentrations of citrullinated (⋄) or noncitrullinated (□) filaggrin. Diluted sera were incubated with soluble citrullinated- or non citrullinated filaggrin for 2 h at 37°C and then tested on citrullinated-rat filaggrin coated plates.
Finally, we have observed that dACRF reactivities were not influenced by the potential interference of RF in this assay (data not shown).
Comparison of rat filaggrin ELISA to indirect immunofluorescence assays for AKA and APF and to ELISA for anti-CCP
AKA, APF, anti-CCP and dACRF were sought in sera from the 314 patients comprising the VErA cohort (Tables 4 and 5). First, we compared the prevalence of the different anti-filaggrin autoAb. The frequency of dACRF was significantly higher than that of AKA (P = 0·02) but significantly lower than that of APF (P = 0·003) and anti-CCP (P = 0·0025) Second, the relationship between the different autoAb populations was studied. Despite a significant positive correlation between results of dACRF and those of AKA, APF and anti-CCP (P < 10−6), we also confirmed the inconsistencies between detection methods cited above, as some sera positive in dACRF ELISA were negative in AKA/APF indirect immunofluorescence assays and/or anti-CCP ELISA and vice versa (Table 4). Nevertheless, the tests exhibiting the greatest concordance were dACRF and anti-CCP autoAb with a kappa value of 0·75. Finally, because of those inconsistencies, we wondered whether combinations of the 4 different assays would significantly improve the detection rate of AFA. As reported in Table 5, the concomitant determination of the 4 tests significantly increased the frequency of AFA (48·9%) as compared to dACRF alone (30·7%).
Table 4.
Comparison of ELISA detection of autoantibodies recognizing citrullinated minus noncitrullinated recombinant rat filaggrin, expressed as differential ACRF (dACRF) to indirect immunofluorescence assays for AKA and APF detection and ELISA detection of anti-CCP antibodies in sera from 314 patients with very early arthritis (group 2)
| dACRF | |||
|---|---|---|---|
| Autoantibody | positive(n = 58) | negative (n = 256) | Kappa-value |
| AKA | 0·66 | ||
| positive (n = 45) | 37 (63·8) | 8 (3·1) | |
| negative (n = 269) | 21 (36·2) | 248 (96·9) | |
| APF | 0·63 | ||
| positive (n = 77) | 48 (82·7) | 89 (11·3) | |
| negative (n = 237) | 10 (17·3) | 227 (88·7) | |
| anti-CCP | 0·75 | ||
| positive (n = 74) | 53 (91·4) | 21 (8·2) | |
| negative (n = 240) | 5 (8·6) | 235 (91·8) | |
Results are expressed in numbers of patients (% of dACRF-positive or -negative patients). AKA, anti-keratin antibodies; APF, anti-perinuclear factor; dACRF, autoAb recognizing citrullinated minus noncitrullinated rat filaggrin; anti-CCP, anti-cyclic-citrullinated peptides.
Table 5.
Diagnostic value of anti-filaggrin autoantibodies and rheumatoid factors for RA in the cohort of very early arthritis patients (group 2)
| Autoantibody | Sensitivity | Specificity | PPV | NPV | Odds ratio [95% CI] |
|---|---|---|---|---|---|
| RF-IgM | 35·2 | 92·1 | 92·5 | 33·7 | 5·8 [2·3–14·6] |
| AKA | 22·7 | 96·8 | 95·2 | 30·9 | 7·3 [2–27·1] |
| APF | 38·1 | 95·2 | 95·7 | 35·5 | 10·6 [3·5–32·6] |
| dACRF | 30·7 | 98·4 | 98·2 | 33·7 | 18·5 [3·5–96·5] |
| Anti-CCP | 39·2 | 95·2 | 95·8 | 35·6 | 11·1 [3·6–34·2] |
| AKA or APF | 39·8 | 92·1 | 93·3 | 35·3 | 7.0 [2·8–17·7] |
| dACRF or anti-CCP | 40·9 | 95·2 | 96 | 36·5 | 12.0 [3·9–36·7] |
| AKA or APF or dACRF | 42·6 | 90·5 | 92·6 | 36 | 6·6 [2·7–15·6] |
| AKA or APF or anti-CCP | 47·7 | 87·3 | 91·3 | 37·4 | 6.0 [2·7–13] |
| AKA or APF or anti-CCP or dACRF | 48·9 | 87·3 | 91·5 | 37·9 | 6·2 [2·9–13·6] |
| (AKA or APF) and RF-IgM | 26·1 | 98·4 | 97·6 | 32·3 | 14·8 [2·8–77·6] |
| dACRF and RF-IgM | 26·1 | 100 | 100 | 32·6 | 45·0 [2·7–746] |
| anti-CCP and RF-IgM | 29·5 | 100 | 100 | 33·6 | 53·5 [3·2–882] |
PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; RF, rheumatoid factor; AKA, anti-keratin antibodies; APF, anti-perinuclear factor; dACRF, autoAb recognizing citrullinated minus noncitrullinated rat filaggrin; anti-CCP, anticyclic citrullinated peptides.
