Abstract
The present study investigates the immunological effects of a combination treatment of mitoxantrone and the cardioprotector dexrazoxane in experimental autoimmune encephalomyelitis (EAE). Mitoxantrone, an anthracycline-derived immunosuppressive drug has been approved recently for treatment of very active multiple sclerosis (MS). Its prolonged use is limited due to its cardiotoxic properties. Dexrazoxane (DZR (S)-(+)-1,2-bis (3,5.dioxopiperazinyl)propane, ICRF-187) is an iron III chelator which in animal models and in cancer patients reduces anthracycline and mitoxantrone induced cardiotoxicity when given immediately before these agents. We examined the immunological effects of dexrazoxane in combination with mitoxantrone in experimental autoimmune encephalomyelitis (EAE) in Lewis rats. EAE was induced by active immunization with myelin basic protein (MBP) or by adoptive transfer of MBP specific T cells (AT-EAE). The clinical course, spinal cord pathology, activity of metalloproteinases (MMP-2 and MMP-9) and T cell apoptosis were assessed. Monotherapy with DZR ameliorated slightly the course of actively induced EAE and AT-EAE. The combination of DZR and mitoxantrone was superior to mitoxantrone given alone. Clinical amelioration ran in parallel with the marked reduction of inflammatory infiltration which was nearly abolished by the combination treatment. DZR did not affect the activity of metalloproteinase 9 and did not increase the proportion of apoptotic lymph node cells ex vivo or T cells in situ. We conclude that in addition to its cardioprotective role, DZR augments mitoxantrone-mediated immunosuppressive effects in animal models of human central nervous system (CNS) autoimmune disease. Clinical trials in MS patients are warranted to evaluate the unexpected immunosuppressive efficacy of DZR as add-on treatment.
Keywords: chelator, dexrazoxane, experimental autoimmune encephalomyelitis, free radical, iron, mitoxantrone, multiple sclerosis
INTRODUCTION
Mitoxantrone (MITOX) is a DNA II topoisomerase inhibitor based on the anthracenedione backbone, which was developed for chemotherapy of malignancies. It turned out to be one of the most effective agents for the treatment of relapsing-remitting and relapsing progressive multiple sclerosis (MS) [1] and has been approved recently to slow worsening of neurological disability in secondary-progressive and relapsing-remitting forms of multiple sclerosis in the United States and Germany. In experimental autoimmune encephalomyelitis (EAE), a commonly used animal model for the study of immunopathological mechanisms in MS, it has been shown that MITOX, in dosages from 0·2 to 5 mg/kg, totally prevented disease in EAE when given before the onset of clinical signs or inhibited the course of the disease significantly [2–5]. MITOX-mediated immunusuppression affects both B and T lymphocytes, with a sustained suppression of B cell functions and an unspecific up-regulation of suppressor functions [6,7]. The prolonged application of this agent is limited due to its cardiotoxic effects which are correlated to its cumulative dosis [8].
Dexrazoxane (DZR, ICRF-187), a iron III chelator, is approved as an cardioprotector [9] which in animal models and in cancer patients reduces anthracycline and mitoxantrone induced cardiotoxicity. No clinical studies have been published so far with a combination of DRZ and MITOX. In MS, add-on treatment with a cardioprotector would be crucial in order to avoid the cardiotoxic adverse effects limiting its application. This experimental study was designed to investigate whether DZR would have intrinsic immunological effects. Moreover, we wished to evaluate its interaction with MITOX in two EAE models that are used commonly for the preclinical assessment of new therapies in MS.
MATERIALS AND METHODS
Animals, induction of EAE and experimental schedule
Female Lewis rats obtained from Charles River Wiga (Sulzfeld, Germany) were 6–8 weeks old and had a body weight of 120–140 g. Animals were housed in plastic cages without grid floors and were given commercial food pellets and water ad libitum. All experiments were carried out in accordance with Bavarian state regulations for animal experimentation and were approved by the responsible authorities. Adoptive transfer EAE (AT-EAE) was induced by i.v. transfer of 8–12 × 106 encephalitogenic MBP-specific T cell line cells that had been activated and isolated as described [10]. Actively induced EAE was elicited by immunization in one hind footpad with 50 µl of a mixture containing equal volumes of guinea pig-MBP in PBS and complete Freund's adjuvant (CFA, Gibco, Eggenstein, Germany) at a final concentration of 0·5 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI, USA) resulting in an inoculum dose of 70 µg GP-MBP per rat. Daily clinical scores were rated according to the following scale: 0 = normal; 1 = limp tail; 2 = mild paraparesis of the hind limbs, unsteady gait; 3 = moderate paraparesis, voluntary movements still possible; 4 = paraplegia or tetraplegia; 5 = moribund.
Dexrazoxane (DZR, Cardioxane®) was obtained from Chiron, Amsterdam, the Netherlands and mitoxantrone (Novantron®) by Wyeth/Lederle, Münster, Germany. DZR was provided as the lyophilized HCl salt and was reconstituted in phosphate buffered saline (PBS) at a stock concentration of 20 mg/ml. The solution was then diluted in PBS and injected at a dose of 5 or 25 mg/kg 30 min prior to injection of MITOX at a dose of 0·5 or 2·5 mg/kg, respectively, into the tail vein of Lewis rats under ether anaesthesia. MITOX dosages were chosen according to earlier EAE data [4,5].
Preventive therapy in actively induced EAE was performed at days 8, 10 and 12 after immunization. Rats received DZR 5 mg/kg followed by 0·5 mg/kg MITOX after an interval of 30 min. In AT-EAE animals were treated i.v. on days 1, 3 and 5 with either DZR (25 mg/kg) followed by MITOX (2·5 mg/kg) after 30 min, MITOX alone, DZR alone or PBS as control.
Morphological analysis, immunohistochemistry
To study spinal cord inflammatory infiltration in EAE, animals were anaesthesized with pentobarbital at 200 mg/kg body weight (Narcoren®, Rhone Merieux, Laupheim, Germany) and perfused through the left cardiac ventricle with Ringer solution (Fresenius, Bad Homburg, Germany) containing 2 × 104 U/l heparin, followed by fixation with 4% paraformaldehyde in 0·1 m phosphate buffer at pH 7·4. The spinal cord was removed, postfixed for 1 h in the same fixative, cut into 5 mm slices, dehydrated and embedded in paraffin.
For immunocytochemistry, 5 µm tissue sections were stained with the monoclonal antibody (MoAb) ED1 (dilution 1 : 1000; Serotec; Wiesbaden, Germany) that labels macrophages, and MoAb B115-1 (dilution 1 : 500; Holland Biotechnology, Leiden, Netherlands) to identify T cells as described by Zettl et al. [11]. We then used the ABC detection system (Dako; Hamburg, Germany) with 3,3′-diaminobenzidine as peroxidase substrate. Endogenous peroxidase activity was suppressed by incubating the sections for 10 min in 3% H2O2 prior to adding the ABC reagents. Finally, sections were counterstained with haematoxylin, dehydrated and mounted in Eukitt (Kindler; Freiburg, Germany).
Analysis of inflammatory infiltrates and of apoptosis was performed by an observer blinded to the treatment, who rated 10 visual fields (1·6 mm2) of half a section of lumbar spinal cord at × 250 magnification, one section per animal. Apoptosis was assessed by labelling by in situ tailing. All photomicrographs were taken with a ZEISS Axiophot 2 (Zeiss; Oberkochen, Germany).
Apoptotic T cells were identified by in situ tailing as described [12]. Double staining for bcl-2 and T cells was performed on 5 µm paraffin sections, which were dewaxed and rehydrated. Preincubation in the microwave oven (850 W) was performed by boiling in 10 mm sodium citrate buffer, pH 6·0, for 30 min. After sections had been cooled to room temperature, they were washed thoroughly with distilled water followed by TRIS-buffered saline. After blocking of non-specific binding with 10% bovine serum albumin (BSA), sections were incubated with a polyclonal rabbit anti-bcl-2 antibody (Pharmingen, Hamburg, Germany) at a dilution of 1 : 750 in TRIS-buffered saline (TBS) with 1% BSA overnight at room temperature. As a secondary antibody, we used a biotinylated goat antirabbit IgG antibody (Vector, via Linaris, Wertheim, Germany), diluted 1 : 50 in TBS with 1% BSA. Visualization was achieved with an alkaline phosphatase AB system (Dako) and Vector Red (Vector) as substrate. After the AB-binding sites had been blocked with an AB-blocking kit (Vector), T cells were detected with a biotinylated horse antimouse IgG antibody, which was preabsorbed with sera from rat, rabbit and goat for 15 min at 37°C and then diluted 1 : 100 in TBS with 1% BSA. The peroxidase-based AB system (Dako) was used with diaminobenzidine–nickel (Vector) as the chromogenic substrate.