Contribution of dACRF to the diagnosis of very early RA
The diagnostic value of dACRF has not yet been assessed in community cases of very early arthritis (group 2). Consequently, the diagnostic values of this marker, alone or in combination with RF-IgM, AKA, APF and anti-CCP, were analysed in 239 patients with arthritis evolving for <6 months, for which the diagnosis was definitively established after a 1-year follow-up (Table 5). The sensitivity of dACRF (30·7%) in the RA subgroup (n = 176) was slightly lower than that of RF (35·2%). Among the 114 RF-negative RA patients, 9 (7·9%) were positive for dACRF and 18 (15·8%) for anti-CCP autoAb. dACRF specificity for RA (98·4%) was higher than that of RF (92·1%), APF (95·2%), AKA (96·8%) and anti-CCP (95·2%). These latter were detected at high levels (>100 AU) in 3 patients with non-RA well-characterized rheumatic diseases including 2 spondylarthropathies and 1 primary Sjögren syndrome while dACRF were only observed in one patient having a spondylarthropathy, with a low titre close to the threshold of positivity. Consequently, the positive predictive value of dACRF was higher than that of the other tests considered independently (Table 5). Finally, the concomitant positivity of RF-IgM and either anti-CCP autoAb or dACRF led to the better diagnostic performances.
DISCUSSION
In the present study, we used a new ELISA to detect AFA. The major characteristics of this assay are the use of a recombinant rat filaggrin as the antigen and to base results on the difference between OD obtained with the same serum tested on citrullinated and noncitrullinated filaggrin. With this approach, we showed that autoAb binding predominantly or exclusively to citrullinated recombinant rat filaggrin are almost exclusively present in RA sera with a frequency similar to that of AKA/APF. Indeed, sera from patients with various CTD and inflammatory arthritis that may contain autoAb reacting with epitopes present on both the citrullinated and the noncitrullinated recombinant protein consistently gave a higher OD on non citrullinated antigen-coated plates than on citrullinated antigen-coated plates. This observation indicates that an ELISA that uses only a citrullinated form of the protein can provide positive results with non-RA sera, thereby limiting the specificity and the diagnostic value of anti-citrullinated filaggrin reactivity. In this regard, previous studies have shown that some sera from patients with CTD like SLE, progressive systemic sclerosis, polymyositis/dermatomyositis, and particularly primary Sjögren's syndrome, reacted with citrullinated peptide variants in solid phase ELISAs [2,9,11]. Thus, in light of the different profiles of reactivity we described herein, testing sera at the same time on both the native and citrullinated forms of recombinant rat filaggrin, appears particularly pertinent to differentiate RA from other rheumatic diseases. The autoAb detected by this ELISA were called differential anti-citrullinated recombinant rat filaggrin (dACRF).