Analysis of inflammatory infiltrates and of apoptosis was performed by an observer blinded to the treatment, who rated 10 visual fields (1·6 mm2) of half a section of lumbar spinal cord at × 250 magnification, three sections per animal taken from identical lumbar levels.
Proliferation assay
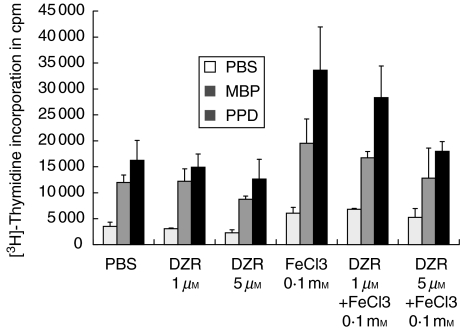
For proliferation studies 2 × 105/well lymph node cells isolated from popliteal lymph nodes 12 days after immunization with MBP were seeded in 100 µl medium in 96-well round-bottomed microtitre plates (Nunc, Wiesbaden, Germany). Cells were activated by myelin basic protein (MBP), purified protein derivative (PPD) (each at 10 µg/ml) or concavalin A (Con A, 2·5 µg/ml). Animals had been treated either with DZR or PBS (see Fig. 1a). Lymph node cells from untreated animals were activated ex vivo in the absence or presence of either DZR or FeCl3 at the indicated concentrations (Fig. 1b). The specific proliferative response was measured by [3H]-thymidine incorporation. Results are expressed as mean cpm + s.d. of triplicates.
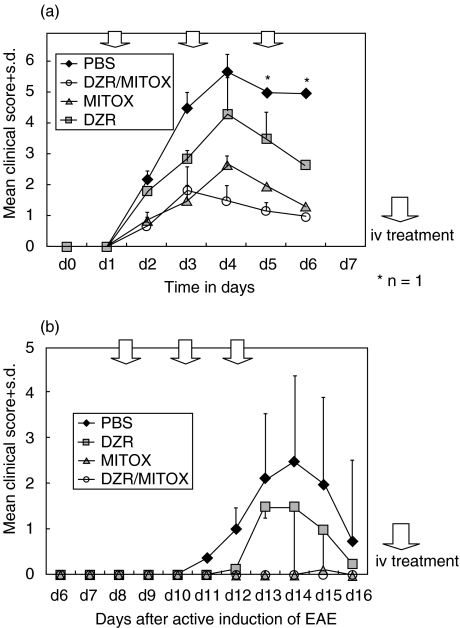
Fig. 1.
Clinical course of adoptive transfer experimental autoimmune encephalomyelitis (AT-EAE; a) and actively induced EAE (b) in the Lewis rat. Statistical significant different disease courses (anova, P < 0·05) were calculated in PBS versus Mitox, Mitox versus DZR, DZR versus PBS and DZR versus Mitox/DZR both in AT-EAE and EAE. Each experiment has been repeated at least twice with identical results.
Gelatine zymography
Gelatinase activity was determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) zymography modified according to the method of Brown et al. [13]. One hundred frozen sections of spinal cord were incubated with 10× ml of Tris/glycine SDS sample buffer (Novex, San Diego, CA, USA), degraded by ultrasound and further homogenized by vortexing for 1 h at 4°C. The samples were applied to a 10% (w/v) polyacrylamide resolving gel containing 0·1% SDS and 0·1% gelatin type A from porcine skin (Sigma, St Louis, MO, USA). Stacking gels were 3% (w/v) polyacrylamide. After electrophoresis gels were washed in renaturing buffer (Novex) containing Triton X-100 to remove any SDS, and incubated in developing buffer (Novex) for 18 h at 37°C. Gels were stained for 6 h in 30% methanol: 10% acetic acid containing 0·5% (w/v) Coomassie brilliant blue G-250 and excessive dye removed by the same buffer without dye. Gelatinase activity was detected as unstained bands on a blue background representing areas of gelatin digestion.
Flow cytometric detection of apoptosis
For annexin V/in situ tailing (IST) double labelling cells were stained according to [14]. Cells, 5 × 105, were suspended in 100 µl binding buffer (10 mm HEPES-NaOH, pH 7·4, 140 mm NaCl, 5 mm CaCl 2) and incubated for 15 min on ice with 2·5 µg/ml annexin V–biotin (Roche Diagnostics, Boehringer Mannheim, Germany). Cells were washed once with binding buffer and fixed for 18 h at 4°C at a density of 2 × 106 cells/ml in 5% formalin/PBS (Merck, Darmstadt, Germany). After washing twice with binding buffer, cells were incubated on ice with 2·5 µl streptavidin–tricolour (0·2 mg/ml; Caltag, Burlingame, USA; 10 min, light protected). After a washing step, cells were end-labelled using the in situ cell death detection kit according to the manufacturer's instructions (Roche Diagnostics) and modified as described [12]. In brief, 5 µl enzyme solution containing TdT was added to 45 µl labelling solution containing fluorescein-labelled nucleotides. The samples were light protected and placed in a rotating shaker (400 r.p.m.) at 37°C for 60 min. Single labelling experiments with annexin-V-fluorescein (Roche Diagnostics) and the IST reaction were performed following the manufacturer's instructions. Propidium iodide (PI; Sigma) staining was performed at a final concentration of 0·1 µg/ml.
Labelled cells were suspended in 300 µl binding buffer after pilot experiments did not show any differences from analyses carried out in PBS as described before [14]. Analyses were performed on a FACScan using Cell Quest software (Becton Dickinson, San Jose, USA). At each time-point, 5000 cells were analysed. Cell debris was excluded by setting appropriate light scatter gates. Quantitative analysis was done by setting region markers for positive cells in the appropriate fluorescence channels.
Statistical analysis
Statistical analysis of the data was performed with Mann–Whitney U-test for unpaired observations considering P < 0·05 to be significant.
RESULTS
AT-EAE
Twelve rats injected with 8–12 × 106 MBP reactive T cells developed clinical signs of EAE. The average day of disease onset was day 2. Whereas PBS-treated rats (n = 3) were moribund and reached a minimum EAE score of 5 at day 4 (Fig. 1a), MITOX treatment significantly ameliorated adoptive transfer EAE to a maximum of score 3 (P = 0·0365; anova). The combination of MITOX and DZR (n = 3) resulted in an additive protective effect, thus reducing mean EAE scores to a maximum of 2 with early recovery after day 3. DZR treatment alone led to an amelioration of EAE (n = 3), albeit not to the same degree as MITOX monotherapy. Weight curves were in accord with the clinical course. In MITOX-containing regimens only a mean maximal weight loss of about 11% occurred at day 6, compared to the PBS or DZR monotherapy group with a more pronounced weight loss of 25% (data not shown). Each experiment has been repeated at least twice with identical results.
Active EAE
In actively induced EAE (Fig. 1b) first clinical signs appeared at day 11. Due to the greater heterogeneity of active EAE only a mean score of 2·5 at disease maximum was reached (n = 3; range 0·5–4·0). Treatment regimen with MITOX (MITOX and DZR/MITOX) given on days 8, 10 and 12 in this experimental model completely prevented the onset of clinical disease. DZR monotherapy (n = 3) reduced clinical severity (P = 0·187 anova). In both experiments, animals treated with a MITOX-containing regimen exhibited less weight loss compared with PBS and DZR treated animals (data not shown).
Immunohistochemical analysis
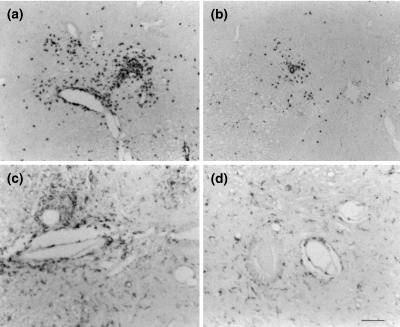
Figure 2 depicts widespread T cell and macrophage infiltration of the spinal cord in the recovery phase of AT-EAE in PBS-treated animals. In a quantitative analysis (Table 1) MITOX treatment significantly reduced macrophage and T cell infiltration (P = 0·049 Mann–Whitney U-test). Combination therapy of MITOX and DZR led to reduction of infiltration by a further 50%. DZR monotherapy reduced macrophage infiltration and T cell infiltration by a similar degree (P = 0·049 Mann–Whitney U-test). In actively induced EAE, T cell infiltration outnumbered macrophage infiltration by about 100% in sham-treated animals at day 15. DZR treatment reduced T cell infiltration only slightly, whereas MITOX almost completely prevented infiltration.