So far, the diagnostic performances of such an ELISA have been evaluated exclusively in patients with well-characterized rheumatic diseases [15]. In our group of well-defined rheumatic diseases, the specificity of dACRF for RA was near 100%. Indeed, in non-RA patients, dACRF were only detected in one patient with primary Sjögren's syndrome. At the same specificity threshold (99–100%), their sensitivity (58%) was comparable with that (55–65%) observed by Vincent et al. [15] using a similar procedure. However, this group did not determine the sensitivity of its assay in very early RA, which prompted us to assess the diagnostic accuracy of dACRF in a cohort of 314 patients with very early arthritides, as well as that of anti-CCP autoAb which has rarely been evaluated in such a population. In this cohort, which is highly representative of daily clinical practice [29], the sensitivity of dACRF was 30·7%. This value is lower than those obtained with ELISAs using citrullinated peptides as the antigen in the present study (39·2%) and in previous reported series in which the sensitivities were 41–66% for early RA [2,11,13,14,30,31]. However, dACRF were shown to have a higher specificity since only 1 patient with spondylarthropathy was dACRF-positive while the presence of anti-CCP autoAb did not allow to exclude the diagnosis of non-RA rheumatic diseases such as spondylarthropathies and primary Sjögren syndrome, in accordance with previous reports [9,32]. In addition, dACRF displayed a higher positive-predictive value for RA than AFA identified by previous tests. In this regard, many groups have demonstrated that only the presence of RF in conjunction with another reactivity like anti-CCP or anti-Sa autoAb reached a specificity for RA near 100%[2,11]. Those data are confirmed by the present study but dACRF alone, with a positive- predictive value of 98·2%, had a diagnostic value similar to those combinations of tests. It is not excluded that the characteristics of our assay are due to the differential procedure which, by subtracting the noncitrullinated filaggrin reactivity frequently observed in normal and non-RA sera, decreased the sensitivity but gave high specificity and positive predictive values. Thus, in light of these findings, one may conclude that AFA detected with the citrullinated-noncitrullinated recombinant rat filaggrin ELISA have a high diagnostic value for very early RA.
When the frequency of dACRF was compared to those of AKA, APF and anti-CCP autoAb in the VErA cohort, we found a dACRF frequency significantly lower than that of APF and anti-CCP and significantly higher than that of AKA. In addition, despite a strong positive correlation between AKA, APF, dACRF and anti-CCP autoAb, several RA sera were positive for only one of these 4 autoAb populations. Thus, the 4 tests gave complementary information since the positivity rate for at least one of them reached 48·9% (vs. 30·7% for dACRF ELISA alone). Therefore, each test does not seem to identify exactly the same autoAb spectrum, which confirms similar findings previously observed by others and the interindividual heterogeneity of AFA specificity [1,9,10,15,33].
In conclusion, our data further demonstrate that dACRF are highly specific for RA. In particular, their diagnostic accuracy in very early arthritides was shown to be comparable to that obtained by the concomitant positivity of RF and of anti-filaggrin autoAb detected by usual assays. Further study is currently underway in our VErA cohort to determine whether this autoAb population may also constitute a predictive marker of severity in patients with very early arthritis.
Acknowledgments
This study was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), and grants from the Fondation de la Recherche Médicale (FRM), the Programme Hospitalier de Recherche Clinique (PHRC 1998) and Pharmacia France for the clinical aspects.
The authors thank Janet Jacobson for correcting the manuscript, Annick Valauney and Marlène Thomas for technical assistance for AKA and APF assays.
REFERENCES
- 1.Vittecoq O, Jouen-Beades F, Krzanowska K, et al. Rheumatoid factors, anti-filaggrin antibodies, and low in vitro interleukin-2 and interferon-gamma production are useful immunological markers for early diagnosis of community cases of rheumatoid arthritis. Preliminary study. Joint Bone Spine. 2001;68:144–53. doi: 10.1016/s1297-319x(00)00244-x. [DOI] [PubMed] [Google Scholar]
- 2.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott DL. Prognostic factors in early rheumatoid arthritis. Rheumatology. 2000;39(Suppl.):24–9. doi: 10.1093/oxfordjournals.rheumatology.a031490. [DOI] [PubMed] [Google Scholar]
- 4.Ménard HA, Lapointe E, Rochdi MD, Zhou ZJ. Insights into rheumatoid arthritis derived from the Sa immune system. Arthritis Res. 2000;2:429–32. doi: 10.1186/ar122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoet RM, van Venrooij WJ. The antiperinuclear factor (APF) and antikeratin antibodies (AKA) in rheumatoid arthritis. In: Smolen JS, Kalden JR, Maini RN, editors. Rheumatoid Arthritis. Berlin/Heidelberg: Springer-Verlag; 1992. pp. 299–318. [Google Scholar]