Fig. 2.
Immunohistochemical analysis of T cell (a,b) and macrophage (c,d) infiltration in PBS (a,c) versus DZR (b,d)-treated AT-EAE animals. Note marked reduction in perivascular infiltrates after DZR alone.
Table 1.
Analysis of infiltrates (cells/mm2) in histological sections of spinal cord of AT-EAE and actively induced EAE (10 sections/animal; three animals/group)
| AT-EAE | Active EAE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Macrophages/mm2 | Macrophages/mm2 | ||||||||
| ED-1-pos.Treatment | PBS | DZR | MITOX | DZR/MITOX | ED-1-pos.Treatment | PBS | DZR | MITOX | DZR/MITOX |
| Mean | 82 | 5·8 | 2·6 | 1·7 | Mean | 15·5 | 0·3 | 0·7 | 0·3 |
| s.d. | 10 | 2·1 | 1·9 | 0·5 | s.d. | 2·5 | 0·04 | 0·1 | 0·05 |
| B115/1-pos. | T-cells/mm2 | B115/1-pos. | T-cells/mm2 | ||||||
| Mean | 81·1 | 3·1 | 0·9 | 0·4 | Mean | 37·9 | 24·1 | 0·07 | 0·04 |
| s.d. | 12 | 0·4 | 0·5 | 0·03 | s.d. | 3·0 | 3·5 | 0·08 | 0·05 |
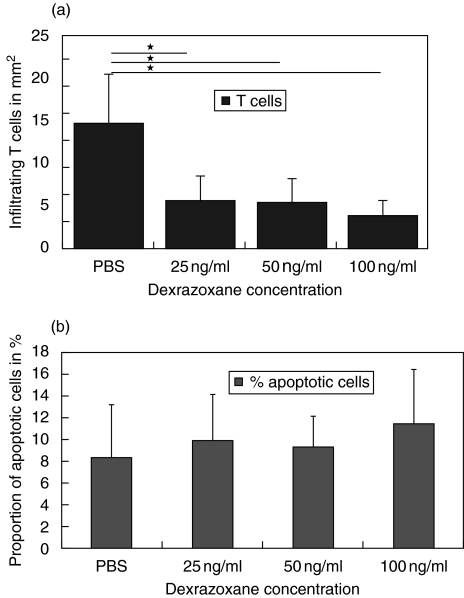
With regard to T cell apoptosis in situ at day 7 after induction of AT-EAE, treatment with DZR did not significantly augment the proportion of apoptotic T cells in dosages of 25–100 ng/ml although it reduced the absolute number of infiltrating T cells by about 66% (P = 0·049 Mann–Whitney U-test; see Fig. 3). No further dose dependence could be observed here.
Fig. 3.
Number of infiltrating T cells (a) and proportion of apoptotic T cells (b) in spinal cord sections (right ordinate) from animals with severe AT-EAE (day 7 p.i.) after treatment with DZR on days 1, 3, 5 (three animals/group, *P< 0·05, Mann–Whitney U-test).
Ex vivo experiments
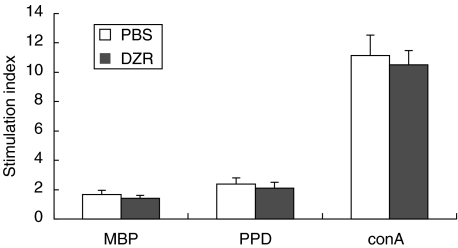
To delineate the mechanism responsible for the observed therapeutic effect of DZR therapy in EAE, proliferation assays with different antigens (MBP and PPD) and mitogen (ConA) were performed. T cells were isolated from lymph nodes of immunized animals treated previously with either DZR or PBS. As shown in Fig. 4 no significant difference in proliferative response to stimulation could be detected in lymph node cells ex vivo, arguing against an effect of DZR treatment on T-cell priming. In a stimulation assay of ex vivo-derived lymph node cells either from naive (data not shown) or gp-MBP immunized Lewis rats (Fig. 5) DZR addition to the culture medium diminished only slightly in vitro reactivity of cells towards the specific antigens. The addition of FeCl3 increased the overall proliferative response, an effect which could be inhibited partially by addition of DZR in a dose dependent manner. This indicates that Fe3+ is a potent co-stimulator of T cell reactivity under our experimental conditions. When calculating the stimulation index, FeCl3 did not affect specific reactivity significantly towards MBP as the autoantigen or the antigen PPD contained in inoculum used for immunization (P = 0·082).
Fig. 4.
Proliferation of T lymphocytes isolated from lymph nodes of Lewis rats treated previously with DZR versus treatment with PBS (two animals each, proliferation in triplicates, differences not significant by using Mann–Whitney U-test).
Fig. 5.
Proliferation of T lymphocytes isolated ex vivo from lymph nodes of Lewis rats immunized with MBP/CFA after addition of the indicated concentrations of DZR and FeCl3 in ordinate scales. (proliferation in triplicate).
Gelatinase activity
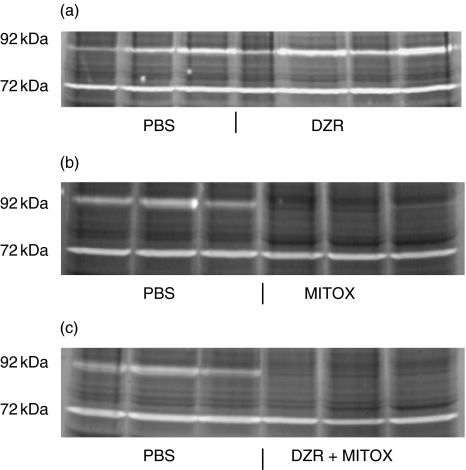
Treatment with DZR did not affect MMP-9 activity in AT EAE animals, whereas MMP-9 activity was abolished completely in MITOX and MITOX + DZR treated animals (see Fig. 6) corresponding to the significant suppression of disease with these treatment regimen. MMP-2 activity was not altered by treatment with MITOX or DZR.
Fig. 6.
Detection of MMP-9 and MMP-2 proteinase activity in spinal cord from Lewis rats by zymography (SDS–substrate gel analysis). Zone of clearing represents proteinase activity. Each lane represents spinal cord tissue from one animal 5 days postinjection of encephalitogenic T cells, treated either with DZR, MITOX or DRZ/MITOX. White areas represent gelatin digestion at levels of 92 kDa and 72 kDa (corresponding to MMP-9 and MMP-2). In contrast, clearing appears to be unchanged at the level of 72-kDa.
Apoptosis of lymph node cells
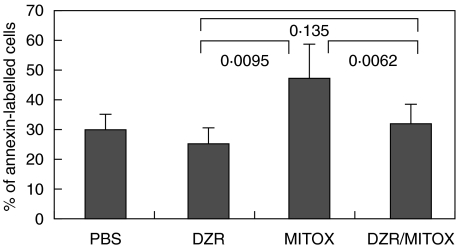
The apoptosis rate of lymph node cells ex vivo during clinical manifestation of EAE was altered significantly when animals were pretreated with MITOX with respect to DZR monotherapy (P = 0·0039, Mann–Whitney U-test) (see Fig. 7).
Fig. 7.
Flow cytometric analysis of apoptotic lymph node cells ex vivo (annexin staining). DZR, MITOX, DZR/MITOX represent results from lymph nodes of six animals at day 13 of EAE (Mann–Whitney U-test).
DISCUSSION
Our experiments studying the combination therapy of DZR and MITOX in an animal model of multiple sclerosis demonstrated an inhibitory effect of DZR monotherapy both on EAE actively induced by MBP and AT- EAE. In AT-EAE experiments an additive effect of DZR in combination with MITOX could be shown with regard to amelioration of clinical disease course and a decrease of cellular inflammation. In actively induced EAE early MITOX monotherapy completely prevented onset of clinical disease. Therefore, no conclusions regarding a synergistic effect of the addition of DZR can be drawn in the active EAE model employed here.
In vivo proliferation assays demonstrated that DZR only slightly inhibited lymph node cell responses. Addition of ferric ions led to a marked increase in reactivity which could be partially reversed by scavenging Fe3+ with DZR. Due to the short half-life of DZR of 2 h [15], these effects on T cell priming were not sustained, because ex vivo isolated T cells of DZR-treated animals did not differ in T cell reactivity when compared to T cells of PBS-treated animals.