- 6.Sebbag M, Simon M, Vincent C, et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95:2672–9. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthelot JM, Maugars Y, Audrain M, Castagne A, Prost A. Impact of using stored cells for immunofluorescence detection of antiperinuclear factor on sensitivity of the method for the diagnosis of rheumatoid arthritis. Rev Rhum. 1995;62:507–12. [PubMed] [Google Scholar]
- 8.Youinou P, Le Goff P, Dumay A, Lelong A, Fauquert P, Jouquan J. The antiperinuclear factor. 1. Clinical and serological associations. Clin Exp Rheumatol. 1990;8:259–64. [PubMed] [Google Scholar]
- 9.Schellekens GA, de Jong BAW, van den Hoogen FHJ, van de Putte LBA, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro) filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- 11.Schellekens GA, Visser H, de Jong BAW, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Palosuo T, Lukka M, Alenius H, et al. Purification of filaggrin from human epidermis and measurement of anti-filaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998;115:294–302. doi: 10.1159/000069460. [DOI] [PubMed] [Google Scholar]
- 13.Aho K, Palosuo T, Matti L, et al. Antifilaggrin antibodies in recent-onset arthritis. Scand J Rheumatol. 1999;28:113–6. doi: 10.1080/030097499442586. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira L, Sebbag M, Vincent C, et al. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 2001;60:882–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent C, Nogueira L, Sebbag M, et al. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay. A highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2002;46:2051–9. doi: 10.1002/art.10436. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 18.Masi AT, Rodnan GP, Medsiger TA, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 19.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 20.Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328–34. [PubMed] [Google Scholar]
- 21.Bohan AT, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 22.Dougados M, van der Linden S, Jublin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–30. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 23.Haydock PV, Dale BA. Filaggrin, an intermediate filament-associated protein. structural and functional implications from the sequence of a cDNA from rat. DNA Cell Biol. 1990;9:251–61. doi: 10.1089/dna.1990.9.251. [DOI] [PubMed] [Google Scholar]
- 24.Cheynet V, Verrier B, Mallet F. Overexpression of HIV-1 proteins in Escherichia coli by a modified expression vector and their one-step purification. Protein Expr Purif. 1993;4:367–72. doi: 10.1006/prep.1993.1048. [DOI] [PubMed] [Google Scholar]
- 25.Letourneur O, Gervasi G, Gaia S, Pagès J, Watelet B, Jolivet J. Characterization of Toxoplasma gondii surface antigen I (SAG1) secreted from Pichia pastoris: evidence of hyper O-glycosylation. Biotechnol Appl Biochem. 2001;33:35–45. doi: 10.1042/ba20000069. [DOI] [PubMed] [Google Scholar]
- 26.Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steiner PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrate trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–16. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- 27.Vincent C, Serre G, Lapeyre F, et al. High diagnostic value in rheumatoid arthritis of antibodies to the stratum corneum of rat oesophagus epithelium, so-called ‘antikeratin antibodies’. Ann Rheum Dis. 1989;48:712–22. doi: 10.1136/ard.48.9.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser H, Gelinck LBS, Kampfraath AH, Breedveld FC, Hazes JMW. Diagnostic and prognostic characteristics of the enzyme linked immunosorbent rheumatoid factor assays in rheumatoid arthritis. Ann Rheum Dis. 1996;55:157–61. doi: 10.1136/ard.55.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Horst-Bruinsma IE, Speyer I, Visser H, Breedveld FC, Hazes JMW. Diagnosis and course of early-onset arthritis: results of a specific early arthritis clinic compared to routine patient care. Br J Rheumatol. 1998;37:1084–8. doi: 10.1093/rheumatology/37.10.1084. [DOI] [PubMed] [Google Scholar]
- 30.Bas S, Perenger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA. Diagnostic tests for rheumatoid arthritis. comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology. 2002;41:809–14. doi: 10.1093/rheumatology/41.7.809. [DOI] [PubMed] [Google Scholar]
- 31.Union A, Meheus L, Humbel RL, et al. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002;46:1185–95. doi: 10.1002/art.10229. [DOI] [PubMed] [Google Scholar]
- 32.Gottenberg JE, Sibilia J, Aucouturier F, et al. Positive anti-filaggrin antibodies should not exclude the diagnosis of primary Sjögren's syndrome [abstract] Arthritis Rheum. 2002;46:S361. [Google Scholar]
- 33.Vittecoq O, Jouen-Beades F, Delpech A, Gilbert D, Le Loët X, Tron F. Autoantibodies in rheumatoid arthritis. Rev Rhum. 1995;62:195S–200S. [Google Scholar]