The mechanism by which DZR reduces disease severity in EAE remains elusive. Although iron chelators are known to induce apoptosis in proliferating T cells [16] and have been proposed to serve as immunosuppressive agents [17], the analysis of apoptosis by immunofluorescence cytometry and immunohistochemical stainings do not indicate that DZR exerted its therapeutic effect by induction of T cell apoptosis in situ, because treatment of EAE with DZR either increased the proportion of apoptotic lymph node cells ex vivo nor increased the proportion of apoptotic T cells in spinal cords in situ.
A mild unspecific immunosuppressive effect as demonstrated by a dose-dependent decrease in proliferation assays obviously may not be instrumental in vivo, due to the short half-life of DZR. Iron has an obligatory role in T lymphocyte activation that may be related to the formation of reactive oxygen species. In addition, iron is believed to influence both migration and function of immune effector cells [18]. The histological analysis of T cell and macrophage infiltrates in spinal cords indicates that blood–brain barrier transmigration and recruitment of inflammatory cells to demyelinating lesions may be reduced significantly with DZR treatment. As shown in our study, these effects may not be mediated by modulation of MMP-9 or MMP-2 activity, as in our experiments the activity of this zinc ion containing enzyme in vivo was not affected differentially by the treatment with dexrazoxane when compared to PBS treatment. The DZR-related iron chelator EDTA had been shown previously to interact with MMP activity [19]. Only under certain conditions physiological concentrations of DZR may inhibit MMP activity (data not shown). Interestingly, in MITOX-treated animals the clinical benefit went parallel with a completely abolished MMP-9 activity, a finding which has not been reported earlier.
A therapeutic effect of iron chelating agents in EAE has been shown previously with desferrioxamine [18,20,21]. It has been stated that this effect requires that the chelating agent traverses the blood–brain barrier, because HES-desferrioxamine in a guinea pig model of EAE could not ameliorate EAE significantly due to its greater molecular weight [21]. DZR, however, does not cross the blood–brain barrier to any significant extent [22]. We therefore propose that DZR therapy in Lewis rat EAE may exert its main action outside the CNS.
Our results indicate that by scavenging iron DZR has a slight beneficial effect on actively induced EAE and AT-EAE, as shown by comparison of disease course and quantitative analysis of inflammatory infiltrates in spinal cords. This effect may depend directly on reducing bioavailability of iron to circulating T cells by DZR. As DZR inhibited only slightly proliferative responses to different antigens in proliferation assays of peripheral T lymphocytes, we suggest that the beneficial effect in EAE is mediated only partly by its immunusuppressive action. If one extrapolates our study to MS treatments, it is helpful to know that the addition of DZR to MITOX in EAE did not interfere negatively with the therapeutic effect of MITOX but rather exhibited additive curative effects. Clinical trials with a combination of MITOX and DZR in multiple sclerosis are now warranted to assess the cardioprotective role with the approved doses of MITOX and to evaluate the intrinsic immunosuppressive effects of DZR.
Acknowledgments
We appreciate the skillful technical assistance of Mrs B. Kugler and A. Amschler. We thank PD Dr B. Kieseier for helpful discussions. This work was supported by a grant from the Deutsche Multiple Sklerose Gesellschaft (DMSG), Hannover, Germany and funds from the state of Bavaria.
REFERENCES
- 1.Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–25. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 2.Baker D, O'Neill JK, Davison AN, et al. Control of immune-mediated disease of the central nervous system requires the use of a neuroactive agent: elucidation by the action of mitoxantrone. Clin Exp Immunol. 1992;90:124–8. doi: 10.1111/j.1365-2249.1992.tb05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson CM, Davison AN, Baker D, et al. Suppression of demyelination by mitoxantrone. Int J Immunopharmacol. 1991;13:923–30. doi: 10.1016/0192-0561(91)90045-9. [DOI] [PubMed] [Google Scholar]
- 4.Lublin FD, Lavasa M, Viti C, et al. Suppression of acute and relapsing experimental allergic encephalomyelitis with mitoxantrone. Clin Immunol Immunopathol. 1987;45:122–8. doi: 10.1016/0090-1229(87)90118-8. [DOI] [PubMed] [Google Scholar]
- 5.Ridge SC, Sloboda AE, McReynolds RA, et al. Suppression of experimental allergic encephalomyelitis by mitoxantrone. Clin Immunol Immunopathol. 1985;35:35–42. doi: 10.1016/0090-1229(85)90075-3. [DOI] [PubMed] [Google Scholar]
- 6.Fidler JM, DeJoy SQ, Smith FD, et al. Selective immunomodulation by the antineoplastic agent mitoxantrone. II. Nonspecific adherent suppressor cells derived from mitoxantrone-treated mice. J Immunol. 1986;136:2747–54. [PubMed] [Google Scholar]
- 7.Fidler JM, DeJoy SQ, Gibbons J., Jr Selective immunomodulation by the antineoplastic agent mitoxantrone. I. Suppression of B lymphocyte function. J Immunol. 1986;137:727–32. [PubMed] [Google Scholar]
- 8.Mather FJ, Simon RM, Clark GM, et al. Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep. 1987;71:609–13. [PubMed] [Google Scholar]
- 9.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Gold R, Giegerich G, Hartung HP, et al. T-cell receptor (TCR) usage in Lewis rat experimental autoimmune encephalomyelitis: TCR beta-chain-variable-region V beta 8.2-positive T cells are not essential for induction and course of disease. Proc Natl Acad Sci USA. 1995;92:5850–4. doi: 10.1073/pnas.92.13.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zettl UK, Gold R, Toyka KV, et al. In situ demonstration of T cell activation and elimination in the peripheral nervous system during experimental autoimmune neuritis in the Lewis rat. Acta Neuropathol. 1996;91:360–7. doi: 10.1007/s004010050437. [DOI] [PubMed] [Google Scholar]
- 12.Gold R, Schmied M, Giegerich G, et al. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71:219–25. [PubMed] [Google Scholar]
- 13.Brown PD, Levy AT, Margulies IM, et al. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990;50:6184–91. [PubMed] [Google Scholar]
- 14.Chan A, Reiter R, Wiese S, et al. Plasma membrane phospholipid asymmetry precedes DNA fragmentation in different apoptotic cell models. Histochem Cell Biol. 1998;110:553–8. doi: 10.1007/s004180050317. [DOI] [PubMed] [Google Scholar]
- 15.Narang PK, Hochester H, Reynolds RD, et al. Does the cardioprotectant dexrazoxane (DZR) affect doxorubicin (DOX) kinetics/dynamics? Proc Am Soc Clin Oncol. 1992;11:126. [Google Scholar]
- 16.Hileti D, Panayiotidis P, Hoffbrand AV. Iron chelators induce apoptosis in proliferating cells. Br J Haematol. 1995;89:181–7. doi: 10.1111/j.1365-2141.1995.tb08927.x. [DOI] [PubMed] [Google Scholar]
- 17.van Reyk DM, Sarel S, Hunt NH. In vitro effects of three iron chelators on mitogen-activated lymphocytes: identification of differences in their mechanisms of action. Int J Immunopharmacol. 1992;14:925–32. doi: 10.1016/0192-0561(92)90092-y. [DOI] [PubMed] [Google Scholar]
- 18.Bowern N, Ramshaw IA, Clark IA, et al. Inhibition of autoimmune neuropathological process by treatment with an iron-chelating agent. J Exp Med. 1984;160:1532–43. doi: 10.1084/jem.160.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quesada AR, Barbacid MM, Mira E, et al. Evaluation of fluorometric and zymographic methods as activity assays for stromelysins and gelatinases. Clin Exp Metastasis. 1997;15:26–32. doi: 10.1023/a:1018480222301. [published erratum appears in Clin Exp Metastasis 1997 May; 15: 339–40] [DOI] [PubMed] [Google Scholar]
- 20.Pedchenko TV, LeVine SM. Desferrioxamine suppresses experimental allergic encephalomyelitis induced by MBP in SJL mice. J Neuroimmunol. 1998;84:188–97. doi: 10.1016/s0165-5728(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 21.Willenborg DO, Bowern NA, Danta G, et al. Inhibition of allergic encephalomyelitis by the iron chelating agent desferrioxamine: differential effect depending on type of sensitizing encephalitogen. J Neuroimmunol. 1988;17:127–35. doi: 10.1016/0165-5728(88)90020-3. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman LR, Spencer CM. Dexrazoxane. A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs. 1998;56:385–403. doi: 10.2165/00003495-199856030-00009. [DOI] [PubMed] [Google Scholar]